Chinese Medicine
Vol. 3 No. 3 (2012) , Article ID: 22712 , 6 pages DOI:10.4236/cm.2012.33024
Activities of Some Nigerian Medicinal Plants against Aedes aegypti
1Department of Pharmacognosy, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Nigeria
2Department of Pharmacognosy and Natural Medicine, Faculty of Pharmacy, University of Uyo, Uyo, Nigeria
3Adibs Pharma Inc., Palmetto Place, Mississauga, Canada
Email: *caadebajo@yahoo.com, aadebajo@oauife.edu.ng
Received May 4, 2012; revised June 4, 2012; accepted June 14, 2012
Keywords: Methanolic Extracts; Nigerian Medicinal Plants; Larvicidal Activities; Aedes aegypti; Plant Larvicides
ABSTRACT
Extracts and constituents of medicinal plants have proven to be biodegradable, had low mammalian toxicity and induction of resistance, and comparable activities to the standard drugs. Therefore, methanolic extracts of some plants that are termite resistant or used ethnomedically as antimalarial and febrifuge were evaluated for activities against 4th-instar larvae of Aedes aegypti. A 61% of these plants with these properties demonstrated larvicidal activities and may confirm the usefulness of these properties in choosing plant larvicides. This is the first report of larvicidal activities of stem barks and leaves of Blighia sapida and Baphia nitida, stem barks of Markhamia tomentosa and Newboldia laevis, and whole plants of Euphorbia macrophylla. Extracts of B. sapida stem bark, Costus specious root and Xylopia aethiopica seed, with LC50 1.71, 1.47 and 1.49 mg/ml at 48 h, respectively, were the most active and had significant activities that were comparable to Endosulphan. Hence, they may be used as plant larvicides in the control of dengue and yellow fever.
1. Introduction
The species of Anopheles, Culex and Aedes are important vectors in the transmission of malaria, filariasis, and dengue, yellow and chikungunya fevers, etc., respectively [1, 2]. These health conditions are endemic in the developing countries of Africa, Latin America and Asia [3]. These mosquitoes have developed resistance against currently used insecticides [4-6], thereby hindering their effective control [7,8] and possibility of eradicating them in areas where they are prevalent. Furthermore, the adverse effects of conventional insecticides on the environment and animals, including human [9], and the limited number of available insecticides [4-6] compel continued search for safer plant insecticides and larvicides [3] that could be used in the control of these diseases. Medicinal plant extracts and their constituents have proved to be biodegradable, have low mammalian toxicity and induction of resistance [3,10] while their activities were similar to those of the standard drugs, such as temephos and methoprene [11,12].
Larvicidal activity is not a traditional method of insect control and therefore there is no plant with such ethnomedicinal claim. Plants that are termite or insect resistant, used as fish poison and folklorically in treating malaria and fever as well as those with reported insecticidal, mosquito repellent and mosquitocidal activities have demonstrated promising larvicidal activities [12,13]. However, no correlation has been established between larvicidal activities and these properties. Therefore, it is hereby proposed that candidate larvicides should be sourced from plants with these properties. Hence, methanolic extracts of the plants with these properties, as listed in Table 1, were investigated for their effects against 4th-instar larvae of Aedes aegypti L. Euphorbia macrophylla that does not have any of the above characteristics was used as an internal control for this hypothesis.
2. Materials and Methods
2.1. Collection of Plant Parts
The different plant parts given in Table 1 were either collected from the Obafemi Awolowo University campus, Ile-Ife, Osun State or Itak Ikot Akap Ikono, Ikono Local Government Area (LGA) of Akwa Ibom state while the seeds of X. aethiopica were bought from Affiong Ekpor market, Etim Ekpo LGA, Akwa Ibom state. All the plant materials were identified by Mr. Oladele, Department of Pharmacognosy, Obafemi Awolowo University, Ile-Ife and confirmed by a taxonomist.
2.2. Plant Extraction
A 1.0 kg each of air-dried and powdered materials of these plant parts was extracted at room temperature with methanol (MeOH) for 3 days. The extract was filtered and concentrated in vacuo. This process was repeated two times and the dried extracts were combined for each plant part.
2.3. Hatching of Aedes aegyptii Eggs
The eggs of Aedes aegyptii (Linn.) (Culicidae) were collected from The National Medical Centre, Yaba, Lagos and were suspended in water for about 24 - 48 hours to hatch. They were fed with rabbit pellets until they reached the fourth instars stage.
