 World Journal of Neuroscience, 2011, 1, 19-27 doi:10.4236/wjns.2011.12003 Published Online August 2011 (http://www.SciRP.org/journal/wjns/ WJNS ). Published Online August 2011 in SciRes. http://www.scirp.org/journal/W JNS Neural correlates of focused attention in cognitively normal older adults Jennifer R. Bowes1, Patr ick Stroman 1,2,3, Angeles Garcia1,4 1Centre for Neuroscience Studies, Queen’s University, Kingston, Canada; 2Department of Diagnostic Radiology, Queen’s University, Kingston, Canada; 3Department of Physics, Queen’s University, Kingston, Canada; 4Department of Medicine, Queen’s University, Kingston, Canada. Email: 6jrb2@queensu.ca Received 28 April 20 11; revised 1 8 May 2011; accepted 22 Jul y 2011. ABSTRACT Focused attention (FA) is among the cognitive func- tions that decline with aging. The Stroop task was used to investigate the neural correlates underlying FA in cognitively normal older adults. Twenty-one participants underwent a novel functional magnetic resonance imaging (fMRI) verbal Stroop task para- digm. Colour words were printed in an incongruent ink colour. Series 1 consisted of four blocks “Read the word” followed by four blocks “Say the colour of the ink”; Series 2 alternated between the two condi- tions. Functional data were analyzed using SPM5 to detect anatomical areas with significant signal inten- sity differences between the conditions. Within-group analyses of the “Say the colour of the ink” minus “Read the word” contrast yielded significant activa- tion in the left supplementary motor area, bilateral inferior frontal gyrus, bilateral precentral gyrus, left insula and right superior frontal gyrus (p < 0.05, un- corrected). These results, using verbal responses, are consistent with previous manual modality Stroop- fMRI studies in older adults. Verbal responses may provide a more suitable modality for older adults and certain patient populations. Keywords: fMRI; Focused Attention; Stroop 1. INTRODUCTION There is a growing interest in studying cognitive do- mains, such as attention and inhibitory control, which decline during the aging process. Older adults appear to be more susceptible to cognitive interference than y- ounger adults [1] and the frontal lobe, which is instru- mental in exercising attention control, is susceptible to some of the earliest and more rapid pathological changes that occur in the aging brain [2]. Consequently, patho- logical changes in the frontal lobe may contribute to the increased susceptibility of older adults to cognitive in- terference. More specifically, attention plays a critical role in restricting the sensory input load that enters our processing system [3], and attentional control is engaged, most importantly, in times of cognitive conflict [4]. Fo- cused attention (FA) biases the information we are ex- posed to by enhancing task-relevant information, and is used as a measure of the ability to inhibit irrelevant in- formation [5]. In order to understand the changes that occur with aging, and with diseases such as Alzheimer ’s Disease (AD), it is therefore very important to under- stand the changes that occur in the processes that control attention. The Stroop test [6] is a well-known experimental para- digm that introduces cognitive conflict by presenting colour names printed in a non-matching (incongruent) ink colour, and is considered the gold standard for as- sessment of FA. Cognitive interference reveals itself in the Stroop task by an increase in response latency and/or higher error rate when naming the ink colour [1,4]. However, the information that can be obtained about at- tentional processes may be significantly enhanced by combining the Stroop task with functional magnetic resonance imaging (fMRI). Finding the ne ural substr ates that underlie the Stroop effect may advance our under- standi ng of how humans maintain attention and perform the Stroop task [7]. The high spatial resolution and non- invasive nature of fMRI makes it a valuable tool for identifying the brain regions mediating FA. Initial Stroop- fMRI studies in young adults have provided the founda- tion to allow further investigations into the functional processes in healthy aging. However, the means by which we study the cognitive processes may require dif- ferent approaches based on the demographics of those being studied. While Stroop-fMRI studies in young  J. R. Bowes et al. / World Journal of Neuroscience 1 (2011) 19-27 20 adults have employed both verbal and manual response modalities, only the manual button press modality has been used with older adults [8-10]. By both self-report and performance, older adults have more difficulty using the button-box than the young adults [10]. The button- box modality adds additional cognitive demands by re- quiring subjects to remember which button corresponds to which colour stimuli. This modality may add task difficulty disproportionately for older adults. Just as cognitively normal older adults appear suscep- tible to cognitive interference, pathological conditions such as AD interfere with cognitive function that in- crease susceptibility to cognitive interference. An as- sessment of cognitive functioning in older adults and certain patient populations, such as those diagnosed with AD and mild cognitive impairment (MCI), are an im- portant part of clinical care. The Stroop task is among the tools used in a clinical setting to assess cognitive function, specifically FA, but has also been studied ex- tensively in experimental paradigms. If cognitively nor- mal older adults