Paper Menu >>
Journal Menu >>
 Journal of Behavioral and Brain Science, 2011, 1, 194-198 doi:10.4236/jbbs.2011.13026 Published Online August 2011 (http://www.SciRP.org/journal/jbbs) Copyright © 2011 SciRes. JBBS Sampling Small Volumes of Saliva for Determination of the Str ess Hormone α-Amylase. A Comparative Methodologica l Study Aristidis Arhakis, Vassilis Karagiannis, Sotirios Kalfas School of Dentistry, Aristotle University of Thessaloniki, Thessaloniki, Greece E-mail: oaristidis@gmail.com Received May 5, 2011; revised June 17, 2011; accepted July 5, 2011 Abstract Salivary alpha amylase was taken into account as an important physiological indicator in psychoneuroendo- crinological research focusing on stress. Two sampling devices that allow saliva collection through absorp- tion to a cotton roll (Salivette®-method) or to small cotton pellets (VectaSpinTM Micro [VSM]-method) were studied. Any loss of salivary alpha-amylase (sAA) activity in relation to the saliva volume absorbed and har- vested by centrifugation was examined. A pooled saliva sample prepared from stimulated whole saliva (col- lected by drooling) of 30 subjects was used. Three different saliva volumes (2.9 ml, 1.5 ml, and 0.8 ml) were tested on cotton rolls and two (0.03 ml, and 0.015 ml) on cotton pellets. The sample sAA activity was deter- mined from the hydrolysis of 2-chloro-4-nitrophenyl-α-D-maltotrioside. In comparison with the original drooling sample, no sAA loss was observed in 1.5 ml samples tested with Salivette, while a significant de- crease of activity was recorded with smaller volumes. VSM collected samples showed a non-volume de- pendent decrease of sAA activity of about 25%. Salivette requires large saliva volumes to allow an accurate sAA estimation. With cases of limited saliva access, VSM may be a suitable sampling device. Keywords: Salivary Alpha Amylase, Enzyme Activity, Method, Saliva Sampling 1. Introduction Saliva is of great clinical significance as a diagnostic fluid and analysis of its components may provide infor- mation on local or systemic diseases and disorders [1-5]. Sampling saliva is clinically more advantageous com- pared to other body fluids since it is performed with non- invasive techniques without the stressful exposure to needles [6-9]. Whole saliva can be sampled by either the draining method, the spitting method, the suction method, or the absorbent (swab) method [10]. A device under the brand name Salivette® (Sarstedt, Numbrecht, Germany) is frequently employed for whole saliva collection by the absorbent method [11]. The com- ponents of the devise are a cotton roll, and a plastic cen- trifuge tube with an inner cylindrical strainer (Figure 1). The cotton roll is placed in the mouth for up to 5 min to absorb saliva till the roll becomes well soaked (about 3 ml