 American Journal of Analyt ical Chemistry, 2011, 2, 456-469 doi:10.4236/ajac.2011.24055 Published Online August 2011 (http://www.SciRP.org/journal/ajac) Copyright © 2011 SciRes. AJAC An Improved High Performance Liquid Chromatographic Method for Identification and Quantization of Polyamin es as Benzoylated Derivatives* Rajat Sethi1#, Sai Raghuveer Chava2, Sajid Bashir2,3§, Mauro E. Castro2# 1Cardiovascular Research and Devel opm ent Laboratory, Department of Pharmaceutical Sciences, Rangel College of Pharmacy, Texas A&M Health Science Center, Kingsville, USA 2Department of Che mistry, Texas A&M University-Kingsville, Kingsville, USA 3Chemical Biol ogy Research Group (CBRG), 700 University Blvd, Kingsville, USA E-mail: #rsethi@tamhsc.edu; #kfmec00@tamuk.edu Received April 25, 2011; revised May 23, 2011; accepted J u ne 23, 2011 Abstract A simple reversed phase high performance liquid chromatography (RP-HPLC) method was developed for the determination of putrescine, cadaverine and spermidine (a class of polyamines) in their benzoylated form from external known standards. In the optimization procedure, a number of parameters were exam- ined: 1) Solvent used in the extraction of standard polyamines (diethyl ether versus chloroform); 2) Sol- vent used in the elution of the polyamine (methanol versus acetonitrile); 3) Mode of derivatization and extraction step(s) (derivatization and extraction performed together versus derivatization and extraction performed separately); and 4) Other instrumental parameters (such as UV detection wavelength, gradient profiles). The advantages of our method, relative to the standard Morgan method are: a) decreased chro- matographic runtime, b) ease of preparation with good resolution, sensitivity, and reproducibility using a standard RP-HPLC method. Keywords: Polyamine, RP-HPLC, Standardization, Benzoylation 1. Introduction Cardiovascular diseases (CVD, *) are a major cause for death of people in North America and have been attributed to a number of epidemic factors [1]. One potential mecha- nism for the increased incidence of CVDs affecting 81million Americans is alteration in polyamine content in the heart [2]. Other risk factors associated with CVD are high blood pressure, poor diet and obesity. Clinical studies have demonstrated that intake of polyamines from external sources can minimize the incidence of CVD [3]. Poly- amines are a class of polycationic compounds, which are ubiquitous in many tissues of mammals and other organ- isms. The three most common members of the polyamine family are: cadaverine, putrescine and spermidine which are low molecular weight aliphatic compounds containing nitrogen, are necessary for cell growth and differentiation in living cells [4-6]. Furthermore, polyamines consist be- tween three to five methylene groups between the terminal nitrogen atoms. Early diagnosis and detection would allow treatment and decrease CVD attributed mortality and a number of clinical and analytical approaches have been utilized towards this end. Previous studies using urine sam- ple of cancer patients allowed determination of polyamine to be conducted using a chromatographic approach, where correlation was observed between patients with and with- out disease and amount