2.4. Larvicidal Test
Larvicidal tests were done according to the standard method of WHO (2005) with slight modifications [26]. Stock solutions (25 mg/ml) of the extracts were prepared and diluted using distilled water to obtain different concentrations of the test agents. Twenty-five larvae at fourth instars stage were dispensed into 50 ml test cups containing 25 ml of different concentrations (0 - 5 mg/ml) of extracts. Toxicity of Endosulphan, a commercial insecticide, was evaluated at 0.312, 0.625, 0.937, 1.25, 1.56 and 1.88 mg/ml while distilled water was the negative control. Dead larvae were those incapable of rising to the surface or without the characteristic diving reaction when the water is disturbed [26]. Mortality was recorded after 24 and 48 h of exposure during which no nutritional supplement was added. Data were evaluated and the LC50 and LC90 values, representing the lethal concentration for 50% and 90% larval mortalities, were predicted using appropriate statistical package. No mortality was observed with the negative control. The number of replicates was six.
2.5. Statistical Analysis
The mean and standard error of the mean, percentage mortalities, LC50 and LC90 values were determined using Microsoft Excel program 2007 [27]. The larvicidal activities of the extracts were compared with that of Endosulphan (positive control) using one way analysis of variance (ANOVA) followed by Bonferonni post-hoc test. p < 0.05 was considered as significant.
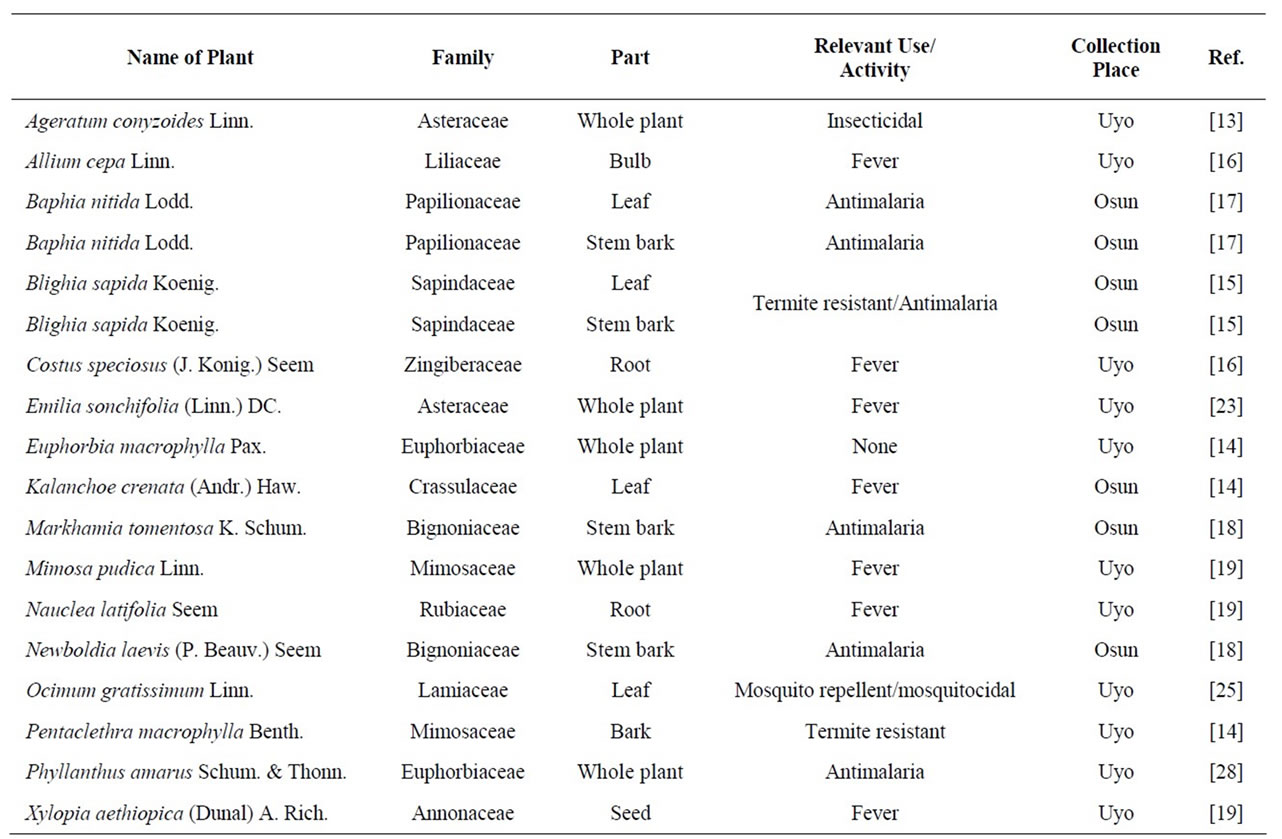
Table 1. List of plants parts used.