report difficulty during a manual mo- dality Stroop-fMRI paradigm, one might expect that pa- tients with cognitive impairments, such as those diag- nosed with AD, would find such a paradigm even more difficult. In order to gain a better understanding of the neural mechanism of FA in these populations, an ex- perimental paradigm suitable to the cognitive abilities of such persons must be applied in order to more accurately ascertain that the cognitive function of FA is being cap- tured. The use of verbal modality in Stroop-fMRI para- digms may be a suitable paradigm that allows one to investigate FA in older adults and in patient populations such as AD and MCI. In order to extrapolate the findings from experimental studies to the clinical setting, one must minimize the differences in the administration of the Stroop task across the two settings. The use of the verbal response modality in the fMRI task would be consistent with the verbal responses required in the pa- per version of the Stroop task that is used in clinical set- tings. Although many variations of the Stroop task paradigm exist, the majority of Stroop-fMRI studies have in- structed subjects to name the ink colour across incon- gruent, congrue nt, neutral or non-lexical stimuli to yield brain regions underlying Stroop interference. However, in fMRI, to best determine the brain regions underlying a given cognitive process, the differences between the two conditions being compared should be minimized as to isolate the cognitive process of interest. In the current study, the colour word stimuli presented are identical in all incongruent trials, with the only difference being the instructions given to the subject (“Read the word” or “Say the colour of the ink”). In this way, the current ex- perimental paradigm isolates the cognitive process of FA better than the previous Stroop-fMRI studies in older adults, and in doing so, improves the similarity between the fMRI Stroop task and the original paper version of the Stroop task. The current study offers a novel Stroop-fMRI para- digm that employed a verbal response modality using incongruent colour-word trials to yield the Stroop effect by contrast ing reading the word from saying the ink co- lour in cognitively normal older adults. 2. METHODS 2.1. Subject Population The study was approved by the University Research Ethics Board. All subjects gav e written informed consent prior to undergoing any study procedures. Twenty-one cognitively normal, independent community dwelling older adults (13 women, 8 men) were recruited. Subjects were all native or highly proficient English-speaking volunteers between the ages of 60 and 85 years (mean age SD, 72 ± 8), who could safely undergo a MRI, with no colour blindness, neurological or other medical conditions that could interfere with the task protocol. 2.2. Cognitive Assessment Subjects underwent a neuropsychological test battery to assess their general cognitive function including the fol- lowing tests : the Mini-Mental State Examinatio n (MMSE) [11], the Montreal Cognitive Assessment (MoCA) [12], the Mattis Dementia Rating Scale (DRS) [13], the Stroop test, the Trail Making Test- Part B (TMT-B) [14], and the California Verbal Learning Test-Version II (CVLT-II) [15]. Subjects were considered to have nor- mal cognitive abilities with the following test scores: MMSE 26, MoCA 26, DRS 123, Stroop colour- word score of > 65 and CVLT-II verbal recall sub-scores > 1.5 SD below the mean for age, sex and years of edu- cation. 2.3. fMRI Experimental Task The fMRI experiment was carried out at the Queen’s University Research MRI Facility. The Stroop test used was an adaptation from the word-colour incongruent task taken from the paper version of the Stroop test. The four colour word stimuli used in the experiment were: blue, green, tan and red. Colour word stimuli were pre- sented visually via back projection onto a screen that was viewed through a mirror attached to the head coil of the scanner. The presentation of the colour word stimuli was run using MatlabR2006b (The Mathworks, Natick, MA). An MR-compatible microphone with noise can- cellation was placed in front of the participant’s mouth to record the verbal responses to the presented colour C opyright © 2011 SciRes. WJNS  J. R. Bowes et al. / World Journal of Neuroscience 1 (2011) 19-27 Copyright © 2011 SciRes. 21 performed twice and h erein referred to as Series 2a and 2 b. word stimuli. Subjects were instructed to speak aloud, but quietly, and without excessive enunciation, in an attempt to minimize head motion while speaking. Verbal reminders to keep their head still and limit any body movements were made at short rest intervals between the series, if movement for a given subject was a concern. Subjects were given headphones to wear to reduce the noise of the MR scanner and to provide a means to communicate with the subject throughout the experiment. Padding was placed between the outside of headphones and the sides of the head coil to reduce the opportunity for the subjects to move their head. The experimental protocol was carried out as a block design with four functional series’ as displayed in Figure 1. Each block was 28 seconds and consisted of 16 colour word stimuli, each of which was presented for 1.75 seconds. Each block was separated by a 14-second rest period. Series 0 consisted of 4 blocks with colour word stimuli presented in black ink and participants were instructed to read the word. The instructions “Read the word” preceded the commencement of the