of polyamine [7]. Endogenous ca- daverine is synthesized using lysine as the starting material via lysine decarboxylase (scheme shown in Figure 1). Similarly, putrescine is syn thesized through a decarboxyla- tion step using ornithine and spermidine was formed by the action of spermidine synthase, which attaches putrescine to an aminopropyl group from decarboxylated S-adenosylme- *This work was supported by grants from United States Environmental Protection Agency Grant (USEPA Grant # IT-83404401-0), Texas A&M Health Science Research Development Grant (Act# 134403-35402) an funds from TAMHSC Research Startup (Act# 13100-35488), Robert A. Welch Foundation (Departmental Grant AC006) and National Science Foundation (NSF) Division of Undergraduate Education (DUE) Progra (9987332) for financial support. §Dedicated to: Rebecca West, Larry Kuchta and Prabin Maharjan. thionine [8-11]. The synthesized polyamines are used by 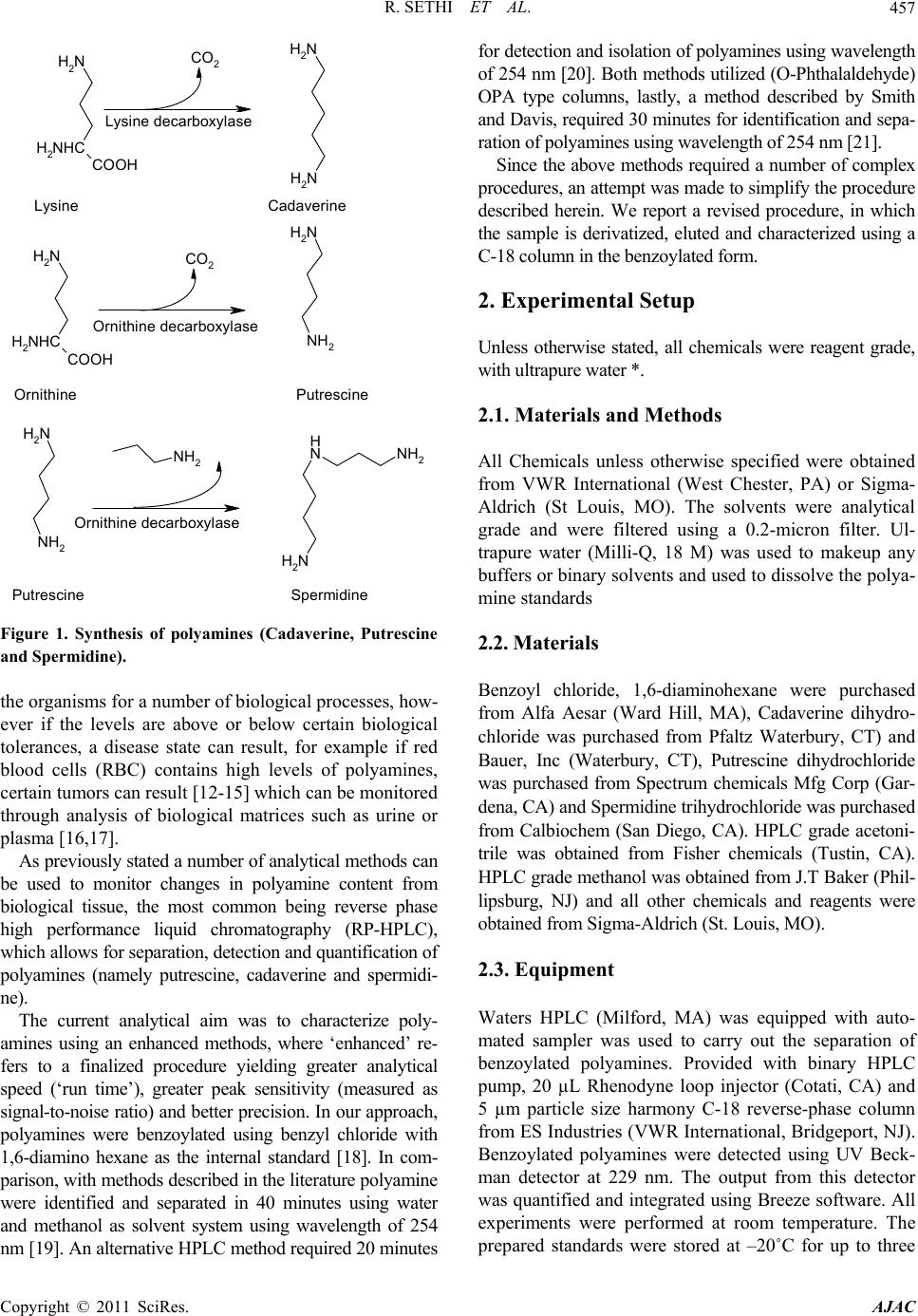 R. SETHI ET AL.457 NH2 H2NHC COOH NH2 NH2 NH2 H2NHC COOH CO2 CO2 NH2 NH2 NH2 NH2 NH2 NH2 N HNH2 Lysine Cadaverine Lysine decarboxylase Orni thine decarbo xylase Ornithine Putrescine Ornithine decarboxylase Putrescine Spermidine Figure 1. Synthesis of polyamines (Cadaverine, Putrescine and Spermidine). the organisms for a number of biological processes, how- ever if the levels are above or below certain biological tolerances, a disease state can result, for example if red blood cells (RBC) contains high levels of polyamines, certain tumors can result [12-15] which can be monitored through analysis of biological matrices such as urine or plasma [16,17]. As previously stated a number of analytical methods can be used to monitor changes in polyamine content from biological tissue, the most common being reverse phase high performance liquid chromatography (RP-HPLC), which allows for separation, detection and quantification of polyamines (namely putrescine, cadaverine and spermidi- ne). The current analytical aim was to characterize poly- amines using an enhanced methods, where ‘enhanced’ re- fers to a finalized procedure yielding greater analytical speed (‘run time’), greater peak sensitivity (measured as signal-to-noise ratio) and better precision. In our approach, polyamines were benzoylated using benzyl chloride with 1,6-diamino hexane as the internal standard [18]. In com- parison, with methods described in the literature polyamine were identified and separated in 40 minutes using water and methanol as solvent system using wavelength of 254 nm [19]. An alternative HPLC method required 20 minutes for detection and isolation of polyamines using wavelength of 254 nm [20]. Both methods utilized (O-Phthalald ehyde) OPA type columns, lastly, a method described by Smith and Davis, required 30 minutes for identification and sepa- ration of polyamines using wavelength of 254 nm [21]. Since the above methods required a number of complex procedures, an attempt was made to simplify the procedure described herein. We report a revised procedure, in which the sample is derivatized, eluted and characterized using a C-18 column in the benzoylated form. 2. Experimental Setup Unless otherwise stated, all chemicals were reagent grade, with ultrapure water *. 