Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio n, 2011, 2, 1014-1021 doi:10.4236/msa.2011.28137 Published Online August 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA Synthesis and Properties of Polythiophène Benzylidene and Their Photovoltaic Applications Reguig Bendoukha Abdelkarim1,3, Ahmed Yahiaoui1*, Aïcha Hachemaoui1, Mohammed Belbachir2, Abdelbasset Khelil3 1Laboratoire de chimie Organique, Macromoléculaire et des Matériaux (LCOMM), Université de Mascara, Faculté des Sciences et de la Technologie, Mascara, Algérie; 2Laboratoire de Chimie des Polymères, Université d’Oran, Oran, Algérie; 3LPCM2E, Universite´ d’Oran Es-Senia, Algérie. Email: *yahmeddz@yahoo.fr Received March 19th, 2011; revised April 11th, 2011; accepted May 9th, 2011. ABSTRACT Research on organic solar cells has a craze importance because they show very interesting properties including their flexibility and the opportunity to be made into large surfaces. However, their stability and performance should be sig- nificantly improved compared to their current state. A nominal output of around 10% will be the goal for the coming years. The use of organic materials for photovoltaic applications is the subject of intense research in recent years. This work is based in part on the development of new conjugated polymers. In this paper, we present the synthesis and characterization of poly [(thiophene-2,5-diyl)-co-(benzylidene)] PTB catalysed by Maghnite-H+, used in the active layer of the solar cell organic heterojunction with PCBM (derivative of C60) was used as a junction of the solar cell: Glas/ITO/BCP/C60/PTB/Au/Al. A current density of short circuit of about Jcc 0.1mA/cm² was obtained for this struc- ture with a yield of around 0.15%. Keywords: Polymerization, Conjugated Polymers, UV-Vis Spectroscopy, IR Spectroscopy, Yield Calculation, Solar Cell Organic Heterojunction. 1. Introduction In the past few decades, conjugated polymers with an extended p-conjugation have received considerable at- tention due to their electronic and photonic applications, such as light-emitting diodes [1,2], photovoltaic cells (PVCs) [3,4], and thin film transistors [5,6]. Polythio- phenes (PTs) are the most promising conjugated poly- mers because of their relatively high charge carrier mo- bility and long wavelength absorption in comparison withother conjugated polymers [7]. For example, regio- regular poly(3-hexylthiophene) (P3HT) have exhibited excellent properties in PVCs [8,9]. However, P3HT only absorbs a part of the visible light and exhibits the rela- tively low open-circuit voltage (Voc) [10]. To further improve the related properties and explore the full poten- tial applications of these materials, chemical modifica- tions of PTs have been performed actively. One success- ful strategy to achieve broader absorption of PTs is based on the pioneering work by Li and coworkers [11,12]. They synthesized PTs with conjugated bi(thienyleneviny- lene) as side chain and made the absorption band in the region from 350 nm to 650 nm, resulting in a good power conversion efficiency (PCE) of 3.18%. PTs with sub- stituents other than alkyl groups have also been investi- gated, among which those with