Paper Menu >>
Journal Menu >>
 Journal of Behavioral and Brain Science, 2011, 1, 111-114 doi:10.4236/jbbs.2011.13015 Published Online August 2011 (http://www.SciRP.org/journal/jbbs) Copyright © 2011 SciRes. JBBS Useful or Not? How Schizophrenic Patients Process the Relevance of a Visual Stimulus Maxime Bubrovszky, Pierre Thomas Department of Psychiatry, University Lille-Nord de France, Lille, France E-mail: maxime.bubrovszky@chru-lille.fr Received May 23, 2011; revised June 17, 2011; accepted June 30, 2011 Abstract Introduction: The impairment of relevant information selection results in clinical symptoms of schizophre- nia. Previous studies assessing visual modality, have reported an impairment of automatic sensory informa- tion processing related to abnormal irrelevant stimuli processing. In healthy subjects, neurophysiologic stud- ies have distinguished two early posterior components which the second could be considered as a deviant processing marker. We propose to explore early selective properties of attention in acute patients using event related potential (ERP) methods. We hypothesize an impairment of the detection of deviant stimuli process- ing, supported by sensory integration neural region. Materiel and Method: Ten patients suffering from an acute episode of schizophrenia and ten controls were assessed with a simple three stimuli oddball paradigm analysed by principal components analysis (PCA) method which allows to separate overlapping components and to evaluate their modifications. Result: PCA distinguished two posterior negative components between 100 and 230 msec. The early one was not different between controls and patients. The later one was signifi- cantly decreased in the patients group. Discussion: Two different physiological components involved in stimuli detection were clearly isolated. The earliest, reflecting elementary perceptive process seems to be pre- served in patients with acute schizophrenia whereas the later component, reflecting integrative processing involved in detection of deviance was impaired. These results could be a clue to understand clinical distrac- tibility. Keywords: Selective Attention, Vision, Event-Related Potentials, Schizophrenia, Principal Component Analysis 1. Introduction The impairment of attentional and pre-attentional proc- esses is associated with the incorrect selection of infor- mation in patients suffering from schizophrenia [1]. Us- ing the visual mismatch negativity (vMMN) paradigm, Urban et al. showed an impairment of automatic sensory information processing among patients with schizophre- nia in visual modality, as it was described in auditory modality [2]. These results suggest that the capacity of the patients to quickly detect deviant stimulus, as a first line of selection of information processing could be im- paired. Recently, Kimura et al., exploring posterior nega- tivity elicited by visual processing before 300 msec in healthy subjects highlighted two distinct components: a forward one associated with refractory effect of visual cor- tex after stimulation and a later one considered as vMMN, index of deviance detection [3]. We propose here to study early stimuli processing in patients with