first block with a fixation cross displayed during the remaining rest periods. A total of 91 images were acquired with a sequence scan time of ap- proximately 3 minutes. Series 1 and 2 consisted of 8 blocks with colour word stimuli presented in an incon- gruent ink colour with the rest period preceding each block displaying the instructions for the subsequent block. A total of 175 images were acquired with a se- quence scan time of approximately 6 minutes for each Series 1 and 2. In Series 1, participants were instructed to “Read the word” (herein referred to as “Word” condi- tion) in the first 4 blocks, and instructed to “Say the colour of the ink” (herein referred to as “Colour” condi- tion) in the last 4 blocks. In Series 2, blocks alternated between “Word” and “Colour” conditions. Series 2 was 2.4. Image Acquisition All imaging was acquired on a 3 Tesla Siemens Magne- tom Trio MRI system (Siemens Medical Systems, Er- lange n, Ge r ma n y) i n c o nj unc t i o n wit h a 1 2 -c ha nnel he ad coil. Motion correction software was used during scan- ning, and in the subsequent data analysis. Structural and functional sequences were acquired. A 176 slice, high- resolution anatomical scan was acquired with a T1- weighted 3D, MP-RAGE sequence (single shot, ascend- ing sequence in the sagittal plane with TR = 1760 msec, TE = 2.2 msec, flip angle = 9˚, FoV = 256 mm, voxel size = 1 mm3). Functional data was acquired along the anterior commissure-posterior commissure (AC-PC) line with blood oxygenation level dependent (BOLD) T2*- weighted echo planar imaging. Thirty-two axial slices (TR = 2000 msec, TE = 30 msec, flip angle = 78˚, FoV = 211 mm, voxel size = 3.3 mm cubic voxels) were ac- quired providing whole-brain coverage using interleaved acquisition. 2.5. Data Analysis Statistical analysis on demographics, cognitive assess- ment and performance accuracy was performed using SPSS, Version 17.0 (SPSS Inc., Chicago, IL). Subject’s verbal responses to colour word stimuli were recorded for analysis of performance accuracy on the Stroop- fMRI task. Condition blocks with less than 75% accu- racy were excluded from fMRI analysis. Errors were classified as incorrect, missed or corrected responses. Spatial pre-processing and statistical analysis of the imaging data was carried out using SPM5 (Wellcome Department of Cognitive Neurology, University College Figure 1. Stroop- fMRI experimental paradigm. In Series 0, 4 blocks of colour word stimuli were present ed in bl ack ink and p arti cipan ts were asked to read th e word. The first rest period displ ayed the instru ction of “Read the word”; t he remaining rest peri ods displayed a fixation cross. In Series 1, 2a, and 2b presented 8 blocks of colour word stimuli presented in an incongruent ink colour, with the rest period displaying the instructions to the subsequent condition block. In Series 1, par- ticipants were asked to ‘Read the word’ for the first 4 blocks, and to “Say the colour of the ink” for the last 4 blo cks. Series 2a and 2b are identical, with bl ocks alternated between t he “Read the word” and “Say the colour of the ink” conditions. WJNS  J. R. Bowes et al. / World Journal of Neuroscience 1 (2011) 19-27 22 London, England). The first two functional scans in each series were discarded from the analyses to allow the lon- gitudinal magnetization to reach a steady state. The fol- lowing pre-processing steps were carried out: realign- ment, co-registration, segmentation, normalization and smoothing. First, functional T2*-weighted images were spatially realigned to the first analyzed volume scanned in the run using a six-parameter rigid-body transforma- tion to compute a mean functional image. Next, the high resolution T1-weighted structural scan was co-registered to the mean realigned functional images. SPM5 com- bines segmentation, spatial normalization and bias cor- rection in a unified model approach [16]. The segmented images were then normalized into the standard stereotac- tic space of the Montreal Neurological Institute (MNI) template. Lastly, the functional images were smoothed with a Gaussian kernel of 8 mm × 8 mm × 8 m full width at half maximum. A 128-second temporal high pass filter (with 1/128 seconds (0.0078 Hz) as the fre- quency cut off) was applied t o the functional data to e x- clude low frequency fluctuatio ns and artifacts. Statistical analysis was performed using the univariate approach of the general linear model [17] whereby the box-car ref- erence function is convolved with the hemodynamic response function to account for the delay in the BOLD signal. For each subject, a design matrix was created with the rest periods, correct “Word” blocks and correct “Colour” blocks applied as regressors, in addition to the six realignment parameters. Error blocks were repre- sented as separate regressors in the design matrix, though not used in any contrasts. Voxel-wise statistical analysis was then carried o ut to dete rmine which voxels were acti- vated by the different experimental conditions. Fixed- effects analysis was applied to each participant resulting in statistical parametric maps (SPMs) for every contrast of interest, using a significance threshold of p = 0.001, uncorrected for multiple comparisons. Individual SPMs from each participant were then pooled together and entered into a random-effects analysis. A one- sample t-test was applied to in vestigate the within-group activa- tion level with a significance threshold of p = 0.05, un- corrected for multiple comparisons. To control for false