saliva). The saliva is then harvested by centrifuging the roll in the tube with the strainer. Salivette® may be difficult or less suitable to use under certain circumstances. Soaking the cotton roll with saliva requires time and the presence of a certain saliva volume in the mouth. To enable saliva collection, many research- ers ask the subjects to chew the cotton roll; however, this is unsuitable when unstimulated saliva has to be col- lected. Occasionally, young children may find the chew- ing procedure cumbersome and refuse to co-operate. Repeated short interval-sampling may suffer by a limited amount of saliva absorbed to the roll, which could affect the composition of the final sample. Indeed, an error vari- ance in the measurement of cortisol, testosterone, DHEA, estradiol, progesterone, and salivary α-amylase (sAA), has been reported, particularly with low sample volumes [12- 14]. The concentration of sAA in saliva is considered as an indicator of the sympathetic adrenal medullar system activation due to stress [15-17]. Saliva for sAA assess- ment has been sampled with Salivette® or by the passive drooling technique. However, these techniques may not be feasible when small sample volumes are to be ex- pected, as mentioned above. To overcome this problem, 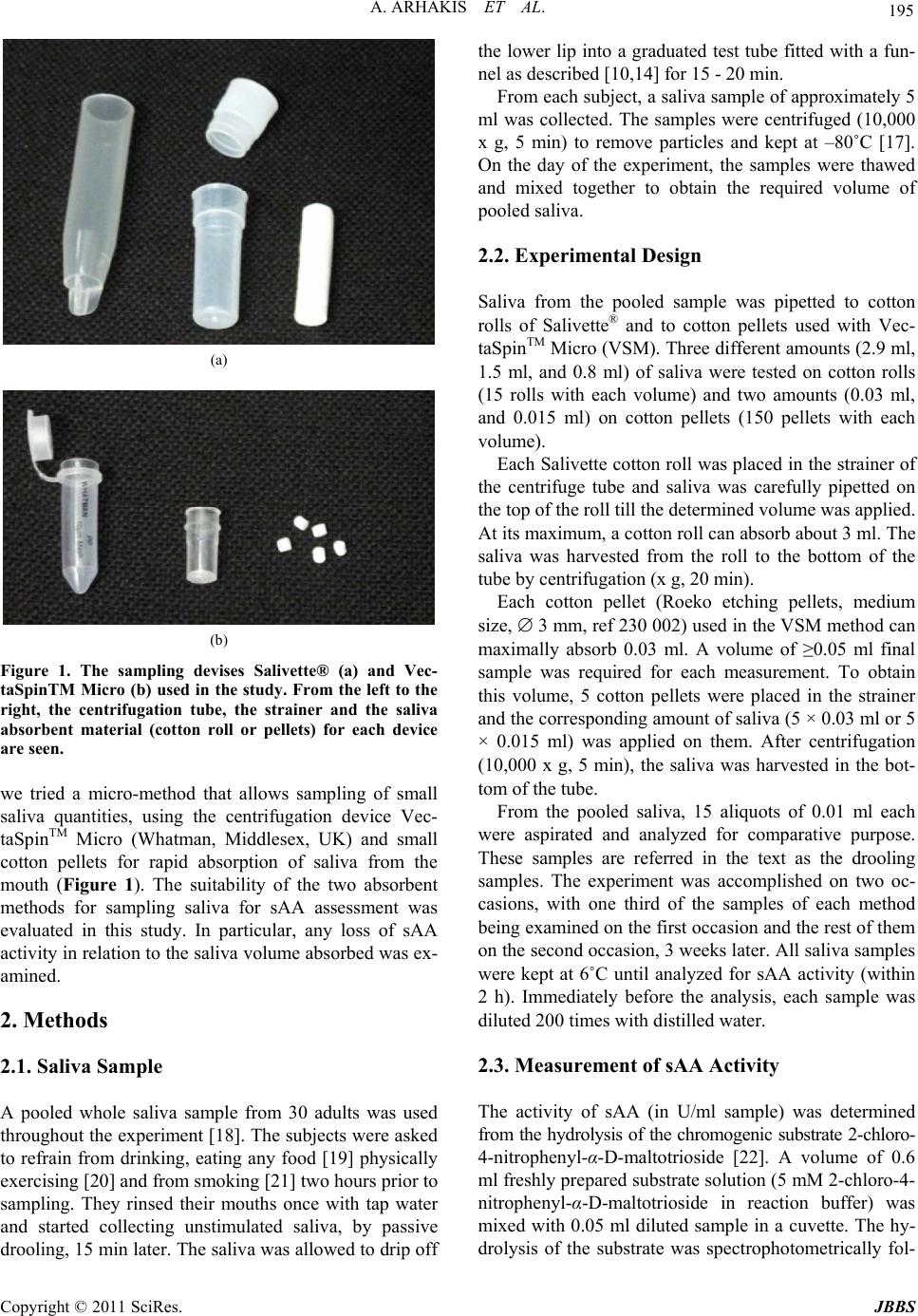 A. ARHAKIS ET AL. Copyright © 2011 SciRes. JBBS 195 (a) (b) Figure 1. The sampling devises Salivette® (a) and Vec- taSpinTM Micro (b) used in the study. From the left to the right, the centrifugation tube, the strainer and the saliva absorbent material (cotton roll or pellets) for each device are seen. we tried a micro-method that allows sampling of small saliva quantities, using the centrifugation device Vec- taSpinTM Micro (Whatman, Middlesex, UK) and small cotton pellets for rapid absorption of saliva from the mouth (Figure 1). The suitability of the two absorbent methods for sampling saliva for sAA assessment was evaluated in this study. In particular, any loss of sAA activity in relation to the saliva volume absorbed was ex- amined. 2. Methods 2.1. Saliva Sample A pooled whole saliva sample from 30 adults was used throughout the experiment [18]. The subjects were asked to refrain from drinking, eating any food [19] physically exercising [20] and from smoking [21] two hours prior to sampling. They rinsed their mouths once with tap water and started collecting unstimulated saliva, by passive drooling, 15 min later. The saliva was allowed to drip off the lower lip into a graduated test tube fitted with a fun- nel as described [10,14] for 15 - 20 min. From each subject, a saliva sample of approximately 5 ml was collected. The samples were centrifuged (10,000 x g, 5 min) to remove particles and kept at –80˚C [17]. On the day of the experiment, the samples were thawed and mixed together to obtain the required volume of pooled saliva. 