electrondonating alkoxy groups have displayed promising optical properties [13]. Actually, the PCE of polymer photovoltaic devices is determined by three main factors: the efficiency of exci- ton generation, the efficiency of exciton dissociation into free charge carriers, and the efficiency of their unhin- dered collection by the electrodes. To increase the device efficiency, the active layer should absorb as many of the incident photons as possible to generate a maximum of excitons [14,15], therefore, low band gap polymers are necessary since the absorption of the active layer should match the solar spectrum well. The factors that influence the bandgap of a polymer are conjugated length, solid state ordering, and the presence of electron withdrawing or -donating moieties. The effective conjugated length, which is dependent upon the torsion angle between the repeating units along the polymer backbone, can be con- trolled by choosing sterically hindered units along the polymer back-chain or by introducing bulky side-chains  Synthesis and Properties of Polythiophène Benzylidene and Their Photovoltaic Applications1015 to twist the units out of plane [16,17]. While, the Voc of a PVCs based on polymer and (6,6)-phenyl C61-butyric acid methyl ester (PCBM) blend system is determined by the difference between the HOMO of the polymer and lowest unoccupied molecular orbital (LUMO) of PCBM [18,19]. Therefore, the HOMO level is also an important parameter to be considered when designing a new elec- tron-donating polymer. Triphenylamine (TPA) is a preferred electrondonating moiety with excellent hole transporting properties and their derivatives have been widely investigated for al- most two decades [20,21]. Owing to the noncoplanarity of the three phenyl substituents, TPA derivatives can be viewed as 3D systems and the amorphous character of these materials offers possibilities to developactive mate- rials for PVCs with isotropic optical and charge-transport properties [22]. Recently, Roncali, J. and coworkers [23] synthesized various series of star-shaped molecules based on TPA small molecules with combinations of thienylenevinylene conjugated branches and lectron- withdrawing indanedione or dicyanovinyl groups, which have been applied to organic PVCs as donor materials and got PCE of 1.20%.To our knowledge, the application of TPA-based polymers for photovoltaic devices has been scarcely considered. Here, we report the synthesis and photovoltaic proper- ties of a new conjugated polymer derived from PT, poly [(thiophene-2,5-diyl)-co-(benzylidene)] (PTB) a novel soluble obtained in a single step polymerization through a very simple acid-catalyzed condensation of thiophene and benzaldehyde in the presence of an exchanged clay montmorillonite called Mag-H as catalyst. The PTB was used for the photoactive layer; ITO and aluminum (Al) are used as metal electrodes. Poly [(thiophene-2,5-diyl)-co-(benzylidene)]PTB is soluble in dichloromethane CH2Cl2, was characterized by infrared spectroscopy and Ultra violet spectroscopy. 