positives, a spatial extent cluster threshold of 3 voxels was applied. The coordinates of voxels that survived the statistical threshold were entered into MRICro software [18], which uses an automated anatomical labeling (AAL) template [19] to report the localization of the activation. Significant voxels reported in SPM5 are re- ported in MNI space, and the AAL template was created based on the anatomical parcellation of the spatially nor malized si ngle- subj ect, se gmented high re solutio n T1 volume provided by MNI. Realignment parameters of motion-corrected and non motion-corrected functional data were used to estimate subject motion during the ex- perimental task. 3. RESULTS 3.1. Behavioural Results Demographic descriptives and mean neuropsychological test battery scores are listed in Table 1. In the paper ver- sion of the Stroop task administered as part of the test battery, all subjects were significantly better reading the word than nami ng the c olour of t he ink (p < 0.001). Both non-motion corrected and motion corrected data were viewed to inspect subject motion parameters. The average subject movement was estimated based on the rotation about and the translation along each of the 3 coordinate axes (x, y, and z). There was a mean (SD) translation of 2.85 1.193 mm and a rotation of 2.964 1.454 degrees for non-motion-corrected acquired images. For the motion corrected acquired images, the mean ( SD) translation was 1.786 0.681 mm and a rotation of 1.905 0.785 degrees. Performance accuracy during the Stroop-fMRI ex- periment was assessed. The mean (±SD) numberof total errors across all series was 8.00 ± 6.834. The mean (± SD) number of errors in Series 1, Series 2a and Series 2b were 1.38 ± 2.397, 3.57 ± 4.976 and 3.05 ± 4.511, re- spectively. There were no significant differences in the numbers of errors between Series 1 and 2a (p = 0.107), Series 1 and 2b (p = 0.144) and Series 2a and 2b (= 0.725). The mean (±SD) numbers of errors in each of the “Word” and “Colour” conditions were 2.90 ± 3.961 and 4.86 ± 4.983, respectively. Across all of the series, there Ta b l e 1 . Demographic descriptives and mean neuropsychologi- cal test battery scores. Cognitively Normal Older Adults N = 21 Mean (SD) Gender (M:F) 8:13 Age (years) 71.24 (5.682) Education (years) 16.10 (4.170) MMSE 29.62 (0.498) MoCA 27.00 (1.949) DRS Total Score 141.90 (2.047) Stroop Word Score Colour Score 112.00 (0.000) 96.24 (11.528) TMT-B (seconds)* 86.00 (20. 039) CVLT-II SDFR Raw Score LDFR Raw Score 10.191 (3.750) 10.429 (3.749) M = Male; F = Female; MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment; DRS = Dementia Rating Scale; TMT-B= Trail Making Test-Part B; CVLT-II = California Verbal Learning Test- Version II; SDFR = Short Delay Free Recall; LDFR = Long Delay Free Recall; *N = 20. C opyright © 2011 SciRes. WJNS 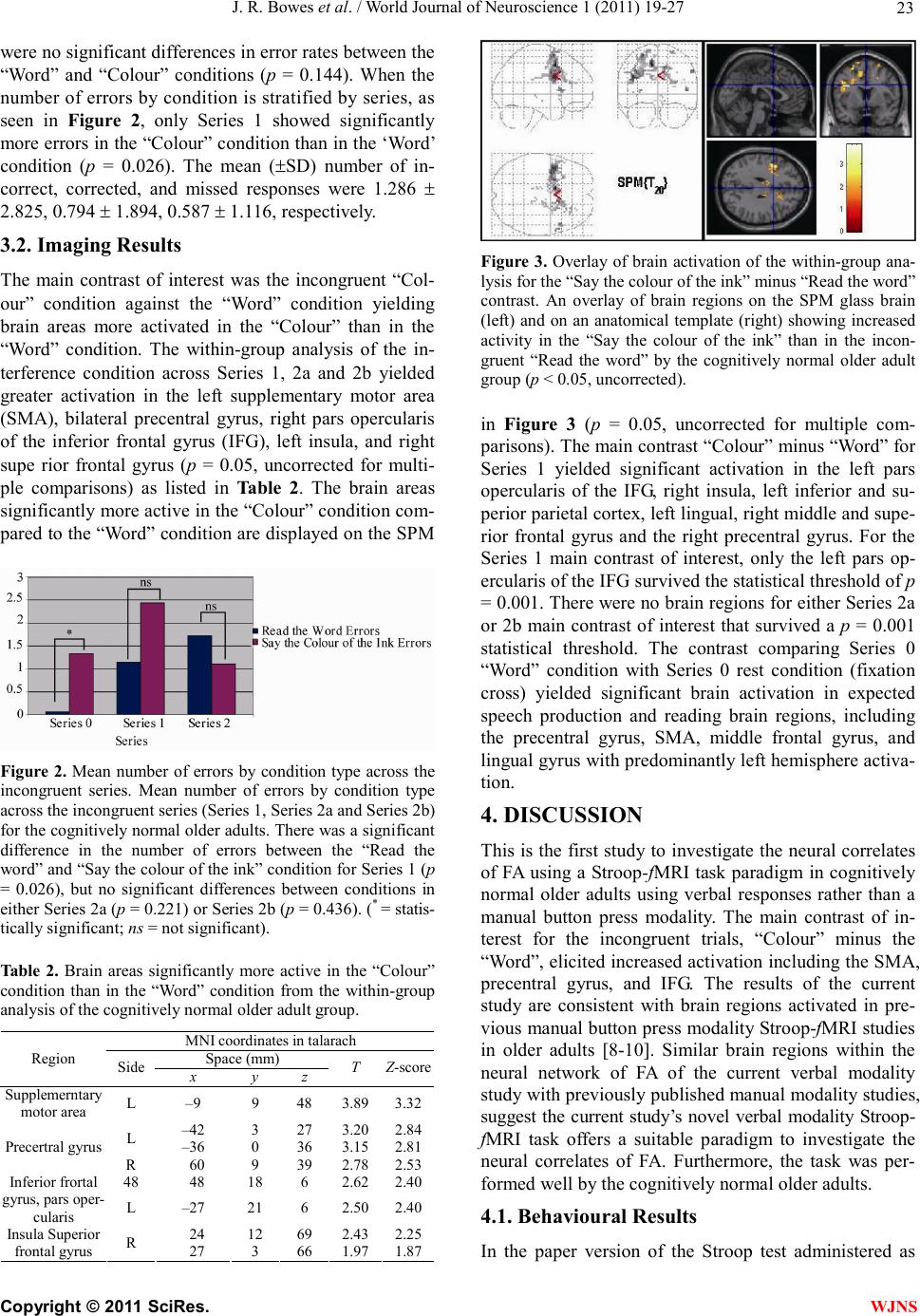 J. R. Bowes et al. / World Journal of Neuroscience 1 (2011) 19-27 23 were no significant differences in error rates between the “Word” and “Colour” conditions (p = 0.144). When the number of errors by condition is stratified by series, as seen in Figure 2, only Series 1 showed significantly more errors in the “Colour” condition than in the ‘Word’ condition (p = 0.026). The mean (SD) number of in- correct, corrected, and missed responses were 1.286 2.825, 0.794 1.894, 0.587 1.11 6, respect ively. 3.2. Imaging Results The main contrast of interest was the incongruent “Col- our” condition against the “Word” condition yielding brain areas more activated in the “Colour” than in the “Word” condition. The within-group analysis of the in- terference condition across Series 1, 2a and 2b yielded greater activation in the left supplementary motor area (SMA), bilateral precentral gyrus, right pars opercularis of the inferior frontal gyrus (IFG), left insula, and right supe rior frontal gyrus (p = 0.05, uncorrected for multi- ple comparisons) as listed in Table 2. The brain areas significantly more active in the “Colour” condition com- pared to the “Word” condition are displayed on the SPM Figure 2. Mean number of errors by condition type across the incongruent series. Mean number of errors by condition type across the incongruent series (S eri es 1, Series 2a an d Series 2b) for the cognitively normal older adults. There was a significant difference in the number of errors between the “Read the word” and “Say the colour of the ink” co ndition for S eries 1 (p = 0.026), but no significant differences between conditions in either Series 2 a (p = 0.221) or Series 2b (p = 0.43 6). ( * = st atis- tically significant; ns = not significant). Ta b l e 2. Brain areas significantly more active in the “Colour” condition than in the “Word” condition from the within-group analysis of the cognitively normal older adult group. MNI coordinates in talarach Space (mm) Region Side x y z T Z-score Supplemerntary motor area L –9 9 48 3.893.32 –42 3 27 3.202.84 L –36 0 36 3.152.81 Precer tral gyru s R 60 9 39 2.782.53 48 48 18 6 2.622.40Inferior frort al gyrus, pars oper- cularis L –27 21 6 2.502.40 24 12 69 2.432.25Insu la Superior frontal gyrus R 27 3 66 1.971.87 Figure 3. Overlay of brain activation of the within-group ana- lysis for the “Say the col our of the ink” minus “Read the word” contrast. An overlay of brain regions on the SPM glass brain (left) and on an anatomical template (right) showing increased activity in the “Say the colour of the ink” than in the incon- gruent “Read the word” by the cognitively normal older adult group (p < 0.05, uncorrected). in