2.1. Materials and Methods All Chemicals unless otherwise specified were obtained from VWR International (West Chester, PA) or Sigma- Aldrich (St Louis, MO). The solvents were analytical grade and were filtered using a 0.2-micron filter. Ul- trapure water (Milli-Q, 18 M) was used to makeup any buffers or binary solvents and used to dissolve the polya- mine standards 2.2. Materials Benzoyl chloride, 1,6-diaminohexane were purchased from Alfa Aesar (Ward Hill, MA), Cadaverine dihydro- chloride was purchased from Pfaltz Waterbury, CT) and Bauer, Inc (Waterbury, CT), Putrescine dihydrochloride was purchased from Spectrum chemicals Mfg Corp (Gar- dena, CA) and Spermidine trihydrochloride was purchased from Calbiochem (San Diego, CA). HPLC grade acetoni- trile was obtained from Fisher chemicals (Tustin, CA). HPLC grade methanol was obtained from J.T Baker (Phil- lipsburg, NJ) and all other chemicals and reagents were obtained from Sigma-Aldrich (St. Loui s, MO). 2.3. Equipment Waters HPLC (Milford, MA) was equipped with auto- mated sampler was used to carry out the separation of benzoylated polyamines. Provided with binary HPLC pump, 20 µL Rhenodyne loop injector (Cotati, CA) and 5 µm particle size harmony C-18 reverse-phase column from ES Industries (VWR International, Bridgeport, NJ). Benzoylated polyamines were detected using UV Beck- man detector at 229 nm. The output from this detector was quantified and integrated using Breeze software. All experiments were performed at room temperature. The prepared standards were stored at –20˚C for up to three Copyright © 2011 SciRes. AJAC  R. SETHI ET AL. Copyright © 2011 SciRes. AJAC 458 months, – 7 0˚C for one year use. 2.4. Benzoylation Procedure To a sample (of 1 ml) containing 50 µmole amines 1 molar equivalent (5 µl, HCl) (1 molar equivalent) was added. The sample was shaken briefly using a vortex mixer and to this, the basic internal standard was added. This consisted of 5 µl of 1,6-diamino hexane (internal standard) and 995 µl of 2 N sodium hydroxide (base). The basic polyamine was derivatized using (5 ml) which benzoyl chloride was added to the solvent mixture and was mixed for about 20 minutes at constant temperature. The derivatized polyamine containing both organic and aqueous compounds was separated using centrifugation (× 15,000g, 15 min). After centrifugation, the upper or- ganic phase was removed and used in the extraction of the polyamines. The organic layer was then extracted twice with diethyl ether (2 ml × 2) and the organic layers combined. The extracted layers were dried in a stream of nitrogen. The remaining residue was dissolved in ace- tonitrile (100 µl). The extracted polyamines were ap- proximately of 50 µmoles and diluted to 0.5 µmoles in 1 mL. 20 µl of the polyamines was characterized using RP-HPLC at wavelength of 229 nm [22,23]. 2.5. HPLC Analysis 20 µl (< 50 µmoles) was injected and separated using the C-18 (harmony, VWR International) column, initially eluted using a gradient elution profile. The elution profile began with 70% of solvent A (acetonitrile) to 100% A over 2 minutes, then to 100% of A over 13 minutes and finally to 100% of A to 70% of A over 2 minutes. The flow rate was 1 mL/min and the total run time with method 1 was 15 min [24]. The polyamines were ex- tracted with either diethyl ether as described previously or with chloroform (2 ml × 2) with everything else remain- ing the same and both characterized using the same chromatographic method. 