2.2. Experimental Design Saliva from the pooled sample was pipetted to cotton rolls of Salivette® and to cotton pellets used with Vec- taSpinTM Micro (VSM). Three different amounts (2.9 ml, 1.5 ml, and 0.8 ml) of saliva were tested on cotton rolls (15 rolls with each volume) and two amounts (0.03 ml, and 0.015 ml) on cotton pellets (150 pellets with each volume). Each Salivette cotton roll was placed in the strainer of the centrifuge tube and saliva was carefully pipetted on the top of the roll till the determined volume was applied. At its maximum, a cotton roll can absorb about 3 ml. The saliva was harvested from the roll to the bottom of the tube by centrifugation (x g, 20 min). Each cotton pellet (Roeko etching pellets, medium size, 3 mm, ref 230 002) used in the VSM method can maximally absorb 0.03 ml. A volume of ≥0.05 ml final sample was required for each measurement. To obtain this volume, 5 cotton pellets were placed in the strainer and the corresponding amount of saliva (5 × 0.03 ml or 5 × 0.015 ml) was applied on them. After centrifugation (10,000 x g, 5 min), the saliva was harvested in the bot- tom of the tube. From the pooled saliva, 15 aliquots of 0.01 ml each were aspirated and analyzed for comparative purpose. These samples are referred in the text as the drooling samples. The experiment was accomplished on two oc- casions, with one third of the samples of each method being examined on the first occasion and the rest of them on the second occasion, 3 weeks later. All saliva samples were kept at 6˚C until analyzed for sAA activity (within 2 h). Immediately before the analysis, each sample was diluted 200 times with distilled water. 2.3. Measurement of sAA Activity The activity of sAA (in U/ml sample) was determined from the hydrolysis of the chromogenic substrate 2-chloro- 4-nitrophenyl-α-D-maltotrioside [22]. A volume of 0.6 ml freshly prepared substrate solution (5 mM 2-chloro-4- nitrophenyl-α-D-maltotrioside in reaction buffer) was mixed with 0.05 ml diluted sample in a cuvette. The hy- drolysis of the substrate was spectrophotometrically fol- 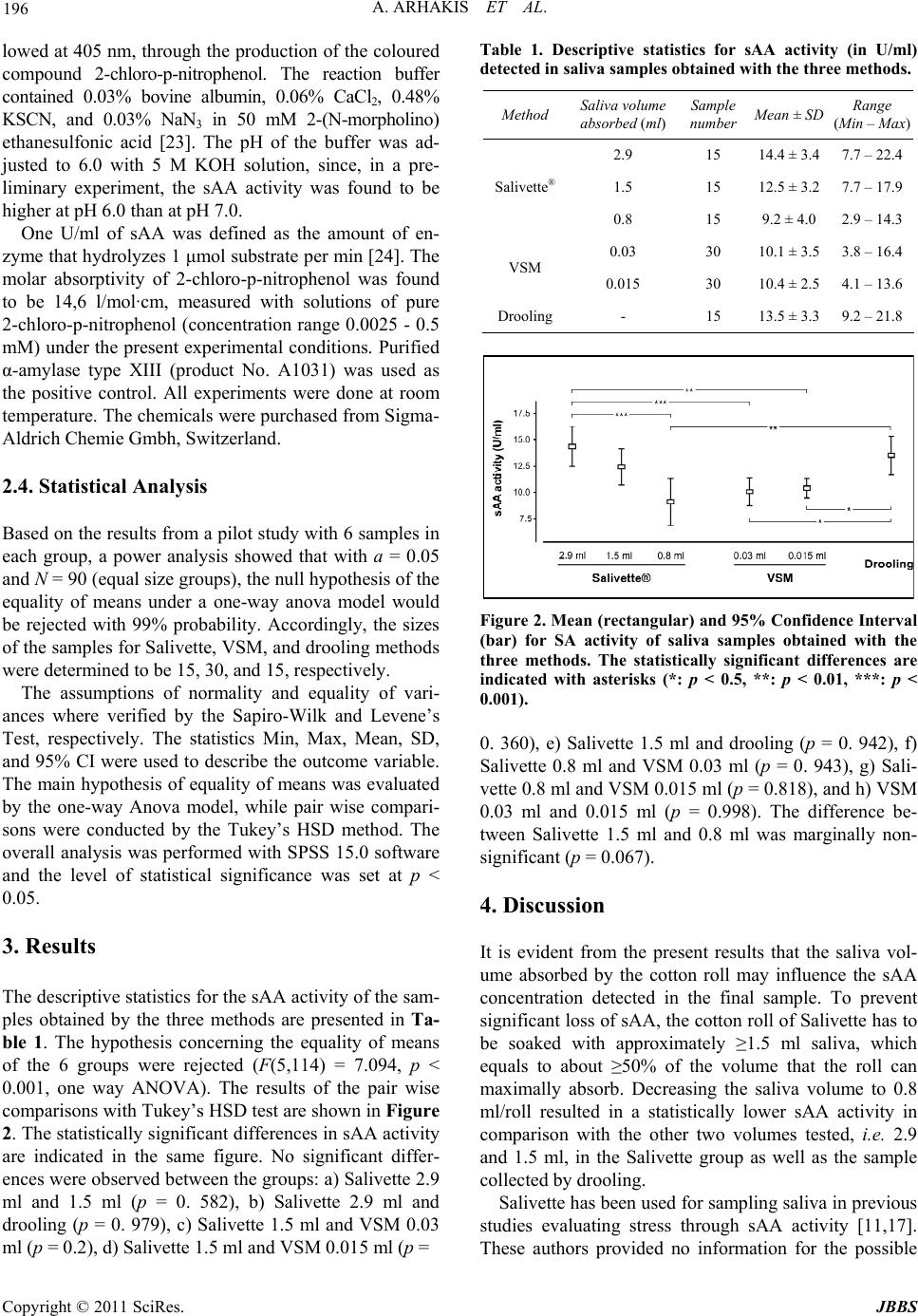 A. ARHAKIS ET AL. Copyright © 2011 SciRes. JBBS 196 lowed at 405 nm, through the production of the coloured compound 2-chloro-p-nitrophenol. The reaction buffer contained 0.03% bovine albumin, 0.06% CaCl2, 0.48% KSCN, and 0.03% NaN3 in 50 mM 2-(N-morpholino) ethanesulfonic acid [23]. The pH of the buffer was ad- justed to 6.0 with 5 M KOH solution, since, in a pre- liminary experiment, the sAA activity was found to be higher at pH 6.0 than at pH 7.0. One U/ml of sAA was defined as the amount of en- zyme that hydrolyzes 1 μmol substrate per min [24]. The molar absorptivity of 2-chloro-p-nitrophenol was found to be 14,6 l/mol·cm, measured with solutions of pure 2-chloro-p-nitrophenol (concentration range 0.0025 - 0.5 mM) under the present experimental conditions. Purified α-amylase type XIII (product No. A1031) was used as the positive control. All experiments were done at room temperature. The chemicals were purchased from Sigma- Aldrich Chemie Gmbh, Switzerland. 