2. Experimental 2.1. Reagents Dichloromethane and methanol (98%) were purchased from Aldrich and used as received. Thiophene: was pur- chased from Aldrich Chemical Co. and distilled under reduced pressure. Benzaldehyde: was purchased from Aldrich Chemical. Raw-Maghnite (Algerian montmoril- lonite clay) was procured from BENTAL (Algerian So- ciety of Bentonite). 2.2. Polymer Preparatio The condensation of benzaldehyde with thiophene in the presence of Mag-H+ as a catalyst was carried out by condensation in bulk under nitrogen for 6 h. Each mixture was prepared with a weighted quantity of Mag-H+ dried just before use for 1 h in a drying oven at 100˚C. Benzaldehyde (6 × 10–3 mol) and thiophene (6 × 10–3 mol) were mixed and 1g of Mag-H+ was added. The reaction was carried out at 25˚C for 6 h. The poly- mer film was washed with water and methanol several times, and finally dried under vacuum at room tempera- ture for 24 h. The yield was 50%. The resulting polymers were characterized by FTIR and UV-Visible spectros- copy. Anal. Calcd for (C22H14S2)n C, 77.16; H, 4.12; S, 18.72. Found: C, 78.63; H, 4.53; S, 15.93. IR (film on NaCl, cm–1): 3027, 1598, 1492, 1443, 1294, 1231, 1176, 1155, 1105, 1073, 1028, 901, 801, 751, 696, 668 (Figure 1). 2.3. Development of the Photovoltaic Cell Photovoltaic cells consist of a molecular active layer sandwiched between an anode of ITO (thickness 100 nm) and an aluminum cathode. The cell size was determined by the size of the aluminum cathode (evaporated through a mask of 0.25 cm²). A layer 260Å béthocuproine PCO (C26H2ON2), sandwiched between the ITO layer and the acceptor is primarily intended to protect this last layer of oxygen diffusion from ITO. Toward the cathode, a layer of 20Å of gold (Au) avoids the recombination of excitons in organic-metal interface (Figure 3). The bathocuproine BCP thickness and C60 respectively 260 and 1650Å were being deposited on the ITO layer in the laboratory by the technique of thermal evaporation (TE). We conducted the following cells, using the active layer in the donor-acceptor pair TMP-C60: The layer of PTB was dissolved in a solution of di- chloromethane and deposited onto the layer of C60 by the method of spin coating. So we made a solar cell junction with the following: Glass/ITO/BCP/ C60/PTB/Au/Al 3. Results and Discussion 3.1. Spectroscopic Characterization: The Figure 1 presents the FTIR spectrum of PTB and shows the appearance of a strong absorption at 1640 cm–1 which is attributed to the stretching vibration of conju- gated C=C and the stretching vibration of aromatic in thiophene .A distinct peack near 737,37 cm–1 is due the out of plane vibration C -H characteristic of the -linkage in thiophene rings . The UV-vis absorption spectra were recorded with an OPTIZEN UV-2120 spectrophotometer (Figure 2) shows the optical absorption spectrum of polymer: PTB in CH2Cl2 solution. The colours of the polymer solutions were almost grey or black. The absorption spectrum in Copyright © 2011 SciRes. MSA 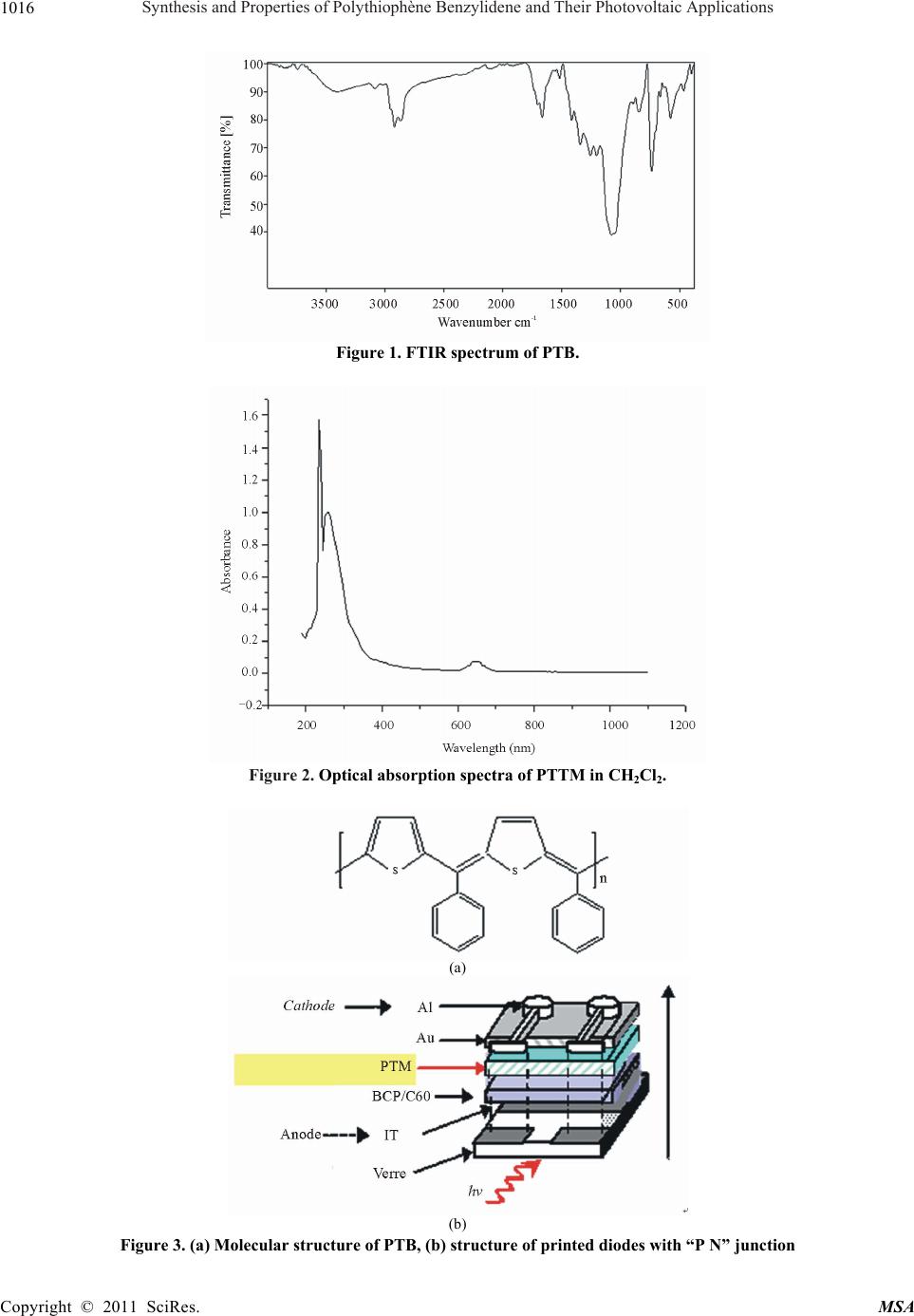 Synthesis and Properties of Polythiophène Benzylidene and Their Photovoltaic Applications 1016 Figure 1. FTIR spectrum of PTB. Figure 2. Optical absorption spectra of PTTM in CH2Cl2. (a) (b) Figure 3. (a) Molecular structure of PTB, (b) structure of printed diodes with “P N” junction Copyright © 2011 SciRes. MSA  Synthesis and Properties of Polythiophène Benzylidene and Their Photovoltaic Applications 1017 Figure 2 shows two major absorption bands. The band in range of 280 - 300 nm is assigned to the π - π* transition of the aromatic heterocyclic since it corresponds to the same band as its precursor, and the band in the range of 620 - 650 nm is assigned to the π - π* band gap transition [9]. 