Journal of Applied Mathematics and Physics
Vol.04 No.08(2016), Article ID:70422,11 pages
10.4236/jamp.2016.48177
Asymptotic Solutions of the Kinetic Boltzmann Equation and Multicomponent Non-Equilibrium Gas Dynamics
S. A. Serov1, S. S. Serova2
1Fundamental Researches Department, Russian Federal Nuclear Centre, All-Russian Scientific Research Institute of Experimental Physics, Sarov, Russia
2St. Petersburg State University, St. Petersburg, Russia



Received 16 June 2016; accepted 24 August 2016; published 31 August 2016

ABSTRACT
In the article correct method for the kinetic Boltzmann equation asymptotic solution is formulated, the Hilbert’s and Enskog’s methods are discussed. The equations system of multicomponent non- equilibrium gas dynamics is derived, that corresponds to the first order in the approximate (asym- ptotic) method for solution of the system of kinetic Boltzmann equations.
Keywords:
Kinetic Boltzmann Equation, Multicomponent Non-Equilibrium Gas Dynamics

1. Introduction
In 1912 Hilbert considered the kinetic Boltzmann equation for one-component gas as an example of integral equation and proposed a “recipe” for its approximate (asymptotic) solution (see [1], Chapter~XXII). Hilbert’s “recipe” was inconvenient for practical use, because the five arbitrary functional parameters of the first and the following approximations of the velocity distribution function had to be found by solving the differential equations in partial derivatives (equations of gas dynamics of the first and higher orders). Five years later Enskog proposed to use zero conditions, conditions with zero right-hand sides, to determine the five arbitrary functional parameters of the first and following approximations of the velocity distribution function. The imposition of the zero conditions leads, in fact, to using different comparison scales in the asymptotic expansion of the velocity distribution function and in the asymptotic expansion of the particle number density, the mean (mass) velocity and the temperature, that are derived from the asymptotic expansion of the velocity distribution function by integration over velocities with different weighting functions. As a result of paralogism of the method of successive approximations (one has to set variable coefficients of the same terms of the unified comparison scale equal to each other) partial time derivatives vanish in the necessary conditions of solutions existence of integral equations of higher orders (see below) and with them terms of gas-dynamic equations, corresponding to viscosity, heat conduction, … vanish. Enskog “improved” the situation by the introducing (see, for example, [2], Chapter 7, § 1, Section 5) of the unsubstantiated expansion of partial time derivative:
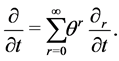 (1)
(1)
The approach of Struminskii, who had proposed in 1974 in [3] his approximate (asymptotic) method of solution of the system of kinetic Boltzmann equations for multicomponent gas, differs from the approach of Enskog to asymptotic solution of the Boltzmann equations system for gas mixture in that, how the infinitesimal parameter is introduced in the Boltzmann equations system for gas mixture, i.e. the solution is constructing in another asymptotic limit. In substance, Struminskii’s method of solution of kinetic equations system is the same as Enskog’s method (Struminskii used the partial time derivative expansion, as Enskog did).
In section 2 below will be proposed the correct method of asymptotic solution of the kinetic Boltzmann equations system for multicomponent gas mixture for the approach, that combines Enskog’s and Struminskii’s approaches; in particular, it will be shown, how one has to modify Enskog’s method: in addition to asymptotic expansion of the velocity distribution function i-component particles of gas mixture it is necessary to determine and to use the expansion of the particle number density 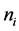 of i-component, mean mass velocity
of i-component, mean mass velocity 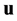 and temperature T of the gas mixture.
and temperature T of the gas mixture.
Further, in the Section 3 the system of infinitesimal first order equations of multicomponent non-equilibrium gas dynamics, appearing during the process of the solution of the system of Boltzmann equations by successive approximations method in the Section 2 as necessary condition of the existence of approximate (asymptotic) solution of the integral equations system, is considered in more detail.
This article is condensed version of our article arXiv:1303.6275. Notations, used below, are close to notations in [2]; it is assumed, that all regarded functions are continuous and continuously differentiable so many times as it is necessary, if their derivatives are considered, and all regarded integrals converge.
2. Correct Method of Solution of the Kinetic Boltzmann Equations System
The Boltzmann equations system, that describes change of dependent on t and spatial coordinates, prescribed by radius-vector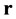 , the velocity distribution functions
, the velocity distribution functions 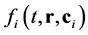 due to collision with particles of other components of mixture of rarefied monatomic gases, where
due to collision with particles of other components of mixture of rarefied monatomic gases, where 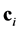 are the velocities of particles of i-component of the mixture {see [2], Chapter 8, Equation (1.1); discussion of the derivation of the Boltzmann equations system and its applicability range see, for example, in [2], Chapters 3 and 18, [4], Chapter 7, § 1; below the central interaction of molecules are considered only, when the force, with which each molecule acts on the other, is directed along the line, connecting the centers of the molecules}, could be written as:
are the velocities of particles of i-component of the mixture {see [2], Chapter 8, Equation (1.1); discussion of the derivation of the Boltzmann equations system and its applicability range see, for example, in [2], Chapters 3 and 18, [4], Chapter 7, § 1; below the central interaction of molecules are considered only, when the force, with which each molecule acts on the other, is directed along the line, connecting the centers of the molecules}, could be written as:
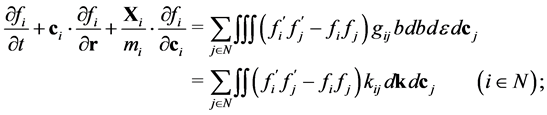 (2)
(2)
in (2) N is a set of indexes, that are numbering components of the mixture; 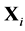 is an external force, which acts on the molecule of the i-component;
is an external force, which acts on the molecule of the i-component; 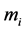 is the mass of the molecule of the i-component;
is the mass of the molecule of the i-component; 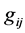 is the modulus of the relative velocity of colliding particles
is the modulus of the relative velocity of colliding particles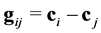 ; b is the impact distance,
; b is the impact distance, 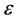 is the azimuth angle,
is the azimuth angle, 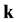 is the unit vector, directed to the center of mass of the colliding particles from the point of closest approach―see [2], Chapter 3, Figure 3; the scalar function
is the unit vector, directed to the center of mass of the colliding particles from the point of closest approach―see [2], Chapter 3, Figure 3; the scalar function 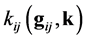 is determined by equality
is determined by equality
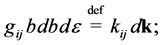 (3)
(3)
by prime in (20) and below the velocities and the functions of velocities after the collision are denoted.
Let us introduce following notations:
 (4)
(4)