3. Results The relative merits of the extraction approach will pre- sented and for the preferred extraction method the ana- lytical accuracy, precision, signal-to-noise is presented followed by a comparison of the characterization of the polyamines standards with a comparison with current literature methods concluding with benefits of our ap- proach (see Tables 1, 2). Linear regression was employed which was mainly deals with summarizing the significance of the obtained data with two or more variables. To fit a curve to the given data, a combination of statistical techniques along with regression analysis for determination of valid data points within a data were used. The regression analysis was used for prediction and forecasting. The regression analysis fit was linearlized as an equation “Y= m X + C” where “m” is the slope of the line and “C” the y-intercept which are constants [25,26]. Table 2 indicates the linear regression parameters of a set of individual standards. The polyamines standards were analyzed using the new HPLC method which gave more accurate results (summarized in Table 1). Table 1 gives information regarding various different retention characteristics of three polyamines found in biological tissues and also in body fluids. Good separa- tion of these polyamines was achieved (separation of polyamine mixture is shown in Figure 2, one example of improvement in peak intensity with methanol or acetoni- trile extracted with diethyl ether, or chlo roform is shown in Figure 3 and 4 respectively), values with excellent precision. The improvements in peak intensity were ob- served with all of the polyamines evaluated (raw chro- matograph for the other polyamines are shown similarly extracted with diethyl ether is also shown in Figures 5-8). Two possible sources of instrumental artifacts could af- fect the measured values. These can be attributed to the ageing of the column may result in increased line width of the pea ks. If there was an obs truc tio n in the colu mn, it will only result in delayed values with an increase in pressure. But, as the same resolution was measured and the samples were centrifuged and filtered, the two aforementioned instrumental artifacts were not observed. 3.1. Standard Curves The correlation coefficients were more than 0.997 for the concentrations which were investigated. The linearity for all the polyamines were exhibited using absolute amount to that of area under the curves (Table 2).The concentra- tions which were investigated were 0.5, 0.25, 0.125, 0.0625, 0.0312, 0.0156, 0.0078, 0.0036 micromoles for Putrescine (Put), Cadaverine (Cad) and Spermidine (Spd) [27]. 