Figure 3 (p = 0.05, uncorrected for multiple com- parisons). The main contrast “Colour” minus “Word” for Series 1 yielded significant activation in the left pars opercularis of the IFG, right insula, left inferior and su- perior parietal cortex, left lingual, right middle and supe- rior frontal gyrus and the right precentral gyrus. For the Series 1 main contrast of interest, only the left pars op- ercularis of the IFG survived the statistical threshold of p = 0.001. There were no brain regions for either Series 2a or 2b main contrast of interest that survived a p = 0.001 statistical threshold. The contrast comparing Series 0 “Word” condition with Series 0 rest condition (fixation cross) yielded significant brain activation in expected speech production and reading brain regions, including the precentral gyrus, SMA, middle frontal gyrus, and lingual gyrus with pred ominantly left hemisp here activa- tion. 4. DISCUSSION This is the first study to investigate the neural correlates of FA using a Stroop-fMRI task paradigm in cognitively normal older adults using verbal responses rather than a manual button press modality. The main contrast of in- terest for the incongruent trials, “Colour” minus the “Word”, elicited increased activation including the SMA, precentral gyrus, and IFG. The results of the current study are consistent with brain regions activated in pre- vious manual button press modality Stroop-fMRI studies in older adults [8-10]. Similar brain regions within the neural network of FA of the current verbal modality study with previo usly publis he d manual modalit y studie s, suggest the current study’s novel verbal modality Stroop- fMRI task offers a suitable paradigm to investigate the neural correlates of FA. Furthermore, the task was per- formed well by the cognitively normal older adults. 4.1. Behavioural Results In the paper version of the Stroop test administered as C opyright © 2011 SciRes. WJNS  J. R. Bowes et al. / World Journal of Neuroscience 1 (2011) 19-27 24 part of the neuropsychological test battery, as expected, subjects were significantly better at reading the word than naming the colour of the ink. Interestingly, in the Stroop-fMRI experimental task, when errors were col- lapsed across all series, subjects did not make signifi- cantly more errors in the “Colour” condition than in the “Word” condition. This is likely a reflection of the ex- perimental design, although overall, very few errors were made during the Stroop-fMRI task. In Series 1, which consisted of four blocks of “Word” followed by four blocks of “Colour”, subjects made significantly more errors when naming the ink colour. Whereas, in Series 2a and 2b, which consisted of alternating “Word” and “Colour” conditions, there was no significant dif- ference in errors between the two conditions, and may reflect the difficulty subjects had in remembering which set of instructions was given. The majority of the “Word” errors were made in the alternating Series 2a and 2b. That is, prior to the introduction of the “Colour” condition, reading the word is a fairly automatic process for the subjects. However, once the “Colour” condition is introduced, reading the word becomes less automatic, and FA is likely required for both conditions because what is considered the irrelevant colour dimension, changes each block. 4.2. Imaging Results The main contrast of interest for the current study was the subtraction of the incongruent “Word” condition from the incongruent “Colour” condition. Significant activation was observed in the left SMA, bilateral pre- central gyrus, and right pars opercularis of the IFG, left insula and right superior frontal gyrus. The neuroimag- ing results of the study are in keeping with the results from previous Stroop-fMRI studies of older adults. Com- mon activated regions between the work of Zysset et al. [9] and the current findings include the IFG and superior frontal gyrus. In the Stroop-fMRI study of Milham et al. [8], older adults activated a network of structures in- cluding the IFG, as found in the current study, and the SMA showed increased activity specific to the presence of co nflicti ng colour i nfor mat ion. The study’s findi ng of right frontal gyri was also found in the Stroop-fMRI study of Langenecker et al. [10]. Similar regions of brain activation were found be- tween the current study and previous Stroop-fMRI stud- ies of older adults despite methodological differences. Chief among the methodological differences was the use of a verbal response modality in this study. The greatest concern with use of verbal responses in an experimental paradigm is the increased susceptibility to motion arti- fact inherent with verbal responses. However, means to account for and correct some of the movements made by subjects during the experimental task were undertaken to min imi ze subj ec t mo tio n and to mi ni miz e it s effe ct on the imaging data in the present study, as described in the Methods section. Motion-corrected software, which in- cluded the automated image registration for motion cor- rection of fMRI time-series during the pre-processing of the functional data, was also applied to reduce some of the effects caused by changes in position between suc- cessive images. Although uncorrected motion can con- found functional data by causing signal changes, it is those voxels that lie close to tissue boundaries that are most susceptible [20]. Given our knowledge of the neu- ral substrates that underlie the Stroop task, the brain re- gions expected to be