3.2. Photovoltaic Properties To investigate the photovoltaic properties of the poly- mers, the bulk heterojunction solar cells with a structure of ITO/BCP/C60/PTB/Au/Al were fabricated where the polymer was used as donor and C60 as acceptor. The active layers were prepared by spin coating Current-voltage characteristics of solar cells in the dark and under illumination of 100 mW/cm2 white light from a xenon lamp (Jobin Yvon, FL-1039) were meas- ured on the computer-controlled Keithley 2400 Sour- ceMeter system measurement. All measurements were carried out under ambient atmosphere at room tempera- ture. The Current–voltage characteristics in the dark and under illumination were measured. One can see that in our case (Figure 4) a photovoltaic effect is revealed but with a small but significant yield was obtained. This structure has a good recovery, a VOC of 0.26 V and a JSC 1,6 mA/cm². In addition, there is a clear improve- ment of the FF (36%), giving a yield of around 0.15%. After a performing a photovoltaic cells, and in order to well understand and control the key physical processes that determine the performance of organic solar cells, we must know all the physical parameters, such as series resistance, parallel resistance, the different saturation currents, photo-current and ideality factor. We were led to use electrical models [24], equivalent circuit to a diode, which allowed us to model our solar cells in the dark and under illumination. 3.3. Electrical Equivalent Circuit of a Photoelectric Cell Solar cells are generally equivalent to a simple circuit with a single diode in parallel with a resistor Rp, and a series resistance Rs . In literature, the most frequent expression is: exp 1 SS S Tp VRI VRI II nu R (1) where n is the ideality coefficient. The solution of equation (1) is: exp pSp S SppS T TT RIR I I VRRIRInuW nu nu (2) where W is the Lambert function defined by . Wx Wxe x 3.4. In the Case of the Diode Under Illumination PN junction under illumination can be diagrammed by a current generator Iph (current proportional to incident light) in parallel with the diode delivering a current -2000200 400 -5,0 -2,5 0,0 2,5 5,0 7,5 10,0 12,5 15,0 17,5 20,0 22,5 25,0 Densit?de courant (mA/cm2) Tension (V) Figure 4. Current–voltage characteristics of polymer photovoltaic cells based on PTB (Substrate/ ITO / PCB /C60 /PTB/ Al) blend system in the dark (----) and underillumination (----) of 100 mW/cm2 white light. Copyright © 2011 SciRes. MSA 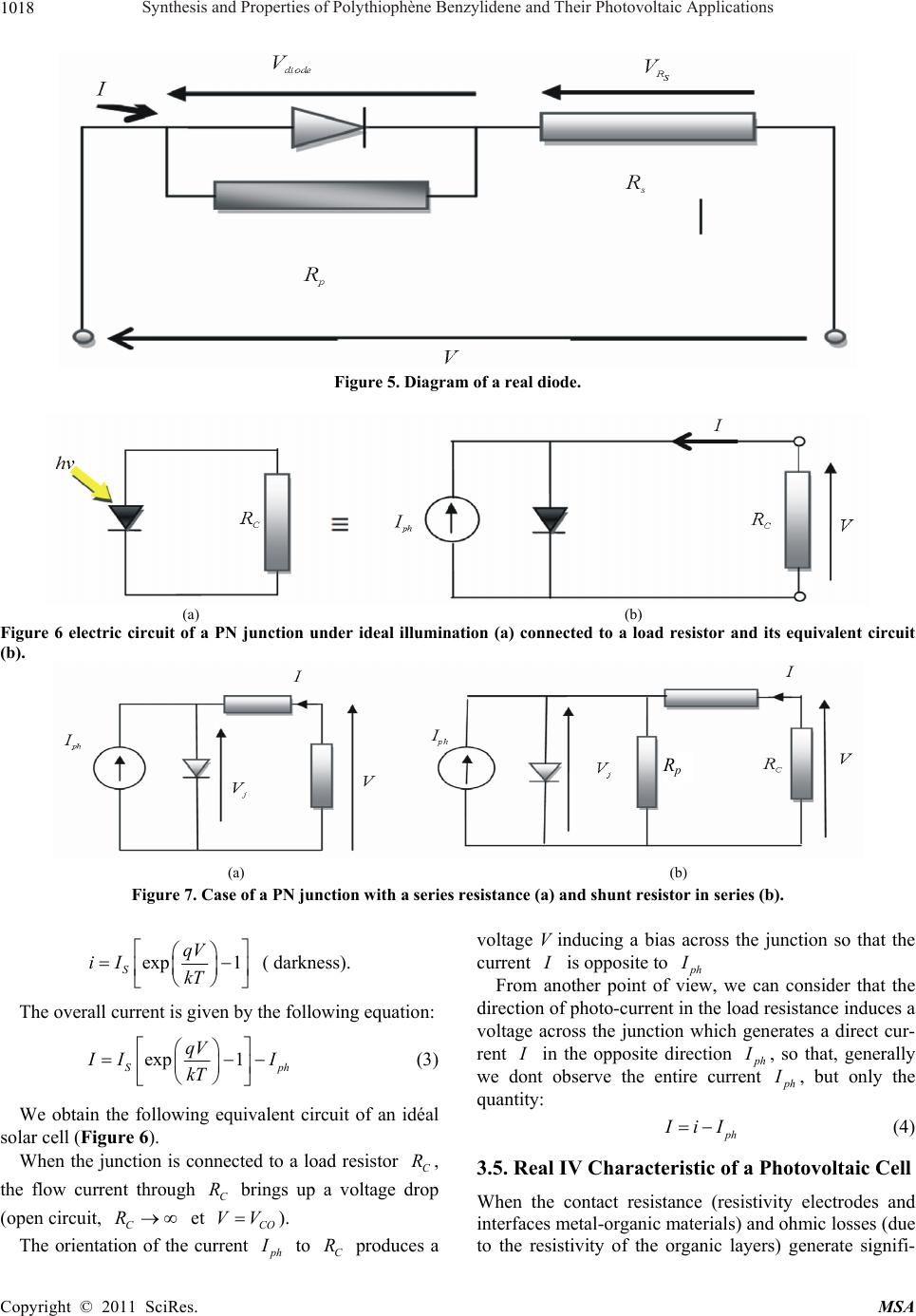 Synthesis and Properties of Polythiophène Benzylidene and Their Photovoltaic Applications 1018 Figure 5. Diagram of a real diode. (a) (b) Figure 6 electric circuit of a PN junction under ideal illumination (a) connected to a load resistor and its equivalent circuit (b). R p (a) (b) Figure 7. Case of a PN junction