 American Journal of Anal yt ical Chemistry, 2011, 2, 491-499 doi:10.4236/ajac.2011.24059 Published Online August 2011 (http://www.SciRP.org/journal/ajac) Copyright © 2011 SciRes. AJAC Sequential Injection Analysis of Ampicillin in Pharmaceuticals by Using Potentiometric Detectors Based on PVC and Sol-Gel Membranes Nestor Zárate1, Alberto N. Araujo2, M. Conceiçao B. S. M. Montenegro2, Ricardo Pérez-Olmos1* 1Dpto. Química Analítica, Escuela Universitaria de Ingeniería Técnica Industrial, Universidad del País Vasco, Plaza de la Casilla 3, 48012, Bilbao, 2REQUIMTE, Dpto. de Química Física, Faculdade de Farmacia, Universidade do Porto, Rua Anibal Cunha 164, 4050 Porto, Portugal E-mail: qappeolr@lg.ehu.es Received April 1, 2011; revised May 12, 2011; accepted May 26, 2011 Abstract This work describes the construction and the evaluation of the general performance of new ampicilli- nate-selective electrodes based on manganese (III) tetraphenylporphyrin [Mn(III) TPP-Cl] as ionophore, in- corporated in both PVC and sol-gel membranes and directly applied onto a conductive graphite/epoxy resin support. The units were constructed without inner reference solution adopting conventional configuration and in the case of PVC membrane the tubular configuration was also adopted. The good working character- istics of these electrodes, made possible its application to the determination of ampicillin in pharmaceuticals formulations, both in conventional batch analysis and in flow conditions, when the electrodes were coupled to a SIA system. In the last case the potentiometric sensors presented linear response towards ampicillin concentration between 5.0 × 10–4 and 5.0 × 10–2 mol·l–1 with slopes of –57.4 and –63.5 mV·dec–1 for the PVC and sol-gel membranes, respectively. The developed procedures enable mean relative standard deviations better than 3% for all the samples analysed. The obtained results do not statistically differ from those fur- nished by applying the HPLC reference method. Keywords: Ampicillin, SIA, Potentiometric Detection, Pharmaceutical Formulations 1. Introduction Several PVC membranes based on metalloporphyrins have been used on the construction of selective elec- trodes for the potentiometric determination of organic species with pharmaceutical interest such us: salicylate [1], penicillin-G [2], valproate [3,4], ibuprofen [5], 2-hydroxybenzyhydroxamate [6] and diclofenac [7]. The use of plasticized PVC membranes for electrodes con- struction has the disadvantage of the gradual leaching of the plasticizer and the electroactive components from the membrane into the sample solution. This fact deteriorates some sensor characteristics like slope and signal-to-noise ratio, thus limiting the durability of these membranes [8], being this effect more important when the electrode is used in flow conditions. Siloxane polymers are very promising materials for the development of ion-selective membranes by the way of sol-gel technology which have permitted to design hybrid inorganic-organic materials, giving an access to the fabrication of glasses and films of network structure with a controlled porosity and thick- ness which showed high physical and chemical stability [9]. Sol-gel process has been employed to prepare ISEs selective to chloride [10,11] and copper [12] by using tridodecylmethylammonium chloride and thiosemicar- bazone, respectively, as ionophores. Recently, a val- proate-selective electrode based on Mn (III)-tetrapheny- lporphyrin incorporated in a sol-gel matrix was previ- ously reported [4]. Some of these sol-gel sensor mem- branes were used as potentiometric detectors in FIA [11] and SIA [4] systems. Ampicillin is one of the most important antibiotics since exerts an effective bactericidal action against both Gram-positive and Gram-negative organisms. Potenti- ometric determination of ampicillin has been initially carried out by using ISEs sensitive to ampicillinate with  N. ZÁRATE ET AL. 492 membranes based on different ionophores such as: qua- ternary ammonium salts [13,14], ampicillin-phosphotun- gstate ion pair [15] and azacompounds and metal phta- locyanines [16]. The use of penicillinase have been pro- posed to construct enzyme electrodes which were applied to the determination of various -lactamic antibiotics [17,18]. The use of conventional and enzyme potenti- ometric detectors in pharmaceutical analysis has been receantly reviewed [19,20]. In this work, a novel ampicillinate selective electrode has been constructed and evaluated using the metal- loporphyrin [Mn (III)-TPP-Cl]. The solid-contact elec- trodes were prepared both with PVC and sol-gel mem- branes, with conventional configuration. In the case of PVC membrane a tubular configuration was also per- formed. The constructed detectors were coupled to a se- quential injection analysis (SIA) system and applied to the analytical control of ampicillin in pharmaceutical formulations (injectables). 