involved with this task are, for the most part, not in close proximity to areas of high con- trast or tissue boundaries. Analyzing the motion pa- rameters and visually inspecting the motion data allows one to consider the influence the motion has on the func- tional results. Subject inter-scan movement can be esti- mated using the motion parameters obtained from the spatial realignment step in pre-processing, whereby each image in the time series is compared to the first, refer- ence image of that time series. The time series images of translation and rotation motion parameters were visually inspected to exclude any subject with excessive motion. Given the verbal modality of the experimental task, ex- cessive motion was considered if both translation and rotation measures were >5 mm and degrees, respectively. Using this cut-off criterion, no subjects were excluded from the analysis. The motion-corrected functional data were used in subsequent analyses and likely reflects the influence of subject motion on the imaging results. However, the non-motion corrected data provides the best estimate of subject motion during the experimental run. By decreasing the length of the experimental para- digm and by giving more reminders to subjects to re- main still, the motion parameters for the older adults may be lowered to improve the integrity of the imaging results. The current study had a larger older adult sample size than previous Stroop-fMRI studies by Milham et al. [8] and Langenecker et al. [10]. The mean age of the older adults in the current study (71 years) was comparable to the mean age of older adults in the Langenecker et al. [10] study (71.1 years) and just slightly older than those in the Milham et al. [8] study (68 years). Among the network of brain regions commonly activated by older adults are the anterior cingulate cortex (ACC)/pre-sup- plementary motor area (pre-SMA), middle, superior and inferior frontal gyri, and superior and inferior parietal lobules. Older adults have shown increased activation, predominantly in the left hemisphere, in the prefrontal cortex, pre-SMA and left inferior frontal gyrus relative C opyright © 2011 SciRes. WJNS  J. R. Bowes et al. / World Journal of Neuroscience 1 (2011) 19-27 25 to younger adults. These results suggest that older adults rely on, and recruit additional brain areas, particularly pre- frontal regions during Stroop task performance [21]. Further more, Milham et al. [8], suggested that the parie- tal cortex has greater involvement in younger than older adults, with older adults reliant on the more anterior, frontal brain regions. This is supported by the findings of the current study where the majority of brain regions underlying Stroop performance lay within the frontal cortex. Implementation of cognitive control may rely on modulation of bilateral inferior frontal regions [22] with activation of the left inferior frontal region consistently found in Stroop-fMRI studies. The inferior frontal region may reflect phonology as this area becomes more active when viewing words and during the retrieval, selection, maintenance or evaluation of semantic knowledge [23]. The inferior frontal region is also home to Broca’s area for speech production, and activation of this region in Stroop interference may reflect the response selection competition involving speech articulatory processes [24]. Stroop interference appears to be mediated by competing subvocal articulatory responses in both manual and ver- bal response modalities suggesting this mediation is not specific to overt speech paradigms [24]. In a positron emission tomography (PET) study, only the left IFG was consistently activated during the interference condition [25]. The left inferior precentral sulcus is located adja- cent to the pars opercularis of the IFG and is thought to play a critical role in processing incongruent Stroop stimuli [24, 25]. The precentral gyrus may correspond to the frontal eye fields [26], which have been shown to be an important area in top-down allocation of attention [27]. The SMA, important for the initiation and control of motor and speech functions [28], and the pre-SMA, involved in encoding and selection of word information [29], are regions found within the medial frontal cortex and have been found to be activated in numerous Stroop- fMRI studies [8,10,30,31]. Located within the pre-SMA region is the superior frontal gyrus, which is also fre- quently activated in the incongruent Stroop condition [8, 10,30,32]. According to Taylor et al. [25], the cingulate sulcus/SMA region is involved in resolving response conflict. Brain regions involved in response generation include the cerebellum, SMA and precentral gyri [32]. Given the current Stroop task whereby subjects are re- quired to initiate a verbal response, the activation of the SMA and precentral gyri support the role these regions may play in response generation. The insula is believed to be involved in verbal working memory and selective attention tasks [7] and contributes to cognitive control [9]. Among the early neuroimaging studies of the Stroop task, the Stroop effect almost unanimously activated the ACC. However, as the Stroop task became used in more studies, and with variations in its paradigm used, the ACC was not consistently activated. This lead Mead et al. [24] to suggest that demonstrating ACC activation may depend on methodological factors in the experi- mental design. In the current study, the main contrast of interest for Stroop interference did not detect