schizophrenia to identify the level (ele- mentary or integrative) of the alteration of visual proc- essing in the disease. Since attentional selective proper- ties may change during the course of the disease, we carefully selected patients suffering from acute schizo- phrenia. We hypothesize that deviant detection process, sup- ported by region involved in sensory integration should by more altered than the activity related to the refractory effect of the visual cortex. Using principal components analysis (PCA) on high density recording, we aimed to extract overlapping components from posterior negativ- ity and reconstruct their sources [4,5]. Moreover, this analysis method should allow us to get information from a simple task, performable by acute patients.  M. BUBROVSZKY ET AL. Copyright © 2011 SciRes. JBBS 112 2. Materiel and Meth od We recruited 10 patients in the acute phase of schizo- phrenia (as diagnosed by both a skilled clinician and a structured interview [SCID]) and 10 controls, according to the guidelines set by recommendations of the local ethics committee. All subjects were over the age of 18. Groups were matched by age, sex and laterality (median for age and sex/laterality repartitions were not signifi- cantly different). Patients were included not later than one week after the introduction or modification of antip- sychotic medications. Visual 3-stimulus oddball paradigm was used, in which 500 stimuli were presented at random in the central vis- ual field over 200 ms (“X” deviant, probability of ap- pearance p = 0.1, “o” target, p = 0.1 and “O” standard, p = 0.8). Inter stimuli interval was between 800 and 1200 ms (pseudo random length). Subjects were asked to press a button as fast as possible when a target appeared. Elec- trophysiological signals were acquired with a 128-elec- trodes cap, 10 - 20 extended system (Electrocap). Re- cordings (at a sampling rate of 1024 Hz, impedances be- low 10 kΩ, bandwith 1.10e-2 to 45 Hz) were recorded using an ANT Amplifier (Advanced NeuroTechnology, Netherlands). Epochs of 800 ms were pre-defined (baseline before trigger: 100 ms). In order to reject artefacts, signal varia- tions of more than 100 µV were excluded. This process was completed by visual inspection for artefacts exclu- sion [6]. We performed for the recordings of the 20 sub- jects temporal PCA followed by spatial PCA (based on covariance matrix with Varimax rotation [6]). This me- thod is known to clearly distinguish different physiolo- gical activities [5]. We selected components with two criteria: posterior topography and latency before 300 ms (i.e., before the latency of the P3 complex, with ERP linked to attentional processing). Then we were able to to reconstruct the cortical sources of components from spa- tial factorial scores with sLORETA algorithm [4]. Facto- rial scores from PCA were used as variables for statisti- cal tests (for each components Friedman test on three conditions (target/standard/deviant), then Mann-Withney for groups comparisons). 