2. Experimental 2.1. Reagents and Solutions The sensor membrane for both PVC and sol-gel elec- trodes were prepared using 5,10,15,20 tetrapehenylpor- phirinate manganese (III) (Mn(III)TPP-Cl) as ionophore. The PVC membrane electrodes consist in a mixture of o-nitrophenyloctylether (o-NPOE) as plasticizer, and high molecular weight PVC as inert matrix, previously dissolved in tetrahydrofuran. The sol-gel membrane elec- trodes were prepared using methyltrietoxysilane (MTES), as silanol precursor, ethanol, hydrochloric acid and poly- ethyleneglycol (PEG 6000), for avoiding a fast mem- brane drying, and deionized water. The conductive sup- port, in both electrodes, was a mixture (1:1.2 w/w) of graphite powder and a non-conductive epoxy resin (Ar- aldite M and hardener HY) respectively. For the PVC electrodes, the stock solution used to prepare the calibration curves in the potentiometric de- terminations was a 1.0 × 10–1 mol·l–1 sodium ampicillin prepared in a 5.0 × 10–2 mol·l–1 dihydrogenphosphate solution (pH 4.5). In the case of the sol-gel electrodes, the sodium ampicillin stock solution was dissolved in a 1.0 × 10–2 mol·l–1 morpholinoethanesulfonic acid solu- tion (pH 3.8). These ampicillin stock solutions were pre- pared daily because of its instability. The 5.0 × 10–2 mol·l–1 dihydrogenphosphate solution was used for the ionic strength and pH adjustment and also as carrier solution in the potentiometric determina- tion with PVC membrane electrodes in flow conditions. Besides the outer chamber of the double junction refer- ence electrode was filled up with the above mentioned solution. In the case of the sol-gel membrane electrodes the 1.0 × 10–2 morpholinoethanesulfonic acid solution was used for the ionic strength and pH adjustment, as carrier solution and also for filling up the outer chamber of the double junction reference electrode. Both ISA/ carrier solutions contained 1.0 × 10–8 mol·l–1 sodium am- picillin for the rapid reestablishment of the potentiomet- ric baseline. For the chromatographic assays the mobile phase A consists in: 0.5 ml of diluted acetic acid solution (1.2 g of glacial acetic acid in 10 ml of deionized water), 50 ml of a 2.0 × 10–1 mol·l–1 dihydrogenphosphate solution and 50 ml of acetonitrile was made up to 1000 ml with de- ionized water. Mobile phase B was prepared in the same way than mobile phase A but in this occasion 400 ml of acetonitrile were added. Both mobile phases were de- gasified and filtered through a Millipore 0.45 µm filter. All solutions were prepared with distilled and deion- ized water with conductivity less than 0.1 µS·cm–1, and analytical grade reagents were used without any addi- tional purification. 2.2. Apparatus Potentiometric measurements were carried out by using a Crison 2002 potentiometer (sensitivity 0.1 mV) cou- pled to an electrode switcher, of the same brand, that recorded the potential difference between an Orion Re- search 92-02-00 double junction reference electrode and the corresponding indicator electrode. To determine the pH value of all solutions an Orion Research 91-02-00 combined glass electrode was used. In constructing the SIA system (Figure 1), a Gilson Minipulse 3 four-channel peristaltic pump was used to ensure flow of the solutions. A CheminertTM C25-3 188D multiposition rotatory valve of 8 ports, electrically actuated, was used to select an adequate solution. With the purpose of guaranteeing the maximum reproducibil- ity of the aspirated and forward volumes, an NResearch 161 T031 three-way solenoid valve (SV) was placed between the peristaltic pump and the selection valve. All of the SIA system components were connected with PTFE tubes (i.d. 0.8 mm). The reaction coil was 50 cm in length and the holding coil was 200 cm and was coiled over a plastic net. The selective and the reference elec- trodes were fitted in home-made plastic supports con- structed as described elsewhere [21] with slight modifi- cations. With the aim of eliminating electric noise, the constructed electrodes were connected to a home-made ground electrode [22]. For the data adquisition a single channel Kipp&Zonen BD11 stripchart recorder was used. All the SIA system operations were controlled by a com- puter through an Advantech Copyright © 2011 SciRes. AJAC 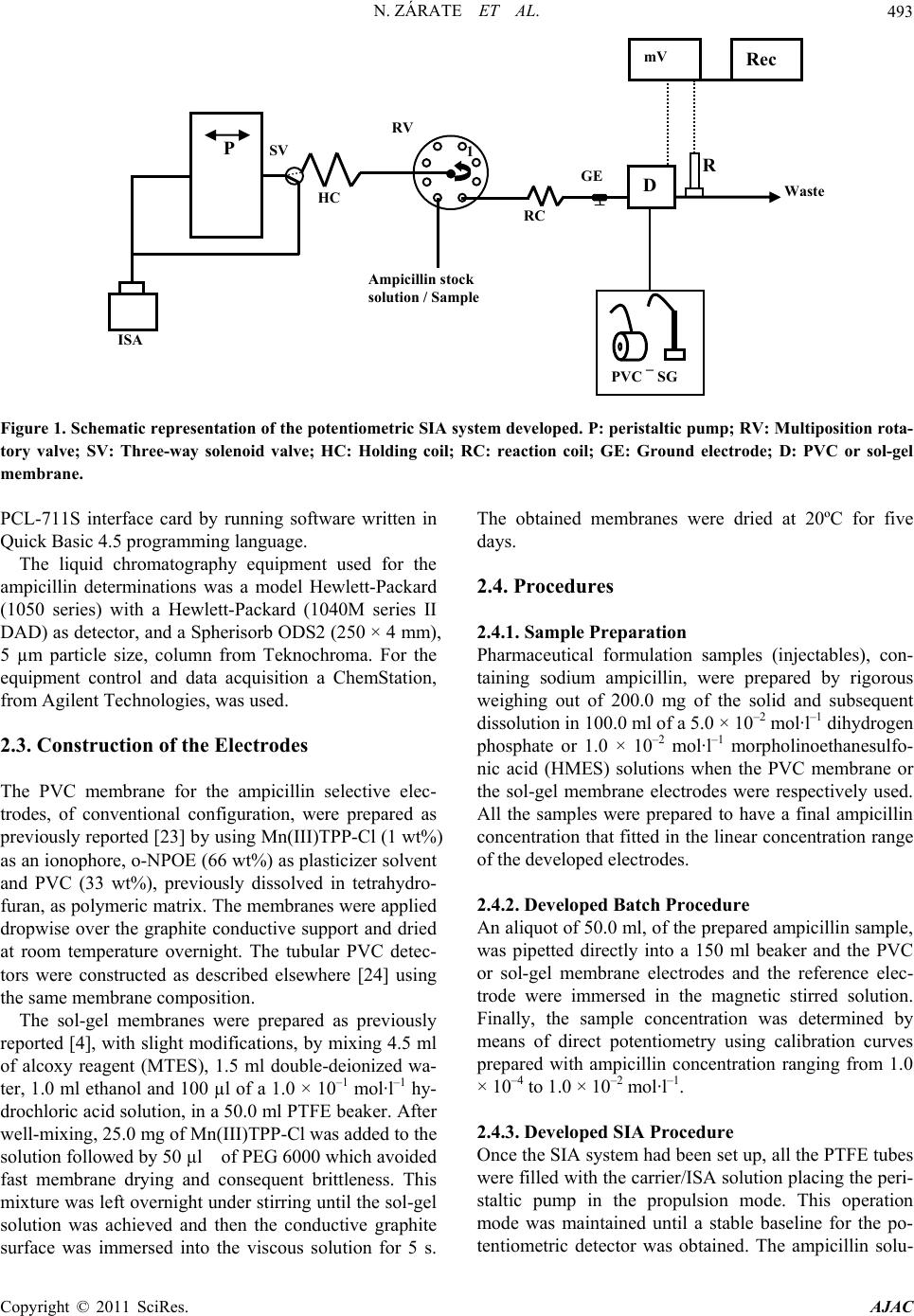 N. ZÁRATE ET AL. Copyright © 2011 SciRes. AJAC 493 Rec mV Figure 1. Schematic representation of the potentiometric SIA system developed. P: per istaltic pump; RV: Multiposition rota- tory valve; SV: Three-way solenoid valve; HC: Holding coil; RC: reaction coil; GE: Ground electrode; D: PVC or sol-gel membrane. PCL-711S