to differ velocities of colliding molecules of the same kind in (22) the one velocity is denoted by 

In Enskog’s approach the differential parts of the Boltzmann Equations (2), that are denoted by 


In Struminskii’s approach to the asymptotic solution of the Boltzmann equations system the differential parts of the Boltzmann Equations (2) and the collision integrals of the particles of i-component with the particles of the other components are considered to be small as compared with the collision integral of the particles of i-component between each other, therefore the indicator of infinitesimality 

It is possible to combine Enskog’s approach with Struminskii’s approach. For this purpose we divide the set of mixture components N into two subsets: the subset of components, that we call formally inner components (we could consider the case, when there are some subsets of inner components, but this case does not fundamentally differ from the one, considered below, the only difference is that the notation become more complicated) and the subset of components, that we call external components. To differ the two groups of mixture components we
denote the subset of indexes of inner components 



intersection of the sets 





Let us write the asymptotic expansion of the velocity distribution function 


The differential parts of the Equations (3) are written as:

where

―cf. with [2], Chapter 7, § 1, Sections 4, 5 and [3]. In (11)-(12) the partial time derivative expansion (1) is not used in contrast to that, how it was made by Enskog and further by Struminskii. As result, described below method for solution of the system of kinetic Boltzmann equations differ fundamentally from Enskog’s method and Struminskii’s method.
Substituting (10) and (11) in (8) and equating coefficients at the same powers of 



Similarly substituting (10) and (11) in (9) and equating coefficients at the same powers of 
functions of particles of external components of gas mixture


Speaking about an order of approximation below, we assume the order to be equal to the value of index r in (14), (16). According to (5), (13), in zero order approximation we have the following system of integral equations to find the velocity distribution functions of particles of inner components of gas mixture

The general solution of the equations system (17) can be written as a set of the Maxwell functions:

where k is the Boltzmann constant.
Particle number density 




in (21) 

that is convenient to use below instead of definition (21).
According to definitions (19), (20), (21), in addition to the asymptotic expansion (10) it is necessary to determine asymptotic expansions for particle number density 

mean mass velocity

and temperature 

Substituting (10) and (23)-(25) in (19), (20), (22) and equating terms of the same infinitesimal order we obtain 