activity within the ACC. Contrasts comparing incongruent col- our words to non-lexical stimuli such as colour blocks, hatches or crosses have shown more consistent ACC activation while contrasts with lexical stimuli, as in the curr ent stud y, ha ve resul ted in less freq uent AC C activa - tion [24]. Some authors have postulated that the atten- tional demands are similarly enhanced on incongruent and congruent trials [33], or that the ACC was activated at approximately the same intensity across the incon- gruent, congruent, and neutral conditions [24]. Further- mor e , M il ha m et al. [8] showed that for older adults, the mere presence of competing colour information of in- congruent trials was enough to produce increases in the ACC. The current experimental paradigm consisted largely of incongruent trials, so if the presence of incon- gruent trials alone produces ACC activation, contrasting the two incongruent conditions will not reveal the com- mon ACC activity in the two conditions. In addition, lesion studies have demonstrated that in some patients with extensive ACC damage, their Stroop performance was unimpaired [21]. These results suggest that although the ACC may be involved in mediating the Stroop effect, it appears that Stroop task performance does not require its activation to resolve the cognitive conflict presented by the Stroop paradigm. The current study used verbal responses and con- trasted incongruent trials of “Read the word” from “Say the colour of the ink” conditions in a Stroop-fMRI task paradigm that more closely mimics the paper, clinical administration of the Stroop task, and whose use of a verbal modality, may be better suited to an older adult population. Changes in cognitive function occur with normal aging, but are exacerbated in pathological dis- eases such as AD. Attentional deficits, including im- pairments in FA, have been observed in patients with AD and MCI [34]. Functional imaging studies with AD and MCI patients would allow for the study of the neural mechanisms underlying their reduced performance on FA tasks such as the Stroop task. However, therein lies the challenge of creating an experimental paradigm to investigate the neural correlates of FA, that patients with diminished cognitive functioning can comprehend and perform as instructed. The Stroop-fMRI task was per- formed well by the older adults, and suggests that this experimental paradigm, with the use of verbal responses instead of the more complicated manual responses, may C opyright © 2011 SciRes. WJNS  J. R. Bowes et al. / World Journal of Neuroscience 1 (2011) 19-27 26 allow the neural correlates of FA in patients with patho- logical diseases, such as AD to be investigated. 5. CONCLUSIONS Combined with preventative measures to minimize mo- tion and its effects on the resulting image data, verbal responses may offer a more appropriate modality for use in an older adult population. The novel Stroop experi- mental paradigm employed in the current study found similar regions of activation than previous Stroop-fMRI studies of older adults using the manual response modal- ity, sugge sting that despite a difference in modality used, the results reveal the neural correlates of FA. Further- more, the verbal task paradigm used in this study may also be useful in studying the neural correlates of FA in patient populations with known impairments of FA. The use of incongruent colour words stimuli across both ex- perimental conditions of the study may provide a means to identify brain areas supporting FA, beyond those, such as the ACC, which may activate upon the presentation of conflicting information o f the incongruent word stimuli. 6. ACKNOWLEDGMENT The study was supported by a Queen’s University CTAQ Endowment Fund grant. REFERENCES [1] Spieler, D.H., Balota, D.A. and Faust, M.E. (1996) Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer’s type. Journal of Experimental Psychology: Human Perception and Performance, 22, 461-4 79. do i:10.1037/0096- 1523.22.2.461 [2] Whelihan, W.M. and Lesher, E.L. (1985) Neuropscy- hological changes in frontal functions with aging. De- velopmental Neuropsychology, 1, 371 - 3 80. doi:10.1080/87565648509540321 [3] Johnson, A. and Proctor, R.W. (2004) Attention theory and practice. SAGE Publications, Inc., Thousand Oak [4] Kane, M.J. and Engle, R.W. (2003) Working-memory capacity and the control of attention: The contribution of goal neglect, response competition, and task set to Stroop interferences. Journal of Experimental Psychology Gen- eral, 13 2, 47-7 0. doi:10.1037/0096-3445.132.1.47 [5] Townsend, J., Adamo, M. and Haist, F. (2006) Changing channels: An fMRI study of aging and cross-modal atten- tion shifts. NeuroImage, 31, 1682 - 16 92. doi:10.1016/j.neuroimage.2006.01.045 [6] Stroop, O.R. (1935) Studies of interference in serial ver- bal reaction. Journal of Experimental Psychology, 18, 643-662. doi:10.1037/h0054651 [7] Leung, H.