interface card by running software written in Quick Basic 4.5 programming language. The liquid chromatography equipment used for the ampicillin determinations was a model Hewlett-Packard (1050 series) with a Hewlett-Packard (1040M series II DAD) as detector, and a Spherisorb ODS2 (250 × 4 mm), 5 µm particle size, column from Teknochroma. For the equipment control and data acquisition a ChemStation, from Agilent Technologies, was used. 2.3. Construction of the Electrodes The PVC membrane for the ampicillin selective elec- trodes, of conventional configuration, were prepared as previously reported [23] by using Mn(III)TPP-Cl (1 wt%) as an ionophore, o-NPOE (66 wt%) as plasticizer solvent and PVC (33 wt%), previously dissolved in tetrahydro- furan, as polymeric matrix. The membranes were applied dropwise over the graphite conductive support and dried at room temperature overnight. The tubular PVC detec- tors were constructed as described elsewhere [24] using the same membrane composition. The sol-gel membranes were prepared as previously reported [4], with slight modifications, by mixing 4.5 ml of alcoxy reagent (MTES), 1.5 ml double-deionized wa- ter, 1.0 ml ethanol and 100 µl of a 1.0 × 10–1 mol·l–1 hy- drochloric acid solution, in a 50.0 ml PTFE beaker. After well-mixing, 25.0 mg of Mn(III)TPP-Cl was added to the solution followed by 50 µl of PEG 6000 which avoided fast membrane drying and consequent brittleness. This mixture was left overnight under stirring until the sol-gel solution was achieved and then the conductive graphite surface was immersed into the viscous solution for 5 s. The obtained membranes were dried at 20ºC for five days. 2.4. Procedures 2.4.1. Sampl e Preparation Pharmaceutical formulation samples (injectables), con- taining sodium ampicillin, were prepared by rigorous weighing out of 200.0 mg of the solid and subsequent dissolution in 100.0 ml of a 5.0 × 10–2 mol·l–1 dihydrogen phosphate or 1.0 × 10–2 mol·l–1 morpholinoethanesulfo- nic acid (HMES) solutions when the PVC membrane or the sol-gel membrane electrodes were respectively used. All the samples were prepared to have a final ampicillin concentration that fitted in the linear concentration range of the developed electrodes. 2.4.2. De ve l op e d Batch Procedure An aliquot of 50.0 ml, of the prepared ampicillin sample, was pipetted directly into a 150 ml beaker and the PVC or sol-gel membrane electrodes and the reference elec- trode were immersed in the magnetic stirred solution. Finally, the sample concentration was determined by means of direct potentiometry using calibration curves prepared with ampicillin concentration ranging from 1.0 × 10–4 to 1.0 × 10–2 mol·l–1. 2.4.3. Developed SIA Procedure Once the SIA system had been set up, all the PTFE tubes were filled with the carrier/ISA solution placing the peri- staltic pump in the propulsion mode. This operation mode was maintained until a stable baseline for the po- tentiometric detector was obtained. The ampicillin solu- RV HC SV Ampicillin stock solution / Sample 1 RC GE Waste R P ISA D PVC ¯ SG  N. ZÁRATE ET AL. 494 tions, of the calibration curve, 5.0 × 10–4, 1.0 × 10–3, 5.0 × 10–3, 1.0 × 10–2 and 5.0 × 10–2 mol·l–1 were prepared from the 1.0 × 10–1 mol·l–1 sodium ampicillin stock solu- tion. Only two steps were required for getting the ana- lytical signal and they were applied to each solution. When the PVC membrane electrodes were used, 165 µl of ampicillin stock solution were aspirated through port 5 of the rotatory valve at a flow of 1.3 ml·min–1 and sent to the detector through port 6 at a flow rate of 1.5 ml·min–1. However, when the sol-gel membrane electrodes were employed, 250 µl of ampicillin stock solution were aspi- rated and sent to the detector at the aforementioned flow rates. The pharmaceutical samples suffered the same procedure. 2.4.4. Reference Procedure The HPLC method used for the determination of am- picillin in the pharmaceutical samples was similar to the chromatographic method proposed by the European Pharmacopoeia [25]. Therefore, 30.0 mg of solid sample were rigorous weighed and dissolved in 10 ml of mobile phase (A/B), formed by 75% of mobile phase A and 25% of mobile phase B. From this solution, 0.1 ml were ex- actly pipetted and transferred to a 25.0 ml volumetric flask and diluted with the mobile phase. Afterwards, an aliquot of 20 l from this solution was injected into the HPLC equipment. The separation and detection were carried out according to the following chromatographic conditions: elution mode, isocratic; flow rate, 1.0 ml min-1; detection wavelength, 254 nm; temperature, am- bient; retention time expected, 3.32 min; analysis time, 5.5 min. 3. Results and Discussion 3.1. Working Characteristics of Electrodes with Conventional Configuration