2.4. Statistical Analysis Based on the results from a pilot study with 6 samples in each group, a power analysis showed that with a = 0.05 and N = 90 (equal size groups), the null hypothesis of the equality of means under a one-way anova model would be rejected with 99% probability. Accordingly, the sizes of the samples for Salivette, VSM, and drooling methods were determined to be 15, 30, and 15, respectively. The assumptions of normality and equality of vari- ances where verified by the Sapiro-Wilk and Levene’s Test, respectively. The statistics Min, Max, Mean, SD, and 95% CI were used to describe the outcome variable. The main hypothesis of equality of means was evaluated by the one-way Anova model, while pair wise compari- sons were conducted by the Tukey’s HSD method. The overall analysis was performed with SPSS 15.0 software and the level of statistical significance was set at p < 0.05. 3. Results The descriptive statistics for the sAA activity of the sam- ples obtained by the three methods are presented in Ta- ble 1. The hypothesis concerning the equality of means of the 6 groups were rejected (F(5,114) = 7.094, p < 0.001, one way ANOVA). The results of the pair wise comparisons with Tukey’s HSD test are shown in Figure 2. The statistically significant differences in sAA activity are indicated in the same figure. No significant differ- ences were observed between the groups: a) Salivette 2.9 ml and 1.5 ml (p = 0. 582), b) Salivette 2.9 ml and drooling (p = 0. 979), c) Salivette 1.5 ml and VSM 0.03 ml (p = 0.2), d) Salivette 1.5 ml and VSM 0.015 ml (p = Table 1. Descriptive statistics for sAA activity (in U/ml) detected in saliva samples obtained with the three methods. Method Saliva volume absorbed (ml) Sample number Mean ± SD Range (Min – Max) 2.9 15 14.4 ± 3.4 7.7 – 22.4 1.5 15 12.5 ± 3.2 7.7 – 17.9Salivette® 0.8 15 9.2 ± 4.0 2.9 – 14.3 0.03 30 10.1 ± 3.5 3.8 – 16.4 VSM 0.015 30 10.4 ± 2.5 4.1 – 13.6 Drooling- 15 13.5 ± 3.3 9.2 – 21.8 Figure 2. Mean (rectangular) and 95% Confidence Interval (bar) for SA activity of saliva samples obtained with the three methods. The statistically significant differences are indicated with asterisks (*: p < 0.5, **: p < 0.01, ***: p < 0.001). 0. 360), e) Salivette 1.5 ml and drooling (p = 0. 942), f) Salivette 0.8 ml and VSM 0.03 ml (p = 0. 943), g) Sali- vette 0.8 ml and VSM 0.015 ml (p = 0.818), and h) VSM 0.03 ml and 0.015 ml (p = 0.998). The difference be- tween Salivette 1.5 ml and 0.8 ml was marginally non- significant (p = 0.067). 