-C., Skudlarski, P., Gatenby, J.C., Peterson, B. S. and Gore, J.C. (2000) An Event-related functional MRI study of the stroop color word interference task. Cerebral Cortex, 10, 552-5 6 0. doi:10.1093/cer cor/10 .6.552 [8] Milham, M.P., Erikson, K.I. Banich, M.T., Kramer, A.F., Webb, A., Wszalek, T. and Cohen, N.J. (2002) Atten- tional control in the aging brain: Insights from an fMRI Study of the stroop task. Brain and Cognition, 49, 277- 296. doi:10.1006 /brcg.2001 .1501 [9] Zysset, S., Schroeter, M.L., Neumann, J. and Von Cramon, D.Y. (2007) Stroop interference, hemodynamic response and aging: An event-related fMRI study. Neuro- biology of Agi ng , 28, 937- 9 46 . do i:10.1016/j.neurobiolaging.2006.05.008 [10] Langenecker, S.A., Nielson, K.A. and Rao, S.M. (2004) fMRI of healthy older adults during Stroop interference. NeuroImage, 21, 192-200. doi:10.1016/j.neuroimage.2003.08.027 [11] Folstein, M., Folstein, S. and McHugh, P.R. (1975) Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Research, 12, 189-198 . do i:10.1016/0022-3956(75)90026-6 [12] Nasreddine, Z.S., Phillips, N.A., Bédirian, V., Charbon- neau, S., Whitehead, V., Collin, I., Cummings, J.L. and Chertkow, H. (2005) The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive im- pairment. Journa l of the A merican Geria trics Soci ety, 53, 695-699. doi:10.1111/j.1532-5415.2005.53221.x [13] Mattis, S. (1988) Dementia rating scale. Professional manual. Psychological Assessment Resources, Odessa. [14] Army Individual Test Battery (1944) Manual of direc- tions and scoring. War Department, Adjunct General’s Office, Washington, DC [15] Delis, D.C., Kramer, J.H., Kaplan, E. and Ober, B.A. (1987) California verbal learning test: Adult version manual. The Psychologic a l Corp o ra tion, San A nto nia . [16] Ashburner, J. and Friston, K.J. (2005) Unified segmeta- tion. Neuroimage, 26, 839-851. doi:10.1016/j.neuroimage.2005.02.018 [17] Friston, K.J., Holmes, A.P., Worsley, K.J., Poline, J.P., Frith, C.D. and Frackowiak, R.S.J. (1994) Statistical pa- rametric maps in functional imaging: A general linear approach. Human Brain Mapping, 2, 189- 210 . doi:10.1002/hbm.460020402 [18] Rorden, C. and Brett, M. (2000) Stereotaxic display of brain lesions. Behavioural Neurology, 12, 191-200. [19] Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., Mazoyer, B. and Joliot, M. (2002) Automated anatomical labeling of acti- vations in SPM using a macroscopic anatomical parcella- tion of the MNI MRI single-subject brain. NeuroImage, 15, 273- 289 . doi:10.1006/nimg.2001.0978 [20] Johnstone, T., Ores Walsh, K.S., Greischar, L.L., Alex- ander, A.L., Fox, A.S., Davidson, R.J. and Oakes, T.R. (2006) Motion correction and the use of motion covari- ates in multiple-subject fMRI analysis. Human Brain Mapping, 27, 779-788. doi:10.1002/hbm.20219 [21] Adleman, N.E., Menon, V., Blasey, C.M., White, C.D., Warsofsky, I.S., Glover, G.H. and Reiss, A.L. (2002) A developmental fMRI study of the Stroop color-word task. NeuroImage, 16, 61-75. doi:10.1006/nimg.2001.1046 [22] Egner, T. and Hirsh, J. (2005) The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage, 24, 539-547. doi:10.1016/j.neuroimage.2004.09.007 C opyright © 2011 SciRes. WJNS  J. R. Bowes et al. / World Journal of Neuroscience 1 (2011) 19-27 Copyright © 2011 SciRes. 27 WJNS [23] Banich, M.T., Milham, M.P., Jacobson, B.L., Webb, A., Wszalek, T., Cohen, N.J. and Kramer, A.F. (2001) Atten- tional selection and the processing of task-irrelevant in- formation: Insights from fMRI examinations of the Stroop task. Progress in Brain Research, 134, 459- 4 70 . doi:10.1016/S0079-6123(01)34030-X [24] Mead, L.A., Mayer, A.R., Bobholz, J.A., Woodley, S.J., Cunningham, J.M., Hammeke, T.A. and Rao, S.M. (2002) Neural basis of the Stroop interference task: Response competition or selective attention? Journal of the Inter- national Neuropsychological Society, 8, 73 5- 7 42. do i:10.1017/S1355 617702860015 [25] Taylor, S.F., Korblum, S., Lauber, E.J., Minoshima, S. and Koeppe, R.A. (1997) Isolation of specific interfer- ence processing in the Stroop task: PET activation stud- ies. NeuroImage, 6, 81-9 2. doi:10.1006/nimg.1997.0285 [26] Paus, T. (1996) Location and function of the human frontal eye-field: A selective review. Neuropsychologia, 34, 475- 483 . doi:10.1016/0028-3932(95)00134-4 [27] Corbetta, M. (1998) Frontoparietal cortical networks for directing attention and the eye to visual locations: Iden- tical, independent, or overlapping neural systems. Pro- ceedings of the National Academy of Sciences, USA, 95, 831-838. doi:10.1073/pnas.95.3.831 [28] Riecker, A., Mathiak, K., Wildgruber, D., Erb, M., Her- trich, I., Grodd, W. and Ackermann, H. (2005) fMRI re- veals two distinct cerebral networks subserving speech motor control. Neurology, 64, 700-7 06. doi:10.1212/01.WNL.0000152156.90779.89 [29] Alario, F., Chainay, H., Lehericy, S. and Cohen, L. (2006) The role of the suppl ementary motor area (SMA) in word production. Brain Research, 1076, 129-143. doi:10.1016/j.brainres.2005.11.104 [30] Derrfuss, J., Brass, M., Neumann, J. and von Cramon, D. Y. (2005) Involvement of the inferior frontal junction in cognitive control: Meta-analyses of switching and Stroop studies. Human Brain Mapping, 25, 22-34. doi:10.1002/hbm.20127 [31] Banich, M.T., Milham, M.P., Atchley, R., Cohen, N.J., Webb, A., Wszalek, T., Kramer, A.F., Liang, Z., Wright, A., Shenker, J. and Magin, R. (2000). fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. Journal of Cogni- tive Neuroscience, 12, 988-1000. doi:10.1162/08989290051137521 [32] Price, C.J., Moore, C.J. and Frackowiak, R.S. (1996) The effect of varying stimulus rate and duration on brain ac- tivity during reading. NeuroImage, 3, 40-52. doi:10.1006/nimg.1996.0005 [33] Melcher, T. and Gruber, O. (2006) Oddball and incongru- ity effects durin g Strop task performance: A comparative fMRI study on selective attention. Brain Research, 1121, 136-149. doi:10.1016/j.brainres.2006.08.120 [34] Parasuraman, R., Greenwood, P.M. and Sunderland, T. (2002) The apolipoprotein E gene, attention and brain function. Neuropsychology, 16, 254-274. do i:10.1037/0894- 4105.16.2.254
|