with a series resistance (a) and shunt resistor in series (b). exp 1 S qV iI kT ( darkness). The overall current is given by the following equation: exp 1 S qV ph I I kT I (3) We obtain the following equivalent circuit of an idéal solar cell (Figure 6). When the junction is connected to a load resistor , the flow current through brings up a voltage drop (open circuit, et C R C R C R CO VV ). The orientation of the current p h I to produces a voltage V inducing a bias across the junction so that the current C R I is opposite to p h I From another point of view, we can consider that the direction of photo-current in the load resistance induces a voltage across the junction which generates a direct cur- rent I in the opposite direction p h I , so that, generally we dont observe the entire current p h I , but only the quantity: p h IIi (4) 3.5. Real IV Characteristic of a Photovoltaic Cell When the contact resistance (resistivity electrodes and interfaces metal-organic materials) and ohmic losses (due to the resistivity of the organic layers) generate signifi- Copyright © 2011 SciRes. MSA 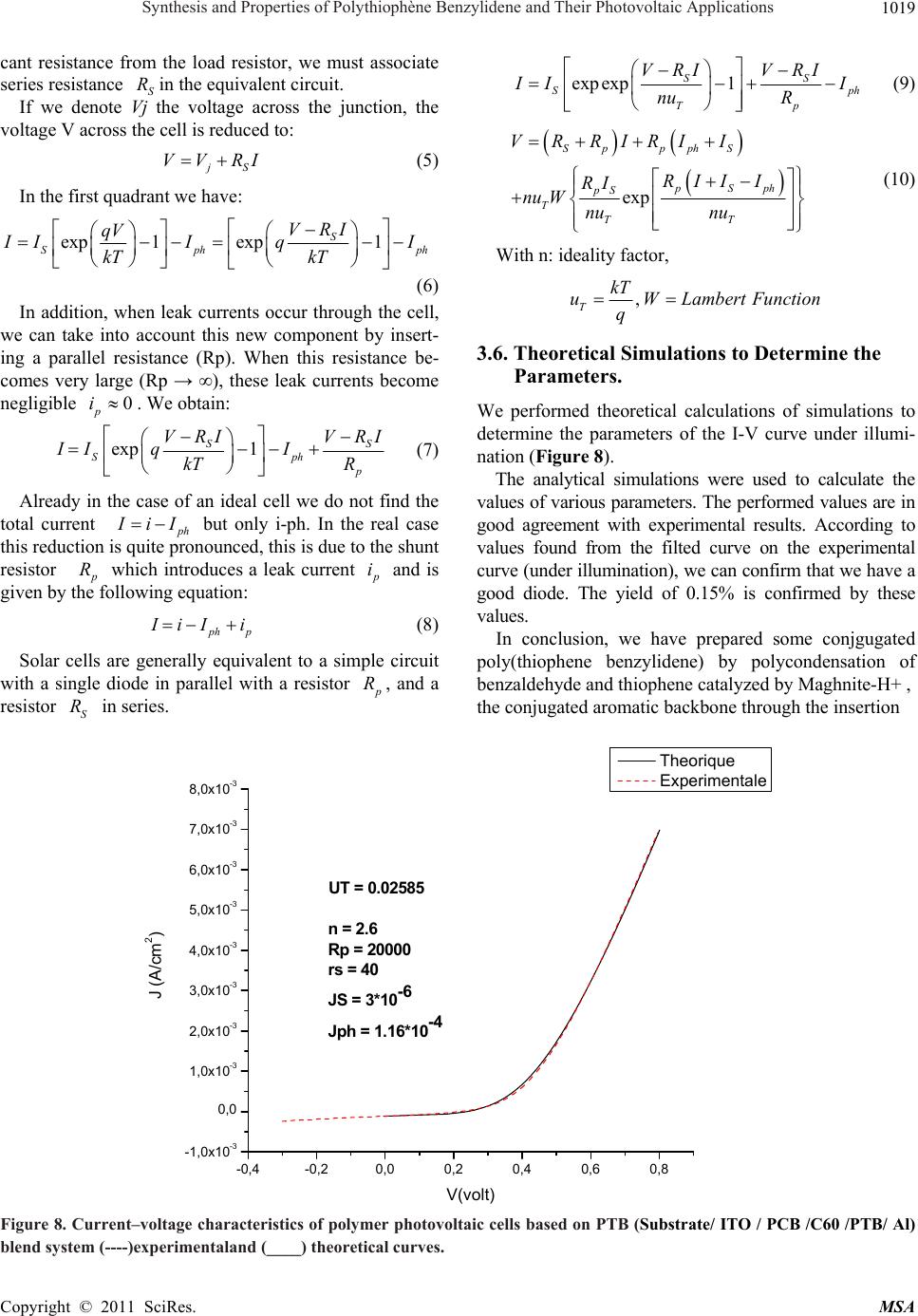 Synthesis and Properties of Polythiophène Benzylidene and Their Photovoltaic Applications1019 cant resistance from the load resistor, we must associate series resistance in the equivalent circuit. S If we denote Vj the voltage across the junction, the voltage V across the cell is reduced to: R j S VV RI (5) In the first quadrant we have: exp 1exp1 S Sph ph VRI qV I IIqI kT kT (6) In addition, when leak currents occur through the cell, we can take into account this new component by insert- ing a parallel resistance (Rp). When this resistance be- comes very large (Rp → ∞), these leak currents become negligible . We obtain: 0 p i exp 1 S Sp p VRI VRI II qI kT R S h (7) Already in the case of an ideal cell we do not find the total current p h but only i-ph. In the real case this reduction is quite pronounced, this is due to the shunt resistor IiI p R which introduces a leak current p i and is given by the following equation: ph p I iI i (8) Solar cells are generally equivalent to a simple circuit with a single diode in parallel with a resistor p R, and a resistor in series. S R expexp 1 SS Sp Tp VRI VRI h I II nu R (9) exp Sp pphS pSph pS T TT VRRIRI I RII I RI nu Wnu nu (10) With n: ideality factor, , T kT uWLambert Function q 3.6. Theoretical Simulations to Determine the Parameters. We performed theoretical calculations of simulations to determine the parameters of the I-V curve under illumi- nation (Figure 8). The analytical simulations were used to calculate the values of various parameters. The performed values are in good agreement with experimental results. According to values found from the filted curve on the experimental curve (under illumination), we can confirm that we have a good diode. The yield of 0.15% is confirmed by these values. In conclusion, we have prepared some conjgugated poly(thiophene benzylidene) by polycondensation of benzaldehyde and thiophene catalyzed by Maghnite-H+ , the conjugated aromatic backbone through the insertion -0,4-0,20,0 0,2 0,4 0,6 0,8 -1,0x10-3 0,0 1,0x10-3 2,0x10-3 3,0x10-3 4,0x10-3 5,0x10-3 