4. Discussion It is evident from the present results that the saliva vol- ume absorbed by the cotton roll may influence the sAA concentration detected in the final sample. To prevent significant loss of sAA, the cotton roll of Salivette has to be soaked with approximately ≥1.5 ml saliva, which equals to about ≥50% of the volume that the roll can maximally absorb. Decreasing the saliva volume to 0.8 ml/roll resulted in a statistically lower sAA activity in comparison with the other two volumes tested, i.e. 2.9 and 1.5 ml, in the Salivette group as well as the sample collected by drooling. Salivette has been used for sampling saliva in previous studies evaluating stress through sAA activity [11,17]. These authors provided no information for the possible  A. ARHAKIS ET AL. Copyright © 2011 SciRes. JBBS 197 effect of a partially soaked cotton roll on the estimation of sAA activity, probably, because they did not face the limitation of a small sample volume. Under certain cir- cumstances, it may be difficult to obtain the saliva amount needed for saturation of the roll. Young children may not be capable or even refuge to provide the required volume. Occasionally, the size of the roll may be disproportional to the small mouth of the child, or the procedure itself may seem unbearable or stressful for a subject who must accept the cotton roll in the mouth for several minutes. Furthermore, repeated sampling with short intervals, e.g. <1 min, is unfeasible, since the flow rate of stimulated saliva is usually <1.5 ml/min. The micromethod VSM helps to overcome these dis- advantages and it is in fact a modification of the Salivette method. The voluminous cotton roll was replaced by small cotton pellets and the VectaSpinTM centrifugation devise was used to harvest the sample. VSM allows a more rapid collection, about 2 - 3 seconds per pellet, the procedure being more comfortable for the subject and demanding a minimum of cooperation. Five saliva-soaked pellets provide a sufficient sample volume for sAA analysis. The sAA activity detected in VSM-collected samples was significantly lower than in the corresponding sam- ples collected by drooling or by Salivette-cotton roll soaked with ≥1.5 ml. Compared to the drooling sample, a 25% and 23% mean loss of sAA activity were found for the volumes 0.03 and 0.015 ml, respectively. The corre- sponding mean values for the Salivette-collected samples of 0.8 and 1.5 ml were 33% and 7%, respectively. Inter- estingly, a higher variation was recorded in sAA activity among the Salivette 0.8 ml samples (Coefficient of va- riation 44%) than among the VSM samples of 0.03 or 0.015 ml (Coefficient of variation 35% and 24%, respec- tively). Although not proved, the loss of sAA activity is probably due to a partial absorption