In (27), (28) the notations are introduced


In particular, for 




density, the mean mass velocity and the temperature of inner components of the mixture:



According to (4), (15), zero order integral equations, from which the velocity distribution functions 

―are simpler than Equations (17) and differ actually from (17) only by lack of summation over components. Therefore, similarly (18), the general solution of the equations system (34) can be written as a set of the Maxwell functions:

where 





Let’s add to the definition of the number density of particles of i-component definitions of mean velocity 



from (19), (36), (37) the equality is obtained:

that is convenient to use below instead of definition (37).
Let’s enter similar (24)-(25) asymptotic expansions of outer 

and outer 

Substituting (10), (23), (39), (40) in (19), (36), (38) and equating terms of the same infinitesimal order we obtain for each 



cf. with (26)-(28). In (42), (43) the notation is used

For 



velocity and the temperature of outer 



For 


into account, can be rewritten in the form

in (49) functions 


The left-hand sides of Equations (49) involves functions, that are known from the previous step of the successive approximations method. Unknown functions 






Multiplying Equations (50) by



From (51) we conclude, that 



where 








To simplify further evaluations according to the expression for

where





where 
Multiplying Equations (55) by 




Among (infinitesimal) set of particular solutions of the system of Equations (55), different from each other on some solution of the system of homogeneous Equations (50), unique solution 


Having substituted expression for

in (26)-(28), taking (18), (29)-(33) and (58)-(59) into account, we obtain a system of 



from which we find expressions for functions


fficients of asymptotic expansions of the particle number density of 



Then the fulfillment of equalities (56)-(57) can be considered as the differential equations, the r-order equations of gas dynamics, for finding


The partial solution of the system of inhomogeneous Equations (55)

coefficients, depending on 
For 


where



The fulfillment of analogous (56)-(57) equalities

can be considered as the differential equations, the r-order equations of gas dynamics, for finding


3. The System of First Order Equations of Multicomponent Non-Equilibrium Gas Dynamics
Let us consider in more detail the system of infinitesimal first order Equations (56)-(57), (71)

To simplify transformations, according to the expressions for velocity distribution functions of particles of infinitesimal zero order (18), (35), functions







for inner components

At transformation of differential parts of the Equations (56)-(57) and (71) we use equalities:



In (75)-(77) the bar above symbol with index 




depending on external forces


After simple transformations from (56)-(57) and (71) 






In accordance with the general definition of pressure tensor of i-component of gas mixture

and with the general definition of i-component heat flux vector

(cf. with [2], Chapter 2, §§ 3, 4) in (79)-(84)

is inner components pressure tensor of zero order, 





is inner components heat flux vector of zero order,

is zero order internal energy of particles of inner components per unit volume, which is equal, in this case, to energy of their translational chaotic motion, however, the energy transfer equations, written in form (81) and (84) can be used in more general cases as well (cf. with [4], Chapter 7, § 6), in (87)-(89) averaging (78) is performed with Maxwell function 

is 



is 

is zero order internal energy of particles of 

General analytic expressions for integrals

System of infinitesimal first order equations of multicomponent non-equilibrium gas dynamics (79)-(84) is proposed to use for describing turbulent flows
Cite this paper
S. A. Serov,S. S. Serova, (2016) Asymptotic Solutions of the Kinetic Boltzmann Equation and Multicomponent Non-Equilibrium Gas Dynamics. Journal of Applied Mathematics and Physics,04,1687-1697. doi: 10.4236/jamp.2016.48177
References
- 1. Hilbert, D. (1912) Grundzüge einer Allgemeinen Theorie der Linearen Integralgleichungen. Teubner, Leipzig and Berlin. (In Ger-man)
- 2. Chapman, S. and Cowling, T.G. (1952) The Mathematical Theory of Non-uniform Gases. Cambridge University Press, Cambridge.
- 3. Struminskii, V.V. (1974) Influence of Diffusion Velocity on Flow of Gas Mixtures. Prikladnaya Mathematica i Me-chanica [Applied Mathematics and Mechanics], 38, 203-210. (In Russian)
- 4. Hirschfelder, J.O., Curtiss, Ch.F. and Bird, R.B. (1954) Molecular Theory of Gases and Liquids. Wiley, New York.