3.2. Recovery and Precision 500 pmol of each compound was combined and recov- ered, from which recovery percentage and quantification Table 1. Different polyamines with retention times. Compound Retention time (min) Variance (%) Putrescine 4.10 0.33 Cadaverine 5.41 0.28 Spermidine 6.24 0.14 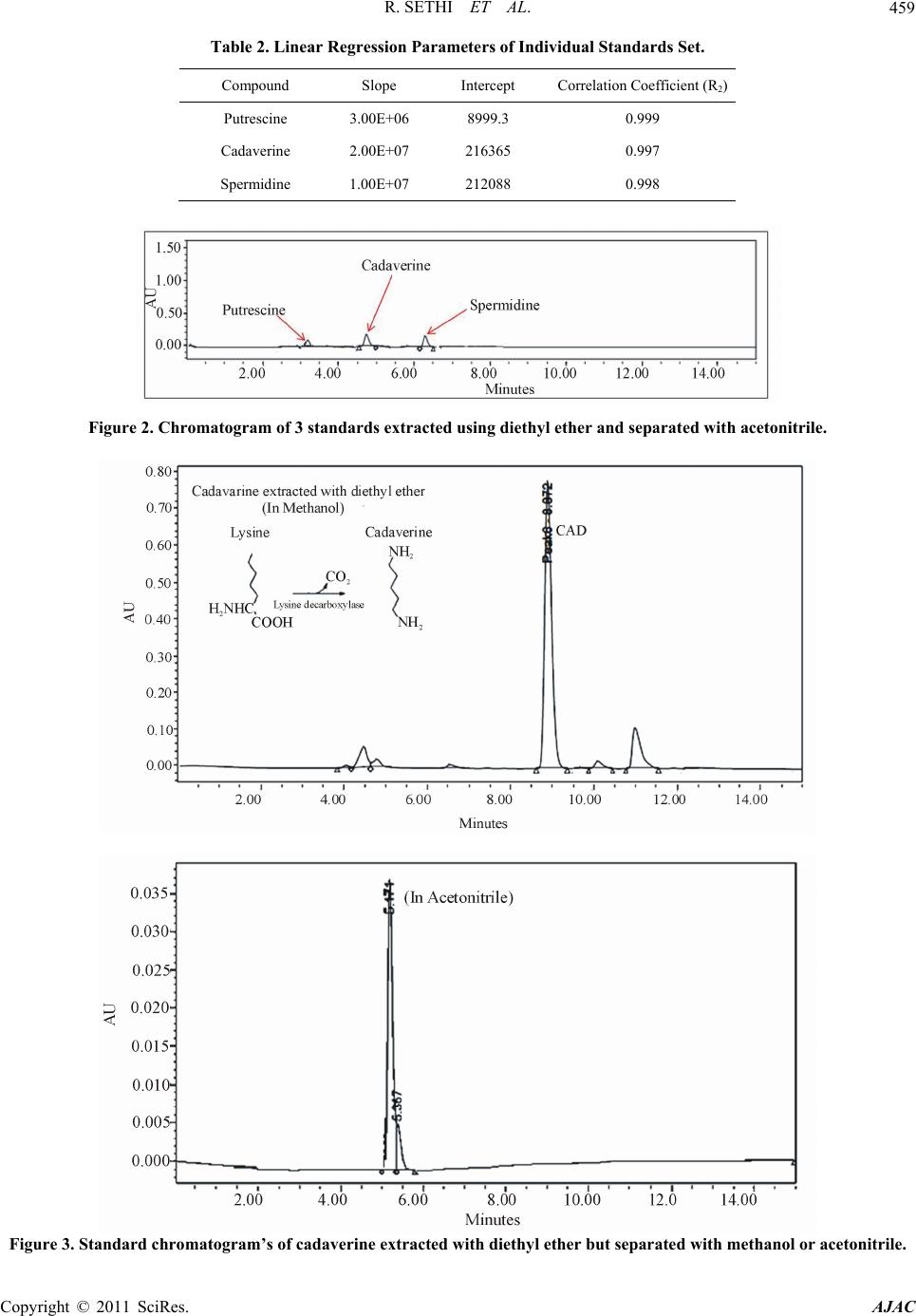 R. SETHI ET AL.459 Table 2. Linear Regression Parameters of Individual Standards Set. Compound Slope Intercept Correlation Coefficient (R2) Putrescine 3.00E+06 8999.3 0.999 Cadaverine 2.00E+07 216365 0.997 Spermidine 1.00E+07 212088 0.998 Figure 2. Chromatogram of 3 standards extracted using diethyl ether and separated with acetonitrile. Figure 3. Standard chromatogram’s of cadaverine extracted with diethyl ether but separated with methanol or acetonitrile. Copyright © 2011 SciRes. AJAC 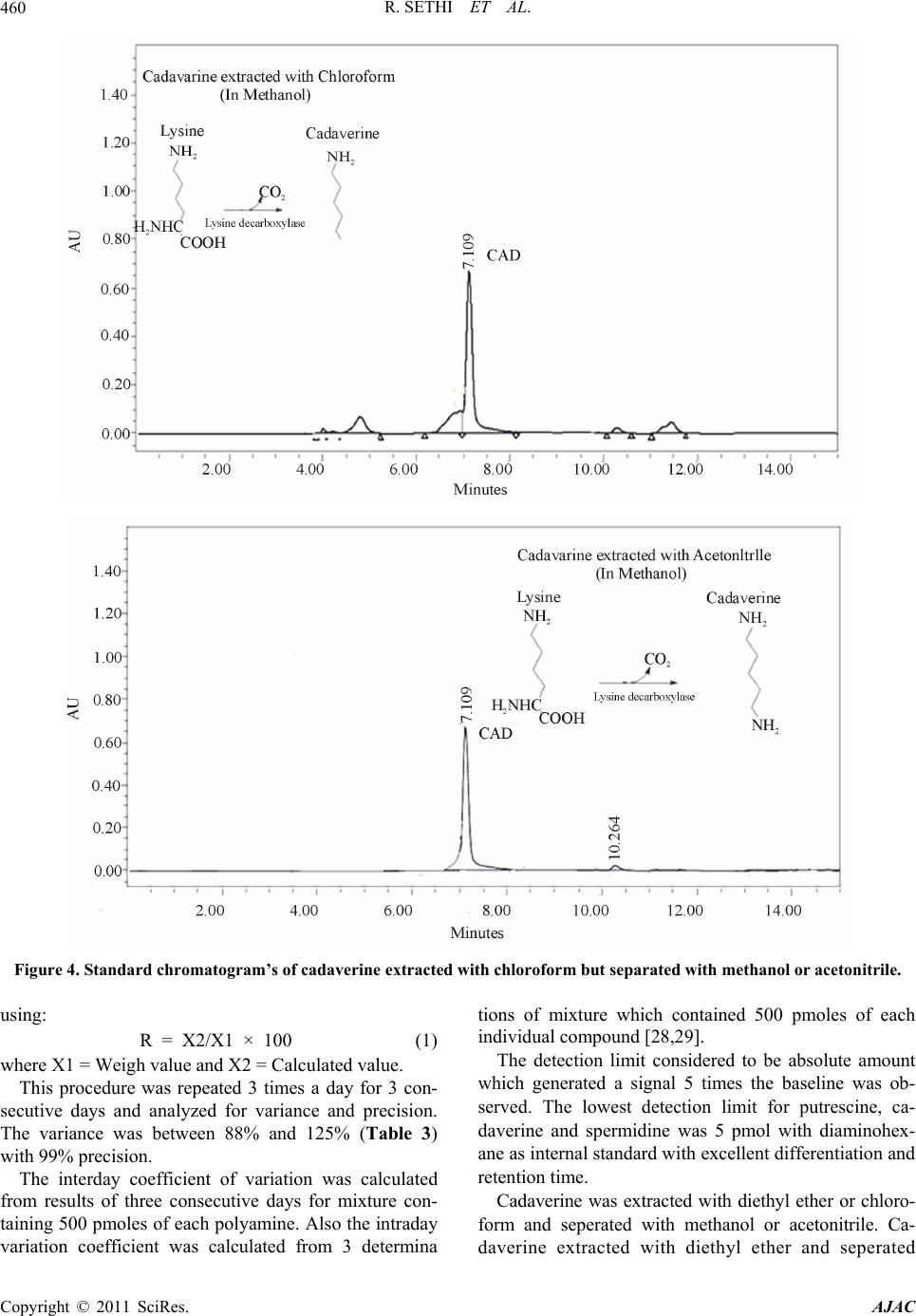 R. SETHI ET AL. Copyright © 2011 SciRes. AJAC 460 Figure 4. Standard chromatogram’s of cadaverine extracted with chloroform but separated with methanol or acetonitrile. using: R = X2/X1 × 100 (1) where X1 = Weigh value and X2 = Calcul at ed value. This procedure was repeated 3 times a day for 3 con- secutive days and analyzed for variance and precision. The variance was between 88% and 125% (Table 3) with 99% precision . The interday coefficient of variation was calculated from results of three consecutive days for mixture con- taining 500 pmoles of each polyamine. Also the intraday variation coefficient was calculated from 3 determina tions of mixture which contained 500 pmoles of each individu al compound [28,29]. The detection limit considered to be absolute amount which generated a signal 5 times the baseline was ob- served. The lowest detection limit for putrescine, ca- daverine and spermidine was 5 pmol with diaminohex- ane as internal standard with excellent differentiation and retention time. Cadaverine was extracted with diethyl ether or chloro- form and seperated with methanol or acetonitrile. Ca- daverine extracted with diethyl ether and seperated  R. SETHI ET AL.461 Copyright © 2011 SciRes. AJAC 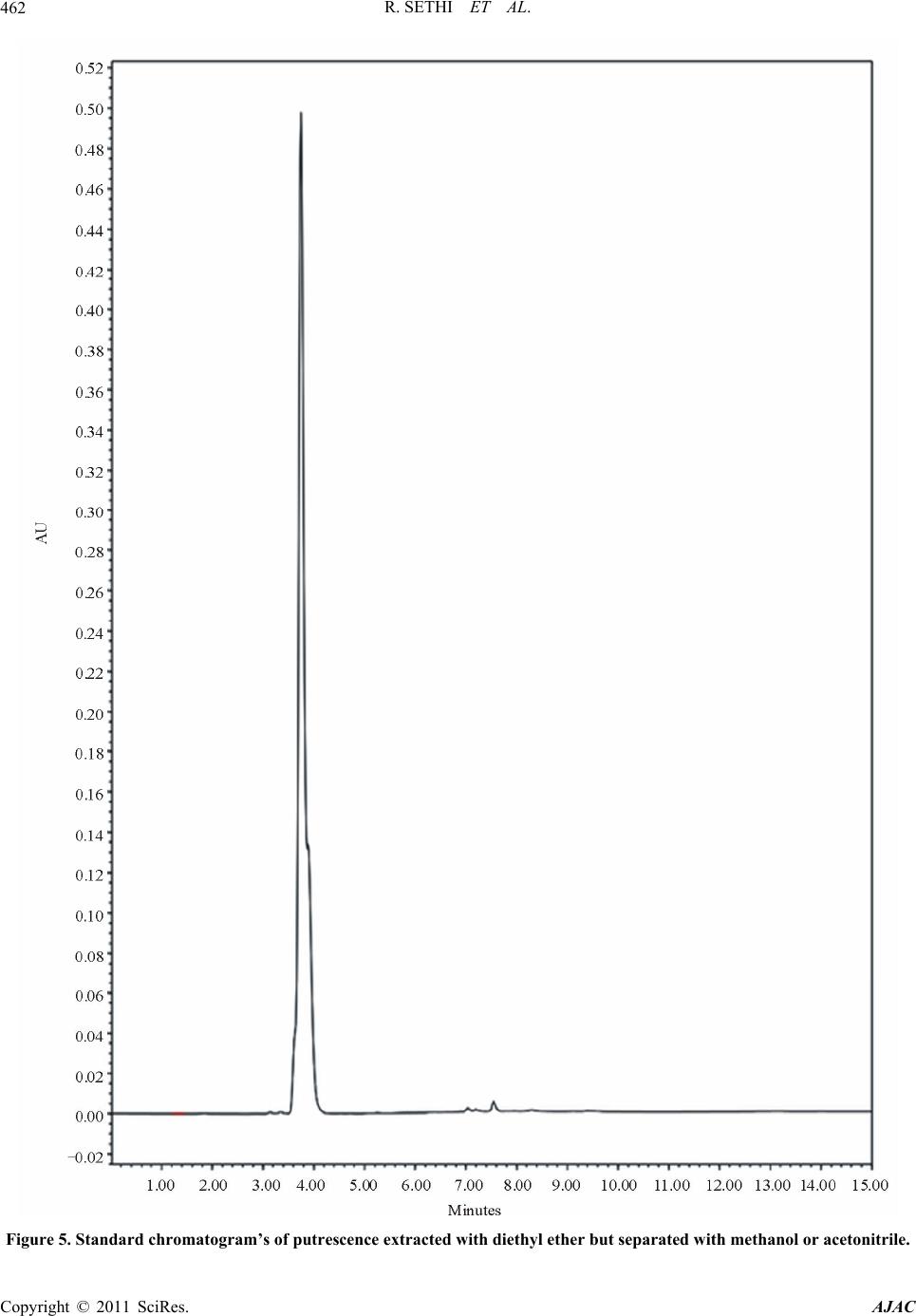 R. SETHI ET AL. 462 Figure 5. Standard chromatogram’s of putrescence extracted with diethyl ether but separated with methanol or acetonitrile. Copyright © 2011 SciRes. AJAC 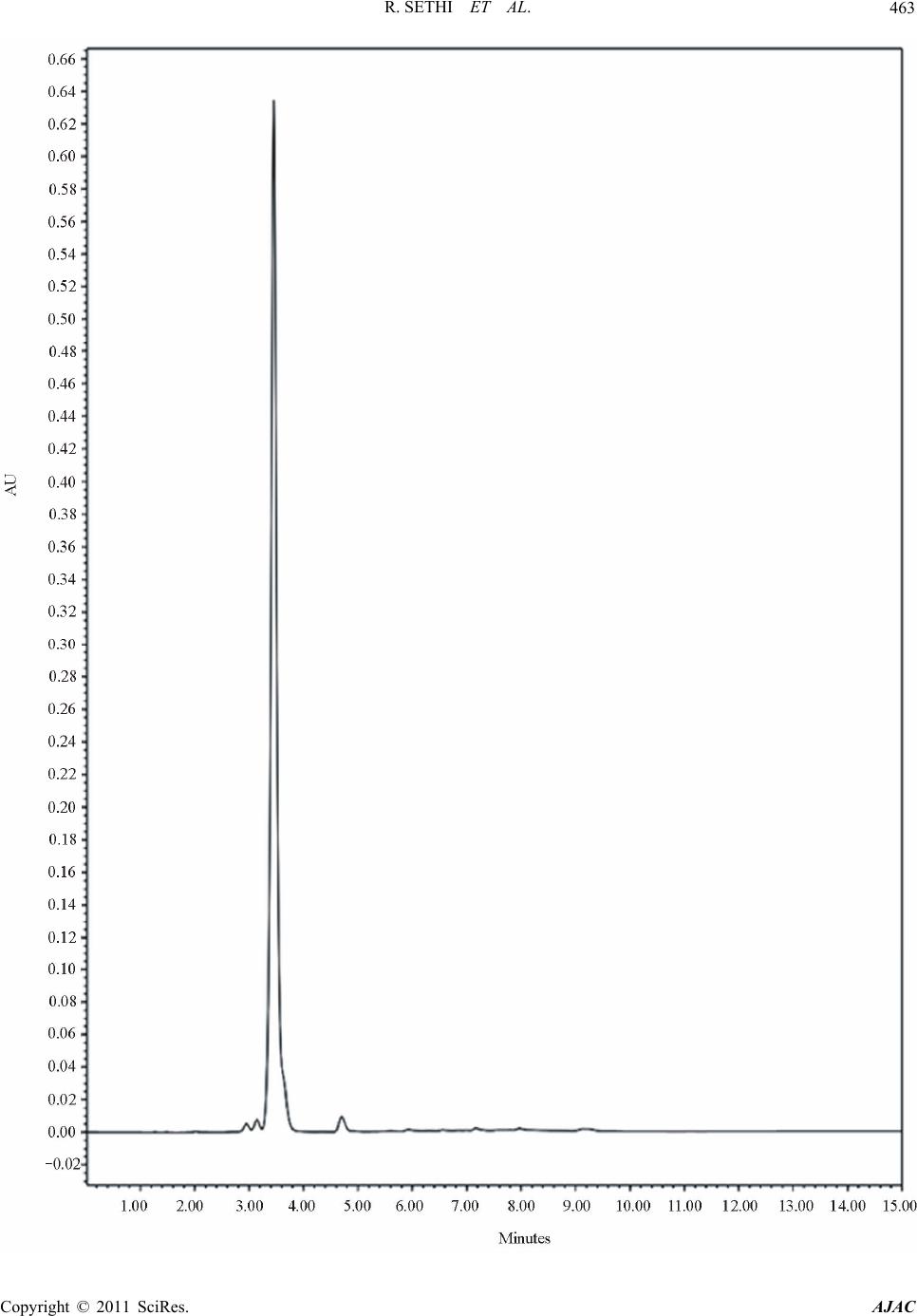 R. SETHI ET AL.463 Copyright © 2011 SciRes. AJAC 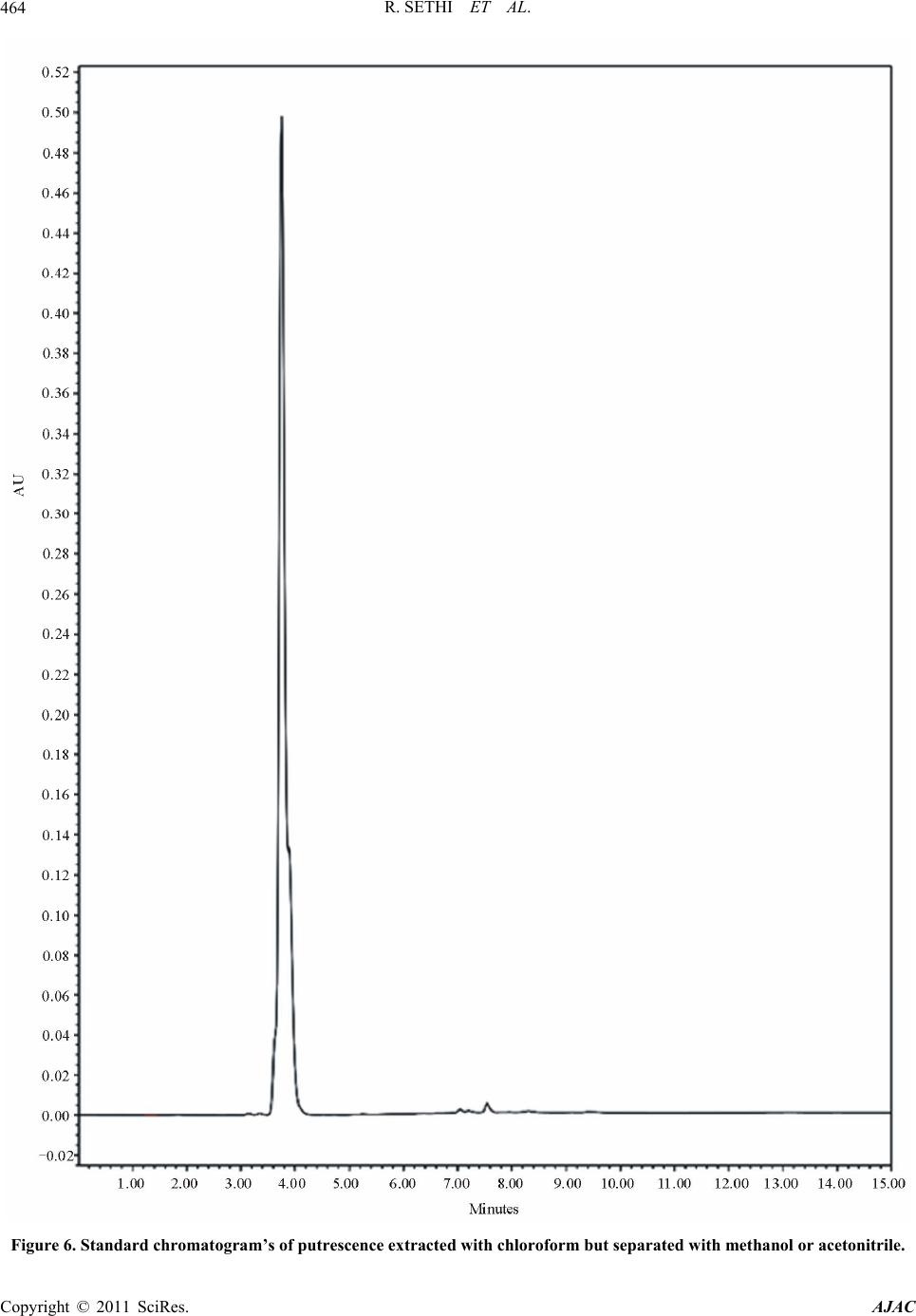 R. SETHI ET AL. 464 Figure 6. Standard chromatogram’s of putrescence extracted with chloroform but separated with methanol or acetonitrile. Copyright © 2011 SciRes. AJAC 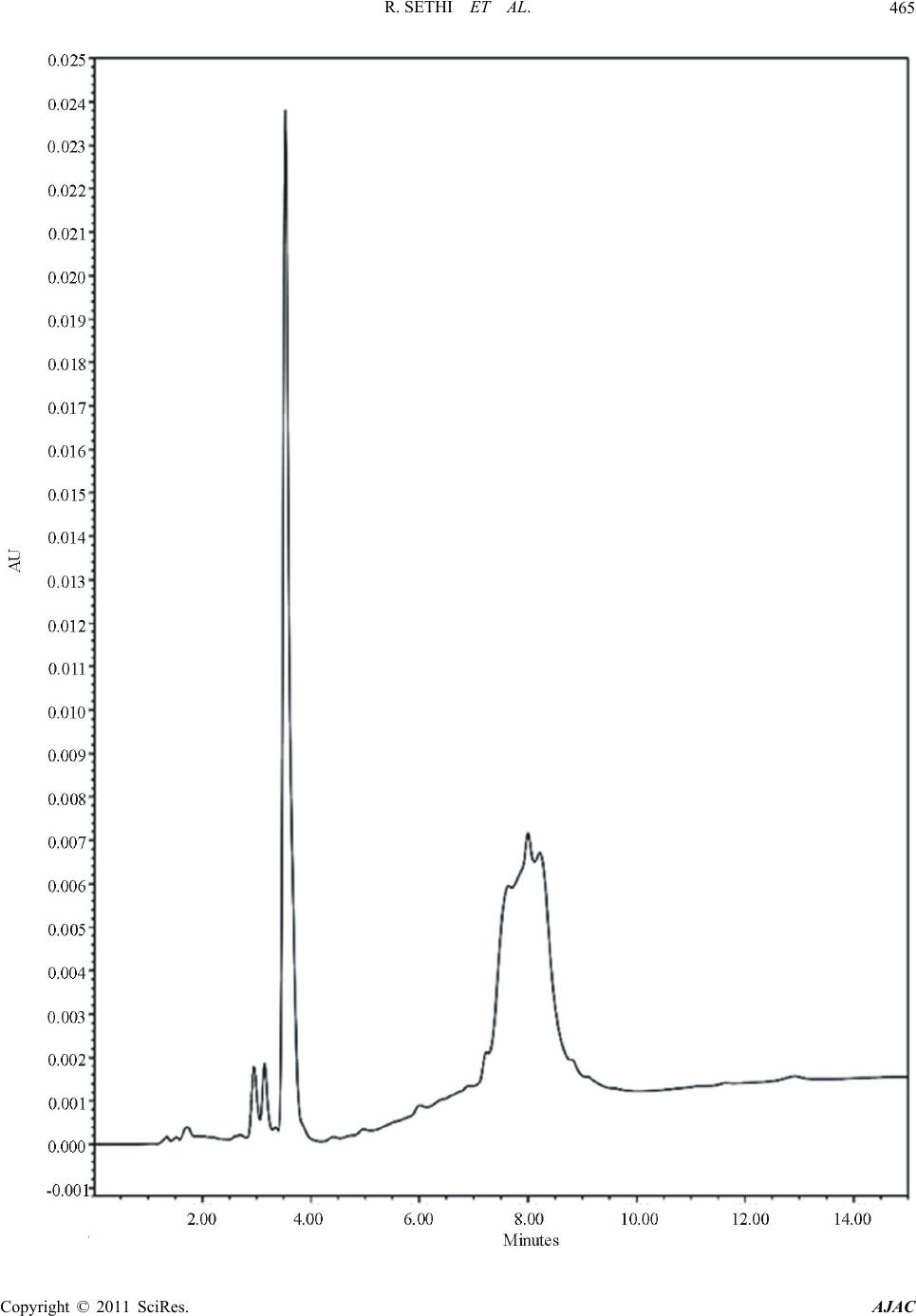 R. SETHI ET AL.465 Copyright © 2011 SciRes. AJAC  R. SETHI ET AL. 466 Figure 7. Standard chromatogram’s of spermidine extracted with diethyl ether but separated with methanol or acetonitrile. Copyright © 2011 SciRes. AJAC  R. SETHI ET AL. Copyright © 2011 SciRes. AJAC 467 Figure 8. Standard chromatogram’s of spermidine extracted with chloroform but separated with methanol or acetonitrile. Table 3. Relative Recovery and Coefficient of variation from nine standards. Compound Intra day Mean CV %Inter day Mean CV %% Recovery Putrescine 2.4 3.0 88.15 Cadaverine 1.9 3.4 102.18 Spermidine 3.0 3.8 125.25 4. Discussions with acetonitrile was comparable to seperations achieved for putrescine and spermidine in giving very good reso- lution, accurately and reproducibility for all extracted polyamines. Accurate analysis of polyamine is important in under- standing pathophysiology in tissue stress. And essential  R. SETHI ET AL. 468 in understanding disease epidemiology and homeostasis. Our method was able to recover 