3. Results All patients (mean age: 33.7 years Standard Deviation (S.D.) 8.7) received atypical antipsychotics (mean chlor- promazine equivalent: 290 mg/day, S.D.:75). The mean PANSS score was 83 (S.D.: 22.4). Reaction times were longer for patients than for controls (medians: 438 ms and 387 ms, p = 0.05). Five temporal components reported 91% of signal va- riance (Figure central panel). According to our a priori criteria, two components were withheld before 300 ms. One temporal component reports alone more than 50% of variance from 185 - 232 ms and a second one from 103 - 150 ms. Spatial PCA isolated a median posterior component (a) from the first temporal component (185 - 232 ms) Figure 1(a) and two symmetrical posterior com- ponents (b) and (c) for the second (103 - 150 ms) Fig- ures 1(b) and (c). Component (a) revealed sources at parieto-occipito-temporal (maximum for Brodman area 7 and 19, Figure 1(a)); components (b) and (c) showed more occipital sources (maximum for Brodman area 19 and 31, Figures 1(b) and (c)). Non-parametrical statistical tests were performed on factorial scores from PCA (Kolmogorov tests for nor- mality do not allow us to retain Gaussian distribution of variables). For the first component (a), a Friedman test on three conditions (target/standard/deviant) showed a sig- nificant difference and so confirmed the specificity of electrical activity evocated by the deviant condition (χ2 = 6.632, dl = 2, p = 0.037 [exact]). The 2 groups differ significantly for this condition (Mann-Whitney test: Z = –2.238, p = 0.017 [exact, bilat- eral]), but not for the target and standard conditions (Z = –0.898, p = 0.4 [exact, bilateral] and Z = –0.245, p = 0.842 [exact, bilateral], respectively). These results con- firm observation of grand average of event-related po- tential as shown on the left panel of the figure. Moreover, amplitude of event-related activity is lower in patient group. Earlier components were not condition-specific (Friedman test for B: χ2 = 2; dl = 2, p = 0.417 [exact] and for C χ2 = 1.684; dl = 2, p = 0.455 [exact]). The groups did not differ for components (b) and (c). 4. Discussion Compared to controls, patients showed an amplitude reduction of the component elicited by deviant condition, for which sources took place in occipito-temporal cortex. Thus, impairment of automatic sensory information pro- cessing already observed [2] would be more specifically related to pre-attentional detection of a deviant visual sti- mulus. The anatomical sources of this component shared a part of vMMN sources, close to areas of perceptual inte- gration (Brodman 19, associative visual cortex, and 7, visuo-perceptive integration cortex). We clearly separate in latency and localization earlier components as Kimura did with a different method [3]. This activity corresponds to a refractory effect on the sensory cortex for Kimura et al. The absence of difference between conditions and the source of the component in occipital cortex strengthens this hypothesis. Links between our data and clinical features could be 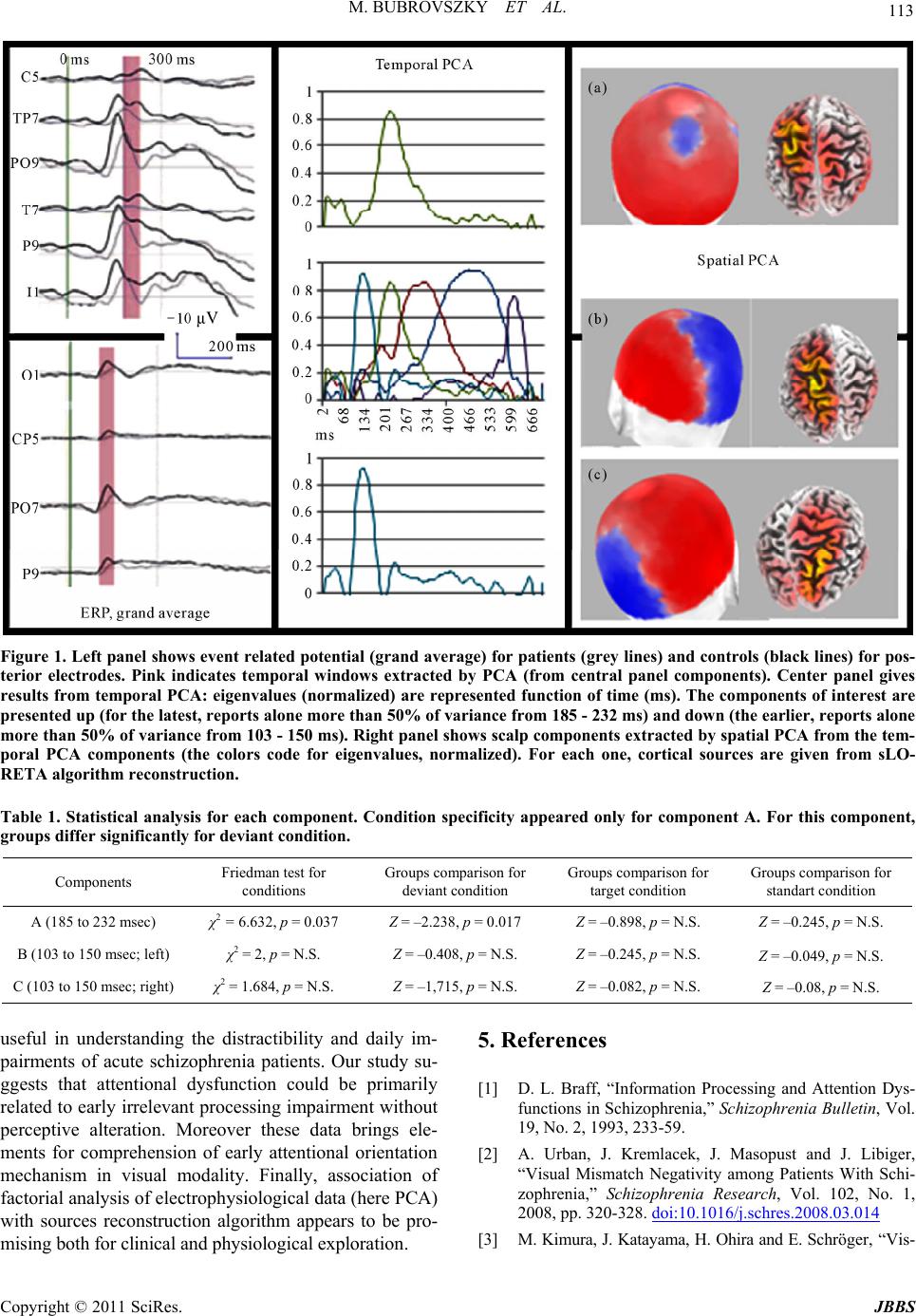 M. BUBROVSZKY ET AL. Copyright © 2011 SciRes. JBBS 113 Figure 1. Left panel shows event related potential (grand average) for patients (grey lines) and controls (black lines) for pos- terior electrodes. Pink indicates temporal windows extracted by PCA (from central panel components). Center panel gives results