6,0x10-3 7,0x10-3 8,0x10-3 J (A/cm2) V(volt) Theorique Experimentale UT = 0.02585 n = 2 .6 Rp = 20000 rs = 4 0 JS = 3*10-6 Jph = 1.16*10-4 Figure 8. Current–voltage characteristics of polymer photovoltaic cells based on PTB (Substrate/ ITO / PCB /C60 /PTB/ Al) blend system (----)experimentaland (____) theoretical curves. Copyright © 2011 SciRes. MSA  Synthesis and Properties of Polythiophène Benzylidene and Their Photovoltaic Applications 1020 Table 1. Photovoltaic Characteristics of our Polymer and those of literature [25]. Voc (V) Jsc (mA/cm2) FF PCE (%) PTB 0.26 1.6 0.36 0.15 P3T-DDTPA 0.72 0.38 0.32 0.086 P1 0.71 0.08 0.24 0.011 P2 0.74 0.12 0.25 0.013 P3 0.74 0.10 0.25 0.019 PTB: poly (thiophene-co-benzylidene 1:1) P3T-DDTPA: Poly ((E)-4-(dodecyloxy)-N-(4-(dodecyloxy)phenyl)-N-(thiophen-3-yl)vinyl)phenyl)aniline) P1: poly ((E)-4-(2-(2,5-Dibromothiophen-3-yl)vinyl)-N,Nbis(4-(dodecyloxy)phenyl)aniline-co-3-hexylthiophene 10:1) P2: poly ((E)-4-(2-(2,5-Dibromothiophen-3-yl)vinyl)-N,Nbisidodecyloxy)phenyl)aniline-co-3-hexylthiophene 2:1) P3: poly ((E)-4-(2-(2,5-Dibromothiophen-3-yl)vinyl)-N,Nbis(4-(dodecyloxy)phenyl)aniline-coi3-hexylthiophene 1:1) P4: poly ((E)-4-(2-(2,5-Dibromothiophen-3-yl)vinyl)-N,Nbis(4-(dodecyloxy)phenyl)aniline-co-3-hexylthiophene 1:4.8) of benzylidene between two thiophene rings. Such results may serve primarily to illustrate a new strategy to increase the low band gap polymers through the arrangement of different aromatic heterocyclic in conjugated polymer backbones. The manufacture of organic solar cell with heterojunc- tion, Glass/ITO/BCP/C60/PTB/Au/Al shows that our cell is Promising with a VOC of 0.26 V and a 1.6-JSC mA/cm². In addition, there is a clear improvement of the FF (36%), giving a yield of about 0.15%. PTB gave the best results compared to the 3-hexylthiophene copolymers [25], with the PCE of 0.15% because of the relative low HOMO level of the polymer. We note here that this high effi- ciency is obtained due to the high Jsc and Voc. photo- voltaic parameters are summarized in Table 1. These results it was confirmed by calculations of theoretical simulations to determine the parameters of the curve under illumination. Further improvements could be made for the PTB from these polymers by optimizing the polymer/C60 entering the morphology of the active layer 4. Acknowledgements The authors wish to thank the National Agency for De- velopment and Research of Algeria for the financial sup- port. REFERENCES [1] A. J. Heeger, “Semiconducting and Metallic Polymers: The Fourth Generation of Polymeric Materials Angew. Chem Int Ed Engl,” National Center for Biotechnology Information, Vol. 40, July 2001, pp. 2591-2611. [2] L. Liao, A. Cirpan, Q. Chu, F. E. Karase and Y. J. Pang, “Synthesis and Optical Properties of Light-Emitting π-Conjugated Polymers Containing Biphenyl and Dithie- nosilole,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 45, No. 10, May 2007, pp. 2048-2058. doi:10.1002/pola.21970 [3] G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, “Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions,” Science, Vol. 270, No. 5423, December 1995, pp. 1789- 1791. doi:10.1126/science.270.5243.1789 [4] E. J. Zhou, Z. A. Tan, Y. J. He, C. H. Yang and Y. F. Li, “Synthesis, Hole Mobility and Photovoltaic Properties of Two Alternating Poly[3-(Hex-1-Enyl)Thiophene-Co- Thioph-Ene]s,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 45, February 2007, pp. 629-638. [5] T. T. M. Dang, S. J. Park, J. W. Park, D. S. Chung, C. E. Park, Y. H. Kim and S. K. Kwon, “Synthesis and Char- acterization of Poly(benzodithiophene) Derivative for Organic Thin Film Transistors,” Journal of Polymer Sci- ence Part A: Polymer Chemistry, Vol. 45, November 2007, pp. 5277-5284. doi:10.1002/pola.22272 [6] E. Lim, Y. M. Kim, J. I. Lee, B. J. Jung, N. S. Cho, J. Lee, L. M. Do and H. K. Shim, “Relationship between the Liquid Crystallinity and Field-Effect-Transistor Be- havior of Fluorene–Thiophene-Based Conjugated Co- polymers,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 44, August 2006, pp. 4709-4721. doi:10.1002/pola.21586 [7] W. S. Shin, S. C. Kim, S.J. Lee, H.S. Jeon, M. K. Kim, B.V.K. Naidu, S.H. Jin, J.K. Lee, J.W. Lee, Y.S. Gal, “Synthesis and Photovoltaic Properties of a Low-Band-Gap Polymer Consisting of Alternating Thio- phene and Benzothiadiazole Derivatives for Bulk-Heterojunction and Dye-Sensitized Solar Cells,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 45, April 2007, pp. 1394-1402. doi:10.1002/pola.21909 [8] M. Reyes-Reyes, K. Kim and D. L. Carroll, “High-Efficie- ncy Photovoltaic Devices Based on Annealed Poly(3-hexyl- thio-phene) and 1-(3-Methoxycarbonyl)-Propyl-1-Phenyl- (6,6) C61 Blends,” Applied Physics Letters, Vol. 87, Au- gust 2005, pp. 83506-83508. doi:10.1063/1.2006986 [9] F. Monestier, J.-J. Simon, P. Torchio, L. Escoubas, F. Flory and S. Bailly, “Remi de Bettignies, Stephane Guil- lerez, Christophe Defranoux, Modeling the Short-Circuit Current Density of Polymer Solar Cells based on P3HT: PCBM Blend,” Solar Energy Materials and Solar Cells, Vol. 91, No. 5-6, 2007, pp. 405-410. doi:10.1016/j.solmat.2006.10.019 [10] W. L. Ma, C. Y. Yang, X. Gong, K. Lee and A. J. Heeger, “Thermally Stable, Efficient Polymer Solar Cells with Nanoscale Control of the Interpenetrating Network Mor- Copyright © 2011 SciRes. MSA  Synthesis and Properties of Polythiophène Benzylidene and Their Photovoltaic Applications1021 phology,” Advanced Functional Materials, Vol. 15, Oc- tober 2005, pp. 1617-1622. doi:10.1002/adfm.200500211 [11] J. H. Hou, Z. A. Tan, Y. J. He, C. H. Yang and Y. F. Li, “Branched Poly(Thienylene Vinylene)s with Absorption Spectra Covering the Whole Visible Region,” Macro- molecules, Vol. 39, No. 14, July 2006, pp. 4657-4662. doi:10.1021/ma060662u [12] J. H. Hou, Z. A. Tan, Y. Yan, Y. J. He, C. H. Yang and Y. F. Li, “Synthesis and Photovoltaic Properties of Two-Dim- ensional Conjugated Polythiophenes with Bi(Thienylenev- Inylene) Side Chains,” Journal of American Chemical Society, Vol. 128, No. 14, April 2006, pp. 4911-4916. doi:10.1021/ja060141m [13] C. J. Shi, Y. Yao, Y. Yang and Q. B. Pei, “Regioregular Copolymers of 3-Alkoxythiophene and Their Photo- voltaic Application,” Journal of American Chemical So- ciety, Vol. 128, No. 27, July 2006, pp. 8980-8986. doi:10.1021/ja061664x [14] F. L. Zhang, M. Johansson, M. R. Andersson, J. C. Hummelen and S. O. Ingana, “Polymer