In order to determine the influence of the immobilizing support on the performance of the electrodes selective to ampicillinate, the metalloporphyrin Mn(III)-TPP-Cl was incorporated both into PVC or ceramic (sol-gel) mem- branes. The working characteristics of both types of conventional electrodes constructed were evaluated on the basis of the IUPAC recommendations [26]. The ex- periments were performed in solutions with the ionic strength adjusted with the 5.0 × 10–2 mol·l–1 dihydro- genphosphate solution when the PVC membranes were evaluated or with the 1.0 × 10–2 mol·l–1 HMES solution in the case of sol-gel membranes. From the data, pre- sented in Table 1, it is possible to affirm that both types of ampicillinate selective electrodes constructed in this work, using the same ionophore, presented similar or better slopes, reproducibilities, response times and prac- tical limits of detection than those reported by other au- thors [13-16]. In spite of having better reproducibility and similar response time, the sol-gel membrane elec- trode showed narrower linear concentration range and shorter life time when compared with that obtained with PVC membrane electrode. The Reilley diagrams obtained for the PVC membrane electrode evidenced an operational pH range between 3.5 and 8.0 for the higher concentration of ampicillinate (1.0 × 10–2 mol·l–1) tested; however, for lower concentration of ampicillinate (1.0 × 10–4 mol·l–1), the operational pH range clearly diminishes, mainly in the basic zone. This is probably due to the fact that with an increase of OH- relatively to ampicillinate, the response towards this ion overlaps the response towards the primary ion. Similar behaviours has been previously described when PVC membranes electrodes selective to other organic com- pounds, using the same metallporphyrin, were con- structed and evaluated by some of us [2,4,27]. For that reason the working pH chosen for all measurement con- dition was 4.5 which guaranteed, according to the peni- cillin pKa (2.5) the complete dissociation of the analyte. In the case of sol-gel membrane electrodes, the Reilley diagrams showed that the potential measurements of the electrode changed according to the pH of the ampicilli- nate solution. This fact previously reported [28], was due to the presence of silanol groups (Si-OH) at the surface of the ceramic membrane which makes these materials sensitive to pH changes. So, when this type of electrode was used, a strict control of sample pH (3.8) was needed. Interference caused by some common inorganic ani- ons was evaluated by carrying out the determination of the selectivity potentiometric coefficients (log Kpot) by the separate solution method at pH 4.5 and 3.8 for the PVC and sol-gel membranes respectively, and in the concentration range from 1.0 × 10–4 to 1.0 × 10–2 mol·l–1 for both primary and interfering ions. The results showed in Table 1 were obtained after two determinations per- formed with three electrodes and reveal that the devel- oped electrodes were fairly selective to ampicillinate over other anions, being their selectivity coefficients similar or better than those obtained by other authors [13,16]. Regarding the Hofmeister selectivity pattern of the classical liquid ion-exchanger, the developed PVC and sol-gel membranes exhibited different selectivity sequences from that one as previously demonstrated when the same metalloporphyrin had been used to con- struct other anion selective membrane electrodes, namely carboxylic ions [2,4,27]. For PVC membrane electrode the sequence SCN– > Sal– > I– > NO3– > NO2– > Cl– > SO42– > H2PO4– > HCO3– was obtained. Regarding the sol-gel membrane electrode the sequence HCO3– > NO2– > NO3– > ≈ SCN– > Sal– > I– > Cl– > H2PO4– > SO42– was Copyright © 2011 SciRes. AJAC  N. ZÁRATE ET AL.495 Table 1. General working characteristics of the developed ampicillinate selective electrodes. Characteristics PVC membrane Sol-gel membrane Linear concentration range (mol·l–1) 1.7 × 10–5 - 1.0 × 10–1 1.5 × 10–4 - 1.0 × 10–1 Practical limit of detection (mol·l–1) 1.0 × 10–4 1.0 × 10–3 Slope (mV·decade–1) 60.2 0.8 65.3 0.3 Potential stability (mV·day–1) 1.5 0.7 Response time (seconds) < 10 < 10 Lifetime (months) 10 1 Working pH rangea 3.0 - 7.5 - PVC membrane Sol-gel membrane Potentiometric selectivity coeffi- cients a (log Kpot) Sulphate –1.37 0.02 –1.38 0.06 Dihydrogenphosphate –1.40 0.03 –1.32 0.05 Hydrogencarbonate –1.65 0.02 0.04 0.04 Chloride –0.41 0.01 –0.65 0.05 Nitrate –0.43 0.03 –0.42 0.14 Nitrite –0.22 0.01 0.12 0.08 Iodide 0.68 0.04 –0.45 0.03 Thiocyanate 1.62 0.07 –0.17 0.07 Salicylate 1.45 0.06 –0.22 0.27 a. Ampicillinate concentration = 1.0 × 10–3 mol·l–1 obtained. Being much more hydrophilic in nature, the sol-gel membrane was less affected by lypophilic anions such as SCN– which confirm the notion that interferent action can be reduced by eliminating the plasticizer in the polymeric membrane [29]. On the other hand, the fact that the interferent action of the salicilate diminished when the sol-gel membrane electrode is used, could led to eliminate significant error for measurement of am- picillin in biological fluid samples from patients who have ingested aspirin. 