88% to 125% of spiked polyamines derivatized with benzoyl chloride using 1, 6-diamino hexane as internal standard. Similar trends were observed in the recovery of the internal standard (1,6-diamino hexane). The limit of detection with ace- tonitrile as solvent was 500 pmoles, which is similar or superior to other published methods which utilize chro- matography. The present study, demonstrates greater sensitivity, resolution and reproducibility with the shorter run time to comparable chromatographic-based methods such as pioneered by Morgan [30]. Other advantages were lower consumption of HPLC elution solvent driv- ing down cost of analy si s. 5. Conclusions In short, we have demonstrated a modified method based on Morgan’s protocol which yielded excellent sensitivity, resolution and a limit of detection of 500 pmoles which is superior to other reported values of 800 pmoles. This method is also more economical by virtue of its lower run time and less consumption of solvents. 6. Author Contribution S. R. Chava undertook all of the experimental work (ex- traction, triplicate analysis) including co-writing of the first draft of the manuscript. S. Bashir assisted in the extraction and the first set of analyses. He also wrote the first and co-wrote the second and submission draft of the manuscript. M. Castro and R. Sethi co-supervised the student towards his Master of Science thesis, and associ- ated publication and research costs respectively. 7. Acknowledgements We are grateful to Mohammad T. Nutan (TAMHSC) for giving permission to use the HPLC and Mr. Don Marek from the Department of Environmental Engineering (TAMUK) for assistan ce wi t h the glo ve bo x. 8. References [1] L. J. Ignarro, M. L. Balestrieri and C. Napoli, “Nutrition, Physical Activity, and Cardiovascular Disease: an up- date,” Cardiovascular Research, Vol. 73, No. 2, January 2007, pp. 326-340. doi:10.1016/j.cardiores.2006.06.030 [2] M. L. Daviglus, J. Stamler, A. J. Orencia, A. R. Dyer, K. Liu, P. Greenland, M. K. Walsh, D. Morris and R. B. Shekelle, “Fish Consumption and the 30-Year Risk of Fatal Myocardial Infarction,” The New England Journal of Medicine, Vol. 336, No. 15, April 1997, pp. 1046-1053. doi:10.1056/NEJM199704103361502 [3] A. H. Lichtenstein, L. J. Appel, M. Brands, M. Carnethon, S. Daniels, H. A. Franc h, B. Franklin, P. Kris-Etherton, W. S. Harris, B. Howard, N. Karanja, M. Lefevre, L. Rudel, F. Sacks, L. Van Horn, M. Winston and J. Wylie- Rosett, “Summary of American Heart Association Diet and Life- style Recommendations revision 2006,” Arteriosclerosis, Thrombosis, and Vascular Biology, Vol. 26, No. 10, Oc- tober 2006, pp. 2186-2191. doi:10.1161/01.ATV.0000238352.25222.5e [4] Y. J. Zhao, C. Q. Xu, W. H. Zhang, L. Zhang, S. L. Bian, Q. Hang, H. L. Sun, Q. F. Li, Y. Q. Zhang, Y. Tian, R. Wang, B. F. Yang and W. M. Li, “Role of Polyamines in Myocardial Ischemia/Reperfusion Injury and Their Inter- actions with Nitric Oxide,” European Journal of Phar- macology, Vol. 563, No. 3, May 2007, pp. 236-246. doi:10.1016/j.ejphar.2007.01.096 [5] J. Martin-Tanguy, “Metabolism and Function of Poly- amines in Plants: Recent Development (New Ap- proaches),” Plant Growth Regulations, Vol. 34, No. 14, May 2001, pp. 135-148. doi:10.1023/A:1013343106574 [6] M. C. White, R. A. Etzel, W. D. Wilcox and C. Lloyd, “Exacerbations of Childhood Asthma and Ozone Pollu- tion in Atlanta,” Environmental Research, Vol. 65, No. 1, April 1994, pp. 56-58. doi:10.1006/enrs.1994.1021 [7] M. D. Denton, H. S. Glazer, D. C. Zellner and F. G. Smith, “Gas–Chromatographic Measurement of Urinary Polyamines in Cancer Patients,” Clinical Chemistry, Vol. 19, No. 8, June 1973, pp. 904-907. [8] D. R. Roberts, M. A. Walker and E. D. Dumbroff, “Mass Spectral Determination of Benzamide Derivatives of Polyamines Separated by HPLC,” Phytochemistry, Vol. 24, No. 5, January 1995, pp. 1084-1090. [9] J. W. Redmond and A. Tseng, “High-Pressure Liquid Chromatographic Determination of Putrescine, Cadaver- ine, Spermidine and Spermine,” Journal of Chromatog- raphy, Vol. 170, No. 2, March 1979, pp. 479-481. doi:10.1016/S0021-9673(00)95481-5 [10] S. Bardocz, “Effect of Phytohaemagglutinin on Intestinal Cell Proliferation. Role of Polyamines,” Archivos Lati- noamericanos de Nutrición, Vol. 44, No. 4, Suppl. 1, December 1996, pp. 16S-20S. [11] A. Gugliucci and T. Menini, “The Polyamines Spermine and Spermidine Protect Proteins from Structural and Functional Damage by AGE Precursors: A New Role for Old Molecules?” Life Science, Vol. 72, No. 23, April 2003, pp. 2603-2616. doi:10.1016/S0024-3205(03)00166-8 [12] S. Wongyai, P. J. Oefner and G. K. Bonn, “High Resolu- tion Analysis of Polyamines and Their Acetyl Derivatives Using RP-HPLC,” Journal of Liquid Chromatography, Vol. 12, No. 12, December 1989, pp. 2249-2261. [13] G. Taibi and M. R. Schiavo, “Simple High-Performance Liquid Chromatographic Assay for Polyamines and Their Monoacetyl Derivatives,” Journal of Chromatography, Vol. 614, No. 1, April 1993, pp. 153-158. [14] P. Torrigiani, A. L. Rabiti, L. Betti, F. Marani, M. Bizzi, N. Bagni and A. Canova, “Improved Method for Poly- amine Determination in TMV, a Rod-Shaped Virus,” Copyright © 2011 SciRes. AJAC  R. SETHI ET AL. Copyright © 2011 SciRes. AJAC 469 Journal of Virological Methods, Vol. 53, No. 1, January 1995, pp. 157-163. [15] J. B. Wehr, “Purification of Plant Polyamines with An- ion-Exchange Column Clean-Up Prior to High-Perfor- mance Liquid Chromatographic Analysis,” Journal of Chromatography A, Vol. 709, No. 2, August 1995, pp. 241-247. doi:10.1016/0021-9673(95)00461-U [16] M. Marcé, D. S. Brown, T. Capell, X. Figueras and A. F. Tiburcio, “Rapid High-Performance Liquid Chroma- tographic Method for the Quantitation of Polyamines as Their Dansyl Derivatives: Application to Plant and Ani- mal Tissues,” Journal of Chromatography B, Vol. 666, No. 2, April 1995, pp. 329-335. doi:10.1016/0378-4347(94)00586-T [17] H. E. Flores and A. W. Galston, “Analysis of Poly amines in Higher Plants by High Performance Liquid Chroma- tography,” Plant Physiology, Vol. 69, No. 3, March 1982, pp. 701-706. doi:10.1104/pp.69.3.701 [18] M. A. Smith and P. J. Davies, “Separation and Quantita- tion of Polyamines in Plant Tissues by High Performance Liquid Chromatography of Their Dansyl Derivatives,” Plant Physiology, Vol. 78, No. 1, May 1985, pp. 89-91. doi:10.1104/pp.78.1.89 [19] R. D. Slocum, H. E. Flores, A. W. Galston and L. H. Weinstein, Improved Method for HPLC Analysis of Polyamines, Agmatine and