of the enzyme by the plastic surfaces of the devise and by the cotton. The ex- tent of protein absorption to a surface is influenced by the chemical composition of the protein-containing solu- tion and the physicochemical properties of the surface. The protein composition of saliva varies depending on the serous or mucous character of the secretory gland. It may also vary among different subjects. A high salivary content of proteins competing with sAA absorption pre- vents extensive loss of sAA and vice versa. To avoid confounding results due to the intra-subject variation, a pooled saliva sample collected from several subjects, was used throughout this study. Under these conditions, the rich protein supply provided by saliva volumes of ≥1.5 ml, seems to sufficiently prevent sAA loss, proba- bly by blocking the protein binding sites in the cotton roll and tube walls of Salivette. On the contrary, the pro- tein content in the 100 times smaller saliva volume ob- tained by the VSM method, appears inadequate to inhibit sAA absorption to the devise surfaces. The same phe- nomenon probably occurs with the 0.8 ml volume in Salivette. The saliva obtained after centrifugation with Salivette or VSM is less turbid and viscous than the initial fluid. This indicates that the cotton roll and pellets retained particles, such as microbes, epithelial cells, and food de- bris, that confer turbidity and also macromolecules, such as mucins that are mainly responsible for the high viscos- ity of saliva. Removal of particles was also achieved by the initial centrifugation step, performed before pooling the individual saliva samples together. The clarified and less viscous saliva obtained by centrifugation and filtra- tion may, occasionally, be advantageous for the follow- ing handling and analysis steps. However, these proce- dures may also affect the compound under test. Removal of microbes also removes sAA bound to the cell surface of certain oral bacterial species [25]. On the other hand, the cotton roll of Salivette seems to retain most of sali- vary mucins but not sAA when adequately soaked with saliva. To conclude, the use of Salivette for collecting saliva samples yields no loss in sAA activity, provided that the sample volume is ≥1.5 ml. When the available saliva volume is small (<1 ml), Salivette cannot be used, how- ever, the micromethod VSM described above may pro- vide a good estimation of sAA activity, the loss being about 20% in the volume range 0.03 - 0.015 ml. Since sAA is a potential indicator of autonomic response to stress a reliable sampling method is required. These find- ings together with the above outlined advantages make the micromethod a suitable choice that may enable saliva sampling under certain circumstances. 