3.2. Behaviour of the Electrodes in the SIA System Both type of constructed electrodes were evaluated when they were coupled in the SIA system depicted in Figure 1 using a 5.0 × 10–2 mol·l–1 dihydrogenphosphate and a 1.0 × 10–2 mol·l–1 HMES solutions as carriers for elec- trodes with PVC or sol-gel membranes, respectively. Initially, the aspiration and propulsion flow rates were estimated by interpolation in graphs obtained by plotting ml·min–1 vs. revolution per minute (rpm) of the peristal- tic pump. The intensity and repeatability of the potenti- ometric signals obtained were studied either by varying the injection volume of the ampicillinate solution in the interval 115 - 450 l, and the propulsion rate in the in- terval 1.2 - 3.5 ml·min–1 in a way, whereby the sampling rate was not diminished. With the increase of the injec- tion volume, an increase in the magnitude of analytical signals was verified up to volumes of 165 and 250 l for PVC and sol-gel membrane electrodes respectively. For greater injection volumes no significant improvement in the signals magnitude was observed requiring more time and greater consumption of reagents. It was also ob- served that volumes smaller than 165 and 250 l pro- vided higher dispersion of the sample zone and conse- quently lack of linearity. These injection volumes were obtained for a maximum flow rate of 1.5 ml·min–1 for both potentiometric detectors. The increments in the flow rate carried an appreciable loss of signal repeatability, possibly caused by inertia to the flow during the aspira- tion stage, and by the dynamic response characteristics of each electrode type used [30]. The general working characteristics of both electrodes were evaluated by means of calibration curves obtained for a range between 1.0 × 10–6 and 1.0 × 10–1 mol·l–1 and they appear reflected in Table 2. From these data, it is possible to state that sol-gel membrane electrode showed narrower linear concentration range and lower life-time than PVC membrane electrode, but the slope resulted to be higher. When compared the behaviour of these units with the results obtained in batch conditions, a small nar- Copyright © 2011 SciRes. AJAC  N. ZÁRATE ET AL. 496 Table 2. Figures of merit of the developed potentiome tr ic SIA system. PVC membrane Sol-gel membrane Linear concentration range (mol·l–1) 7.0 × 10–5 – 1.0 × 10–1 7.5 × 10–4 – 1.0 × 10–1 Practical limit of detection (mol·l–1)b 4.0 × 10–5 4.1 × 10–4 Regression equationa E = –57.4 log[C] + 20.1 E = –63.5 log [C] + 19.3 Quadratic correlation coefficient (R2) 0.9992 0.997 Electrode slope and standard deviation (mV·decade–1) –57.4 0.5 –63.5 0.5 Relative standard deviation (%)c 0.2 0.3 Sampling rate (sample h–1) 40 33 a E: potentiometric signal (mV); log [C]: logarithm of analyte concentration (mol l-1); b Determined according to IUPAC recommendations [26]; c Obtained for 11 replicates at 1.0 x 10-3 mol l-1 analyte standard solutions rowing in the linear concentration ranges, an increase in the practical limit of detection and a diminution of the slopes were observed with both type of electrodes. 3.3. Analytical Applications The potentiometric analysis of five samples of pharma- ceutical formulation (injectables) containing sodium am- picillin, commercialized in Europe, was carried out with conventionally or tubular (PVC membranes) shaped ele- ctrodes according to the batch and SIA procedures. For comparison purposes the samples were simultaneously analyzed by applying the HPLC reference method and the obtained results are summarized in Tables 3 and 4. The precisions of the developed and the reference me- thods were evaluated by their simultaneous application to 6 different samples of the same pharmaceutical product and were expressed in terms of their relative standard deviations. From the obtained data, it was possible to affirm that the precisions of both, batch and SIA, poten- tiometric determinations were good since the mean rela- tive standard deviation were 1.7% (PVC membrane elec- trode) and 1.2% (sol-gel membrane electrode) in the SIA system and 2.1 and 1.6% respectively in batch conditions. To test whether the developed (batch and SIA) and the reference methods differed in their precision, a signifi- cance (two-tailed) F-test was carried out. Since the cal- culated F values were (just with one exception) less than the