Solar Cells Based on MEH-PPV and PCBM,” Synth MetZhang, Vol. 137, April 2003, pp. 1401-1402. doi:10.1016/S0379-6779(02)01059-7 [15] K. F. Cheng, C. L. Liu and W. C. Chen, “Small Band Gap Conjugated Polymers based on Thiophene-Thienopyra- zine Copolymers,” Journal of American Chemical Society, Vol. 45, October 2007, pp. 5872-5883. doi:10.1002/pola.22339 [16] M. R. Andersson, O. W. Thomas, Mammo, M. Svensson, M. Theander and S. O. Ingana, “Substituted Polythio- Phenes Designed for Optoelectronic Devices and Con- ductors,” Journal of Material Chemistry, Vol. 9, 1999, pp. 1933-1940. doi:10.1039/a902859e [17] E. Perzon, X. J. Wang, S. Admassie, S. O. Ingana and M. R. Andersson, “An alternating Low Band-Gap Polyfluo- rene for Optoelectronic Devices,” Polymer, Vol. 47, May 2006, pp. 4261-4268. doi:10.1016/j.polymer.2006.03.110 [18] C. J. Brabec, N. S. Sariciftci and J. C. Hummelen, “Plastic Solar Cells,” Advanced Functional Materials, Vol. 11, February 2001, pp. 15-26. doi:10.1002/1616-3028(200102)11:1<15::AID-ADFM15 >3.0.CO;2-A [19] S. E. Shaheen, C. J. Brabec, N. S. Sariciftci, F. Padinger, T. Fromerz and J. C. Hummelen, “2.5% Efficient Organic Plastic Solar Cells,” Applied Physics Letters, Vol. 78, December 2001, pp. 841-843. doi:10.1063/1.1345834 [20] C. H. Kuo, W. K. Cheng, K. R. Lin, M. K. Leung and K. H. Hsieh, “High-Efficiency Poly(phenylenevinylene)-co- Fluorene Copolymers Incorporating a Triphenylamine as the End Group for White-Light-Emitting Diode Applica- tions,” Journal of Polymer Science Part A: Polymer Chem- istry, Vol. 45, October 2007, pp. 4504-4513. doi:10.1002/pola.22194 [21] S. K. Lee, T. Ahn, N. S. Cho, J. I. Lee, Y. K. Jung and J. Lee, H. K. Shim, “Synthesis of New Polyfluorene Co- polymers with a Comonomer Containing Triphenylamine Units and Their Applications in White-Light-Emitting Diodes,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 45, April 2007, pp. 1199-1209. doi:10.1002/pola.21892 [22] L. J. Huo, C. He, M. F. Han, E. J. Zhou and Y. F. Li, “Alternating Copolymers of Electron-Rich Arylamine and Electron-Deficient 2,1,3-Benzothiadiazole: Synthesis, Char- acterization and Photovoltaic Properties,” Journal of Poly- mer Science Part A: Polymer Chemistry, Vol. 45, Sep- tember 2007, pp. 3861-3871. doi:10.1002/pola.22136 [23] S. Roquet, A. Cravino, P. Leriche, O. Aleveque, P. Frere and J. Roncali, “Triphenylamine−Thienylenevinylene Hybrid Systems with Internal Charge Transfer as Donor Materials for Heterojunction Solar Cells,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 128, March 2006, pp. 3459-3466. doi:10.1021/ja058178e [24] B. Kouskoussa, M. Morsli, K. Benchouk, G. Louarn, L. Cattin, A. Khelil and J. C. Bernede, “On the Improvement of the Anode/Organic Material Interface in Organic Solar Cells by the Presence of an Ultra-Thin Gold layer,” Phy- sical Status Solidity, Vol. 206, February 2009, pp. 311- 315. [25] Y. Li, L. Xue, H. Xia, B. Xu, S. Wen and W. Tian, “Syn- thesis and Properties of Polythiophene Derivatives Con- taining Triphenylamine Moiety and Their Photovoltaic Applications,” Journal of Polymer Science Part A: Poly- mer Chemistry, Vol. 46, 2008, pp. 3970-3984. doi:10.1002/pola.22737 Copyright © 2011 SciRes. MSA |