5. References [1] D. J. Adam, A. A. Milne, S. M. Evans, J. E. Roulston, A. J. Lee, C. V. Ruckley and A. W. Bradbury, “Serum Amy- lase Isoenzymes in Patients Undergoing Operation for Ruptured and Non-Ruptured Abdominal Aortic Aneu- rysm,” Journal of Vascular Surgery, Vol. 30, No. 2, 1999, pp. 229-235. doi:10.1016/S0741-5214(99)70132-1 [2] P. M. Martinez, A. R. Torres, R. Ortiz de Lejarazu, A. Montoya, J. F. Martin and J. M. Eiros, “Human Immu- nodeficiency Virus Antibody Testing by Enzyme-Linked Fluorescent and Western Blot Assays Using Serum, Gin- gival-Crevicular Transudate, and Urine Samples,” Jour- nal of Clinical Microbiology, Vol. 37, No. 4, 1999, pp. 1100-1106. [3] E. Filaire and G. Lac, “Dehydroepiandrosterone (DHEA) Rather than Testosterone Shows Saliva Androgen Re-  A. ARHAKIS ET AL. Copyright © 2011 SciRes. JBBS 198 sponses to Exercise in Elite Female Handball Players,” International Journal of Sports Medicine, Vol. 21, No. 1, 2000, pp. 17-20. doi:10.1055/s-2000-8851 [4] S. P. Humphrey and R. T. Williamson, “A Review of Saliva: Normal Composition, Flow, and Function,” Jour- nal of Prosthetic Dentistry, Vol. 85, No. 2, 2001, pp. 162- 169. doi:10.1067/mpr.2001.113778 [5] C. F. Streckfus and L. R. Bigler, “Saliva as a Diagnostic Fluid,” Oral Diseases, Vol. 8, No. 2, 2002, pp. 69-76. doi:10.1034/j.1601-0825.2002.1o834.x [6] D. B. Ferguson, “Current Diagnostic Uses of Saliva,” Journal of Dental Research, Vol. 66, No. 2, 1987, pp. 420- 424. doi:10.1177/00220345870660020601 [7] D. Malamud, “Saliva as a Diagnostic Fluid,” British Medical Journal, Vol. 305, No. 6847, 1992, pp. 207-208. doi:10.1136/bmj.305.6847.207 [8] I. D. Mandel, “A Contemporary View of Salivary Re- search,” Critical Reviews in Oral Biology & Medicine, Vol. 4, No. 3-4, 1993, pp. 599-604. [9] H. C. Slavkin, “Toward Molecularly Based Diagnostics for the Oral Cavity,” Journal of the American Dental As- sociation, Vol. 129, No. 8, 1998, pp. 1138-1143. [10] M. Navazesh, “Methods for Collecting Saliva,” Annals of the New York Academy of Sciences, Vol. 20, No. 694, 1993, pp. 72-77. doi:10.1111/j.1749-6632.1993.tb18343.x [11] N. Rohleder and U. M. Nater, “Determinants of Salivary Alpha-Amylase in Humans and Methodological Consid- erations,” Psychoneuroendocrinology, Vol. 34, No. 4, 2009, pp. 469-485. doi:10.1016/j.psyneuen.2008.12.004 [12] E. A. Shirtcliff, D. A. Granger, E. Schwartz and M. J. Curran, “Use of Salivary Biomarkers in Biobehavioral Research: Cotton-Based Sample Collection Methods Can Interfere with Salivary Immunoassay Results,” Psycho- neuroendocrinology, Vol. 26, No. 2, 2001, pp. 165-173. doi:10.1016/S0306-4530(00)00042-1 [13] A. G. Harmon, L. C. Hibel, O. Rumyantseva and D. A. Granger, “Measuring Salivary Cortisol in Studies of Child Development: Watch out-What Goes in May Not Come out of Saliva Collection Devices,” Developmental Psy- chobiology, Vol. 