critical F value (7.15), there was no statistically sig- nificant difference between the standard deviations at 95% confidence level [31]. After three additions of kn- own concentration of standard sodium ampicillin, the samples were analysed following the same methods and the percentages of spike recovery calculated were used to evaluate the accuracy. No significant differences were found between the obtained results by applying batch or SIA procedures. Moreover, slight better results were founded when sol-gel membrane electrode, instead of PVC membrane electrode, was used. On the other hand, when the ampicillin commercial samples were simultaneously analyzed by applying the developed potentiometric, batch and SIA, and the HPLC reference method, a very slight tendency to get lower values with the potentiometric methods was observed, since the mean percentage of difference were –1.3% for the PVC membrane electrode and –1.1% for the sol-gel membrane electrode in batch conditions and –0.8% for both type of membrane electrodes when used as poten- tiometric detectors in the SIA system. In order to estab- lish whether the developed potentiometric methods could be accepted as giving reliable results, a two-tailed t-test with multiple sample (paired by differences) was per- formed at a P = 0.05 confidence level. The t-data paired test was applied using the formula: tcalc. = xD X S n, where xD is the mean difference between the methods and Sx is the standard deviation of the differences. When substituting for tcalc., they were found to be –0.62 (PVC membrane electrode) and –1.11 (sol-gel membrane elec- trode) in the flow system, and –0.79 and –1.19 respec- tively in batch analysis. Since all these values were less than the tabulated t-value (2.78) there was no statistically significant difference between both potentiometric de- terminations and the HPLC reference method [31]. The limits of detection of the developed potentiometric procedures were established according to the Analytical Methods Committee [32] and following the criteria ( + 3 blank). The calculated values resulted to be 80.5 mg·l–1 for the PVC membrane electrode and 88.2 mg·l–1 for the sol-gel membrane electrode when used as potentiometric detectors in flow analysis, and 71.7 and 83.8 mg·l–1 re- spectively in batch conditions. The limits of quantification ( + 10 blank) were found to be 81.7 mg·l–1 for the PVC membrane electrode and 90.1 mg·l–1 for the sol-gel membrane electrode when used in the SIA system, and 79.8 and 93.7 mg·l–1 respectively in batch analysis. 4. Conclusions The study undertaken has demonstrated that the use of Mn(III) TPP-Cl as an ionophore for the preparation of Copyright © 2011 SciRes. AJAC 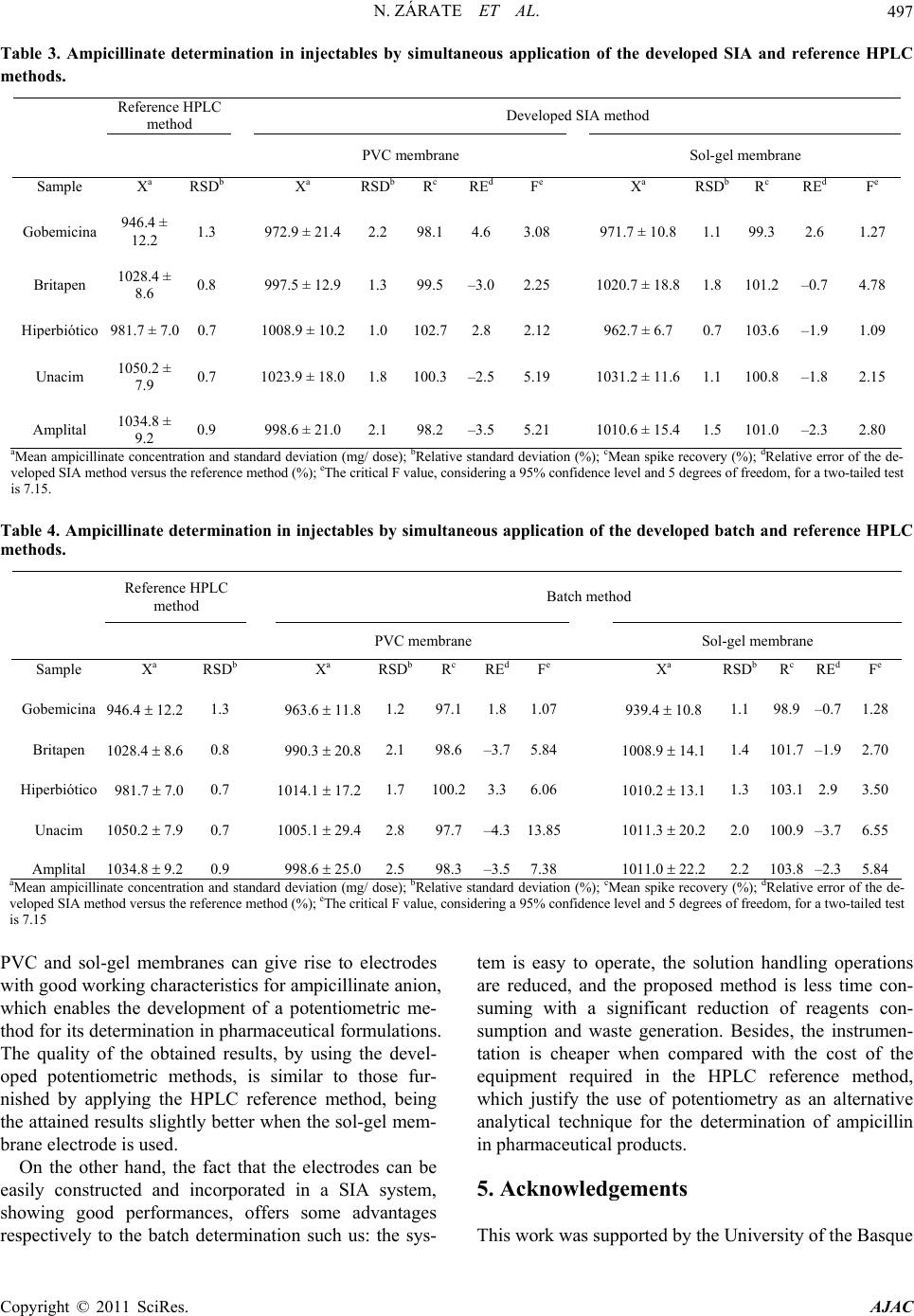 N. ZÁRATE ET AL.497 Table 3. Ampicillinate determination in injectables by simultaneous application of the developed SIA and reference HPLC methods. Reference HPLC method Developed SIA method PVC membrane Sol-gel membrane Sample Xa RSDb Xa RSDbRc REd Fe Xa RSDb R c REd F e Gobemicina 946.4 ± 12.2 1.3 972.9 ± 21.4 2.2 98.14.6 3.08 971.7 ± 10.81.1 99.3 2.6 1.27 Britapen 1028.4 ± 8.6 0.8 997.5 ± 12.9 1.3 99.5–3.02.25 1020.7 ± 18.81.8 101.2 –0.7 4.78 Hiperbiótico 981.7 ± 7.0 0.7 1008.9 ± 10.2 1.0 102.72.8 2.12 962.7 ± 6.70.7 103.6 –1.9 1.09 Unacim 1050.2 ± 7.9 0.7 1023.9 ± 18.0 1.8 100.3–2.55.19 1031.2 ± 11.61.1 100.8 –1.8 2.15 Amplital 1034.8 ± 9.2 0.9 998.6 ± 21.0 2.1 98.2–3.55.21 1010.6 ± 15.41.5 101.0 –2.3 2.80 aMean ampicillinate concentration and standard deviation (mg/ dose); bRelative standard deviation (%); cMean spike recovery (%); dRelative error of the de- veloped SIA method versus the reference method (%); eThe critical F value, considering a 95% confidence level and 5 degrees of freedom, for a two-tailed test is 7.15. Table 4. Ampicillinate determination in injectables by simultaneous application of the developed batch and reference HPLC methods. Reference HPLC method Batch method PVC membrane Sol-gel membrane Sample Xa RSDb Xa RSDbRc REdFe Xa RSDb R c REdFe Gobemicina 946.4 12.2 1.3 963.6 11.81.2 97.11.81.07 939.4 10.8 1.1 98.9 –0.71.28 Britapen 1028.4 8.6 0.8 990.3 20.82.1 98.6–3.75.84 1008.9 14.1 1.4 101.7 –1.9 2.70 Hiperbiótico 981.7 7.0 0.7 1014.1 17.21.7 100.23.36.06 1010.2 13.1 1.3 103.1 2.93.50 Unacim 1050.2 7.9 0.7 1005.1 29.42.8 97.7–4.313.85 1011.3 20.2 2.0 100.9 –3.7 6.55 Amplital 1034.8 9.2 0.9 998.6 25.02.5 98.3–3.57.38 1011.0 22.2 2.2 103.8 –2.3 5.84 aMean ampicillinate concentration and standard deviation (mg/ dose); bRelative standard deviation (%); cMean spike recovery (%); dRelative error of the de- veloped SIA method versus the reference method (%); eThe critical F value, considering a 95% confidence level and 5 degrees of freedom, for a two-tailed test is 7.15 PVC and