Aromatic Monoamines in Plant Tissue,” Plant Physiology, Vol. 89, No. 2, February 1989, pp. 512-517. doi:10.1104/pp.89.2.512 [20] P. F. Hockl, S. M. Thyssen and C. Libertun, “An Im- proved HPLC Method for Identification and Quantitation of Polyamines and Related Compounds as Benzoylated Derivatives,” Journal of Liquid Chromatography & Re- lated Technologies, Vol. 23, No. 5, March 2000, pp. 693-703. doi:10.1081/JLC-100101482 [21] A. Gamarick and R. B. Frydman, “Cadaverine, an Essen- tial Diamine for the Normal Root Development of Ger- minating Soybean (Glycine max) Seeds,” Plant Physiol- ogy, Vol. 97, No. 2, February 1991, pp. 778-785. doi:10.1104/pp.97.2.778 [22] R. M. Adibhatla, J. F. Hatcher, K. Sailor and R. J. Dempsey, “Polyamines and Central Nervous System In- jury: Spermine and Spermidine Decrease Following Transient Focal Cerebral Ischemia in Spontaneously Hy- pertensive Rats,” Brain Research, Vol. 938, No. 1-2, May 2002, pp. 81-86. doi:10.1016/S0006-8993(02)02447-2 [23] W. Paschen, R. Widmann and C. Weber, “Changes in Regional Polyamine Profiles in Rat Brains after Transient Cerebral Ischemia (Single versus Repetitive Ischemia): Evidence for Release of Polyamines from Injured Neu- rons,” Neuroscience Letters, Vol. 135, No. 1, January 1992, pp. 121-124. doi:10.1016/0304-3940(92)90150-6 [24] C. N. Ramchand, I. Das, A. Gliddon and S. R. Hirsch, “Role of Polyamines in the Membrane Pathology of Schizophrenia A Study Using Fibroblasts from Schizo- phrenic Patients and Normal Controls,” Schizophrenia Research, Vol. 13, No. 3, October 1994, pp. 249-253. doi:10.1016/0920-9964(94)90049-3 [25] W. Paschen, L. Csiba, G. Rohn and D. Bereczki, “Poly- amine Metabolism in Transient Focal Ischemia of Rat Brain,” Brain Research, Vol. 566, No. 1-2, December 1991, pp. 354-357. doi:10.1016/0006-8993(91)91726-H [26] C. Strambi, A. Tirard, M. Renucci, P. Faure, P. Charpin, R. Augier and A. Strambi, “Ecdysone Deprivation Af- fects Polyamine Metabolism in the House Cricket Acheta Domesticus,” Insect Biochemistry and Molecular Biology, Vol. 23, No. 1, January 1993, pp. 165-170. doi:10.1016/0965-1748(93)90096-B [27] K. Kotzabasis, M. D. Christakis and K. A. Roube- lakis-Angelakis, “A Narrow-Bore HPLC Method for the Identification and Quantitation of Free, Conjugated, and Bound Polyamines,” Analytical Biochemistry, Vol. 214, No. 2, November 1993, pp. 484-489. doi:10.1006/abio.1993.1526 [28] C. Cann-Moissan, J. Caroff, A. Hourmant, C. Videau and F. Rapt, “Quantitative Analysis of Polyamines at Trace Levels by High Performance Liquid Chromatography in High Salt Solutions. Application to Seawater,” Journal of Liquid Chromatography, Vol. 17, No. 16, December 1994, pp. 1413-1417. doi:10.1080/10826079408013773 [29] C. F. Verkolen, J. C. Romijn, F. H. Schroeder, W. P. Schalkwijt and T. A. Splinter, “Quantitation of Poly- amines in Cultured Cells and Tissue Homogenates by Reversed-Phase High-Performance Liquid Chromatog- raphy of Their Benzoyl Derivatives,” Journal of Chro- matography, Vol. 426, No. 11, November 1988, pp. 41- 54. [30] D. M. L. Morgan, “Determination of Polyamines as Their Benzoylated Derivatives by HPLC,” In: D. M. L. Morgan, Ed., Polyamine Protocols, Humana Press, Totowa, 1997, pp. 111-118. doi:10.1385/0-89603-448-8:111
|