49, No. 5 2007, pp. 495-500. doi:10.1002/dev.20231 [14] J. A. DeCaro, “Methodological Considerations in the Use of Salivary a-Amylase as a Stress Marker in Field Re- search,” American Journal of Human Biology, Vol. 20, No. 5, 2008, pp. 617-619. doi:10.1002/ajhb.20795 [15] J. A. Bosch, H. S. Brand, T. J. Ligtenberg, B. Bermond, J. Hoogstraten and A. V. Nieuw Amerongen, “Psychologi- cal Stress as a Determinant of Protein Levels,” Psycho- somatic Medicine, Vol. 58, No. 4, 1996, pp. 374-382. [16] U. M. Nater, R. La Marca, L. Florin, A. Moses, W. Lang- hans, M. M. Koller and U. Ehlert, “Stress-Induced Changes in Human Salivary Alpha-Amylase Activity- Associations with Adrenergic Activity,” Psychoneuroen- docrinology, Vol. 31, No. 1, 2006, pp. 49-58. doi:10.1016/j.psyneuen.2005.05.010 [17] D. A. Granger, K. T. Kivlighan, M. el-Sheikh, E. B. Gordis and L. R. Stroud, “Salivary Alpha-Amylase in Biobehav- ioral Research: Recent Developments and Applications,” Annals of the New York Academy of Sciences, Vol. 1098, 2007, pp. 122-144. doi:10.1196/annals.1384.008 [18] M. Neu, M. Goldstein, D. Gao and M. L. Laudenslager, “Salivary Cortisol in Preterm Infants: Validation of a Simple Method for Collecting Saliva for Cortisol Deter- mination,” Early Human Development, Vol. 83, No. 1, 2007, pp. 47-54. doi:10.1016/j.earlhumdev.2006.04.003 [19] S. L. Udupa, A. R. Prabhakar and S. Tandon, “Al- pha-Amylase Inhibitors in Foodstuffs,” Food Chemistry, Vol. 34, No. 2, 1989, pp. 95-101. doi:10.1016/0308-8146(89)90077-0 [20] K. T. Kivlighan and D. A. Granger, “Salivary Alpha- Amylase Response to Competition: Relation to Gender, Previous Experience, and Attitudes,” Psychoneuroendo- crinology, Vol. 31, No. 6, 2006, pp. 703-714. doi:10.1016/j.psyneuen.2006.01.007 [21] R. Nagler, S. Lischinsky, E. Diamond, N. Drigues, I. Klein and A. Z. Reznick, “Effect of Cigarette Smoke on Salivary Proteins and Enzyme Activities,” Archives of Biochemistry and Biophysics, Vol. 379, No. 2, 2000, pp. 229-236. doi:10.1006/abbi.2000.1877 [22] E. S. Winn-Deen, H. David, G. Sigler and R. Chavez, “Development of a Direct Assay for Alpha-Amylase,” Clinical Chemistry, Vol. 34, No. 10, 1988, pp. 2005-2008. [23] A. Y. Foo and R. Bais, “Amylase Measurement with 2- Chloro-4-Nitrophenyl Maltotrioside as Substrate,” Cli- nica Chimica Acta, Vol. 272, No. 2, 1998, pp. 137-147. doi:10.1016/S0009-8981(98)00009-6 [24] H. Ben-Aryeh, M. Fisher, R. Szargel and D. Laufer, “Composition of Whole Unstimulated Saliva of Healthy Children: Changes with Age,” Archives of Oral Biology, Vol. 35, No. 11, 1990, pp. 929-931. doi:10.1016/0003-9969(90)90075-L [25] M. Kilian and B. Nyvad, “Ability to Bind Salivary Al- pha-Amylase Discriminates Certain Viridans Group Strep- tococcal Species,” Journal of Clinical Microbiology, Vol. 28, No. 11, 1990, pp. 2576-2577. |