sol-gel membranes can give rise to electrodes with good working characteristics for ampicillinate anion, which enables the development of a potentiometric me- thod for its determination in pharmaceutical formulations. The quality of the obtained results, by using the devel- oped potentiometric methods, is similar to those fur- nished by applying the HPLC reference method, being the attained results slightly better when the sol-gel mem- brane electrode is used. On the other hand, the fact that the electrodes can be easily constructed and incorporated in a SIA system, showing good performances, offers some advantages respectively to the batch determination such us: the sys- tem is easy to operate, the solution handling operations are reduced, and the proposed method is less time con- suming with a significant reduction of reagents con- sumption and waste generation. Besides, the instrumen- tation is cheaper when compared with the cost of the equipment required in the HPLC reference method, which justify the use of potentiometry as an alternative analytical technique for the determination of ampicillin in pharmaceutical products. 5. Acknowledgements This work was supported by the University of the Basque Copyright © 2011 SciRes. AJAC  N. ZÁRATE ET AL. 498 Country (Spain) through Project UPV 171.363-E-15923/ 2004. One of the authors (N.Z.) wishes to thank to this University for the Ph.D. Grant 98/13828/01. We also thank to Laboratorios Normon S.A, Madrid (Spain), for samples kindly provided. 6. References [1] E. Malinowska, J. Niedziólka, E. Rozniecka and M. E. Meyerhoff, “Salicylate-Selective Membrane Electrodes Based on Sn(IV) and O=Mo(V)-Porphyrin: Differences in Response Mechanism and Analytical Performance,” Journal of Electroanalytical Chemistry, Vol. 514, No. 1-2, 2001, pp. 109-117. doi:10.1016/S0022-0728(01)00615-5 [2] E. M. G. Santos, A. N. Araújo, C. M. C. M. Couto, M. C. B. S. M. Montenegro, A. Kejzlarová and P. Solich, “Ion Selective Electrodes for Penicillin-G Based on Mn (III) TPP-Cl and Their Application in Pharmaceutical Formu- lations by Sequential Injection Analysis,” Journal of Pha- rmaceutical and Biomedical Analysis, Vol. 36, No. 4, 2004, pp. 701-709. doi:10.1016/j.jpba.2004.08.006 [3] T. Katsu, K. Ido, A. Moriya, Y. Nakae and I. Sakata, “Valproate-Selective Membrane Electrode Based on Gal- lium(III)Tetraphenylphorfyrin,” Electroanalysis, Vol. 12, No. 16, 2000, pp. 1282-1285. doi:10.1002/1521-4109(200011)12:16<1282::AID-ELAN 1282>3.0.CO;2-D [4] E. M. G. Santos, A. N. Araújo, C. M. C. M. Couto and M. C. B. S. M. Montenegro, “Construction and Evaluation of PVC and Sol-Gel Sensor Membranes Based on Mn(III) TPP-Cl. Aplication to Valproate Determination in Phar- maceutical Preparation,” Analytical and Bioanalytical Chemistry, Vol. 384, No. 4, 2006, pp. 867-875. doi:10.1007/s00216-005-0170-y [5] S. S. M. Hassan, W. H. Mahmoud, M. A. F. Elmosallamy and M. H. Almarzooqi, “Nobel Ibuprofen Potentiometric Membrane Sensors Based on Tetraphenylphorfyrinato Indium(III),” Analytical Sciences, Vol. 19, No. 5, 2003, pp. 675-680. doi:10.2116/analsci.19.675 [6] J. H. A. Badr, M. E. Meyerhoff and S. S. M. Hassan, “Metallophorfyrin-Based Polymer Membrane Electrode with High Selectivity for 2-Hydroxybenzhydroxamate,” Analytica Chimica Acta, Vol. 321, No. 1, 1996, pp. 11-19. doi:10.1016/0003-2670(95)00580-3 [7] E. M. G. Santos, A. N. Araújo, C. M. C. M. Couto and M. C. B. S. M. Montenegro, “Potentiometric Behaviour of Ion Selective Electrodes Based on Iron Porphyrins: The Influence of Porphyrins Substituents on the Response Properties and Analytical Determination of Diclofenac in Pharmaceutical Formulations,” Journal of Pharmaceuti- cal and Biomedical Analysis, Vol. 42, No. 5, 2006, pp. 535-542. doi:10.1016/j.jpba.2006.05.019 [8] O. Dinten, U. E. Spichiger, N. Chaniotakis, P. Gehring, B. Rusterholtz, W. E. Morf and W. Simon, “Lifetime of Neutral-Carrier-Based Liquid Membranes in Aqueous Samples and Blood and the Lipophilicity of Membrane Components,” Analytical Chemistry, Vol. 63, No. 6, 1991, pp. 596-603. doi:10.1021/ac00006a009 [9] J. Lin and C. W. Brown, “Sol-Gel Glass as a Matrix for Chemical and Biochemical Sensing,” Trends in Analyti- cal Chemistry, Vol. 16, No. 4, 1997, pp. 200-211. doi:10.1016/S0165-9936(97)00021-6 [10] W. Kim, S. Chung, S. B. Park, S. Ch. Lee, C. Kim and D. D. Sung, “Sol-Gel Method for the Matrix of Chlo- ride-Selective Membranes Doped with Fridodecyl- Methylammonium Chloride,” Analytical Chemistry, Vol. 69, 1997, pp. 95-98. doi:10.1021/ac9604088 [11] W. Kim, D. D. Sung, G. S. Cha and S. B. Park, “Chlo- ride-Selective Membranes Prepared with Different Ma- trices Including Polymers Obtained by the Sol-Gel Me- thod,” Analyst, Vol. 123, 1998, pp. 379-382. doi:10.1039/a705788a [12] M. Mazloum, M. Salavati-Niasari, M. Khayat and S. M. Ghoreishi, “A Copper Ion-Selective Electrode with High Selectivity Prepared by Sol-Gel Coated Wire Tech- niques,” Analytical and Bioanalytical Chemistry, Vol. 378, No. 6, 2004, pp. 1659-1665. doi:10.1007/s00216-003-2462-4 [13] S. Z. Yao, J. Shiao and L. H. Nie, “Potentiometric De- termination of Penicillins with Ion-Selective Electrodes,” Talanta, Vol. 36, No. 12, 1989, pp. 1249-1252. doi:10.1016/0039-9140(89)80058-X [14] E. G. Kulapina, V. V. Baraguzina and O. I. Kulapina, “Ion-Selective Electrodes for Determining Penicillin An- tibiotics in Biological Fluids and Pharmaceutical Forms,” Journal of Analytical Chemistry, Vol. 59, No. 9, 2004, pp. 971-975. doi:10.1023/B:JANC.0000040704.24966.46 [15] M. S. Rizk, Y. M. Issa and A. F. Shoukry, “New Am- picillin Selective Plastic Membrane and Coated Metal Electrodes Based on Ampicillinium Phosphotungstate Ion Pair,” Analytical Letters, Vol. 27, No. 6, 1994, pp. 1055- 1065. [16] N. V. Shvedene and S. V. Borovskaya, “Determination of β-Lactam Antibiotics by Potentiometry with Ion-Sele- ctive Electrodes,” Journal of Analytical Chemistry, Vol. 58, No. 11, 2003, pp. 1208-1213. doi:10.1023/A:1027393608302 [17] G. J. Papariello, A. K. Muhkerji and C. M. Shearer, “Pe- nicillin Selective Enzyme Electrode,” Analytical Chemis- try, Vol. 45, No. 4, 1973, pp. 790-792. doi:10.1021/ac60326a032 [18] I. S. Park, D. K. Kim and N. Kim, “ Characterization and Food Application of a Potentiometric Biosensor Measur- ing β-Lactam Antibiotics,” Journal of Microbiology and Biotechnology, Vol. 14, No. 4, 2004, pp. 698-706. [19] E. S. Gil and G. R. Melo, “Electrochemical Biosensors in Pharmaceutical Analysis,” Brazilian Journal of Pharma- ceutical Sciences, Vol. 46, No. 3, 2010, pp. 375-391. [20] K. Gupta, et al., “Recent Advances on Potentiometric Membrane Sensors for Pharamaceutical Analysis,” Com- binatorial Chemistry & High Throughput Screening, Vol. 14, No. 4, 2011, pp. 284-302. [21] S. Alegret, J. Alonso, J. Bartrolí, A. A. S. C. Machado, J. L. F. C. Lima and J. M. Paulis, “Construction of Equip- ment for Potentiometric Determinations in Flow Analy- Copyright © 2011 SciRes. AJAC  N. ZÁRATE ET AL. Copyright © 2011 SciRes. AJAC 499 sis,” Química Analítica, Vol. 6, No. 3, 1987, pp. 278-292. [22] J. Alonso, J. Bartrolí, J. L. F. C. Lima and A. A. S. C. Machado, “Sequential Flow-Injection Determinations of Calcium and Magnesium in Waters,” Analytica Chimica Acta, Vol. 179, 1986, pp. 503-508. doi:10.1016/S0003-2670(00)84500-6 [23] J. L. F. C. Lima, M. C. B. S. M. Montenegro and A. M. Roque da Silva, “A Phenobarbital-Selective Electrode without an Inner Reference Solution, an Its Application to Pharmaceutical Analysis,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 8, 1990, pp. 701-704. doi:10.1016/0731-7085(90)80106-Y [24] J. L. F. C. Lima, M. C. B. S. M. Montenegro and A. M. Roque da Silva, “FIA Potentiometric Determination of Salicylate in Pharmaceutical Preparations with a Tubular Detector,” Journal of Flow Injection Analysis, Vol. 7, 1990, pp. 19-33. [25] European Directorate for the Quality of Medicines, “Di- rectorate for the Quality of Medicines of the Council of Europe,” European Pharmacopoeia, 4th Edition, 2002. [26] IUPAC, Pure and Applied Chemistry, Vol. 53, No. 10, 1981, pp. 1907-1912. doi:10.1351/pac198153101907 [27] E. M. G. Santos, C. M. C. M. Couto, M. C. B. S. Monte- negro, M. G. P. M. S. Neves, S. L. H. Rebelo, J. A. S. Cavaleiro and B. F. Reis, “Ion-Selective Electrodes Based on Metalloporphyrins for Gibberellic Acid Deter- mination in Agricultural Products,” Analytical and Bio- analytical Chemistry, Vol. 375, No. 4, 2003, pp. 511-516. [28] A. Caneiro, P. Fabry, H. Khireddine and E. Siebert, “Performance Characteristics of Sodium Superionic Con- ductor Prepared by the Sol-Gel Route for Sodium Sen- sors,” Analytical Chemistry, Vol. 63, No. 22, 1991, pp. 2550-2557. doi:10.1021/ac00022a004 [29] U. Oesch, D. Amman, H. Pham, U. Wuthier, R. Zund and W. J. Simon, “Design of Anions-Selective Membranes for Clinically Relevant Sensors,” Chemical Society Fa- raday Transactions I, Vol. 82, No. 4, 1986, pp. 1179- 1186. doi:10.1039/f19868201179 [30] A. M. Pimenta, A. N. Araújo and M. C. B. S. M. Monte- negro, “A Sequential Injection Analysis System for Po- tassium Clavulanate Determination Using Two Potenti- ometric Detectors,” Journal of Pharmaceutical and Bio- medical Analysis, Vol. 30, No. 4, 2002, pp. 931-937. doi:10.1016/S0731-7085(02)00474-0 [31] J. C. Miller and J. N. Miller, “Statistics for Analytical Chemistry,” 2nd Edition, Ellis Horwood Ltd., Chichester, 1988. [32] Analytical Methods Committee, “Recommendations for the Definition, Estimation, and Use of Detection Limit,” Analyst, Vol. 112, 1987, pp. 199-204. doi:10.1039/an9871200199
|