from temporal PCA: eigenvalues (normalized) are represented function of time (ms). The components of interest are presented up (for the latest, reports alone more than 50% of variance from 185 - 232 ms) and down (the earlier, reports alone more than 50% of variance from 103 - 150 ms). Right panel shows scalp components extracted by spatial PCA from the tem- poral PCA components (the colors code for eigenvalues, normalized). For each one, cortical sources are given from sLO- RETA algorithm reconstruction. Table 1. Statistical analysis for each component. Condition specificity appeared only for component A. For this component, groups differ significantly for deviant condition. Components Friedman test for conditions Groups comparison for deviant condition Groups comparison for target condition Groups comparison for standart condition A (185 to 232 msec) χ2 = 6.632, p = 0.037 Z = –2.238, p = 0.017 Z = –0.898, p = N.S. Z = –0.245, p = N.S. B (103 to 150 msec; left) χ2 = 2, p = N.S. Z = –0.408, p = N.S. Z = –0.245, p = N.S. Z = –0.049, p = N.S. C (103 to 150 msec; right) χ2 = 1.684, p = N.S. Z = –1,715, p = N.S. Z = –0.082, p = N.S. Z = –0.08, p = N.S. useful in understanding the distractibility and daily im- pairments of acute schizophrenia patients. Our study su- ggests that attentional dysfunction could be primarily related to early irrelevant processing impairment without perceptive alteration. Moreover these data brings ele- ments for comprehension of early attentional orientation mechanism in visual modality. Finally, association of factorial analysis of electrophysiological data (here PCA) with sources reconstruction algorithm appears to be pro- mising both for clinical and physiological exploration. 5. References [1] D. L. Braff, “Information Processing and Attention Dys- functions in Schizophrenia,” Schizophrenia Bulletin, Vol. 19, No. 2, 1993, 233-59. [2] A. Urban, J. Kremlacek, J. Masopust and J. Libiger, “Visual Mismatch Negativity among Patients With Schi- zophrenia,” Schizophrenia Research, Vol. 102, No. 1, 2008, pp. 320-328. doi:10.1016/j.schres.2008.03.014 [3] M. Kimura, J. Katayama, H. Ohira and E. Schröger, “Vis-  M. BUBROVSZKY ET AL. Copyright © 2011 SciRes. JBBS 114 ual Mismatch Negativity: New Evidence from the Equi- probable Paradigm,” Psychophysiology, Vol. 46, No. 2, 2009, pp. 402-409. doi:10.1111/j.1469-8986.2008.00767.x [4] R. D. Pascual-Marqui, “Standardized Low-Resolution Brain Electromagnetic Tomography (sLORETA): Tech- nical Details,” Methods and Findings in Experimental and Clinical Pharmacology, Vol. 24, Suppl D, 2002, pp. 5-12. [5] J. Dien, K. M. Spencer and E. Donchin, “Localization of the Event-Related Potential Novelty Response as Defined by Principal Components Analysis,” Brain Research/Cog- nitive Brain Research, Vol. 17, No. 3, 2003, pp. 637-650. doi:10.1016/S0926-6410(03)00188-5 [6] T. W. Picton, S. Bentin, P. Berg, E. Donchin, S. A. Hillyard, R. Johnson, G. A. Miller, W. Ritter, D. S. Ruch- kin, M. D. Rugg and M. J. Taylor, “Guidelines for Using Human Event-Related Potentials to Study Cognition: Recording Standards and Publication Criteria,” Psycho- physiology, Vol. 37, No. 2, 2000, pp. 127-152. doi:10.1111/1469-8986.3720127 |