3. Results
Larvicidal activities of the extracts and that of Endosulphan at 24 and 48 h are given in Figures 1 and 2.
4. Discussion
Plant extracts have been given as an ideal ecofriendly approach for the control of the dengue vector, A. aegypti [28]. The larvicidal activities of the methanolic extracts of the plant parts with the proposed desired properties, as indicated in Table 1, were determined to test the hypothesis of sourcing plant larvicides from these groups of plants. The activities of most of the extracts and Endosulphan at 24 hours were comparable (p > 0.05) to those of 48 hr, indicating that the activities were larvicidal and not larvistatic in nature (Figures 1 and 2). However, significantly (p < 0.05) time dependent larvicidal activities of A. cepa, O. gratissimum, N. laevis, A. conyzoides, P. macrophylla and E. macrophylla extracts may show that these were slow acting and longer exposure to these test agents may be beneficial. This is the first report of larvicidal activities of M. tomentosa, E. macrophylla, N. laevis, stem barks and leaves of B. sapida and B.nitida as well as the first report of K. crenata leaf, whole plants of M. pudica and E. sonchifolia against A. aegyptii (Figures 1 and 2).
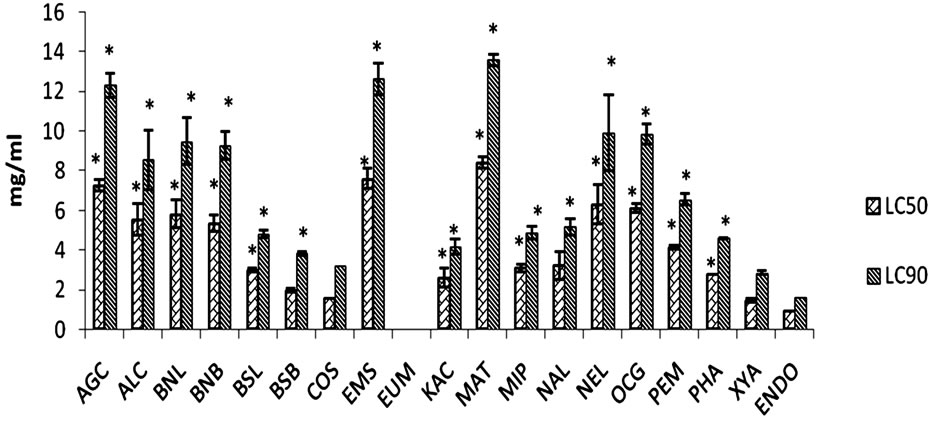
AGC: Ageratum conyzoides whole plant; ALC: Allium cepa bulb; BNL: Baphia nitida leaf; BNB: Baphia nitida bark; BSL: Blighia sapida leaf; BSB: Blighia sapida stem bark; COS: Costus speciosus root; EMS: Emilia sonchifolia whole plant; EUM: Euphorbia macrophylla whole plant; KAC: Kalanchoe crenata leaf; MAT: Markhamia tomentosa stem bark; MIP: Mimosa pudica whole plant; NAL: Nauclea latifolia root; NEL: Newboldia laevis stem bark; OCG: Ocimum gratissimum leaf; PEM: Pentaclethra macrophylla bark; PHA: Phyllanthus amarus whole plant; XYA: Xylopia aethiopica dried seeds; ENDO: Endosulphan (positive control). The larvicidal activities of the extracts were compared with that of Endosulphan (positive control) using one way analysis of variance (ANOVA) followed by Bonferonni post-hoc test. *: p < 0.05 significantly different from Endosulphan. N = 6.
Figure 1. Effects of methanolic extracts of Nigerian medicinal plants against Aedes aegypti at 24 hr.
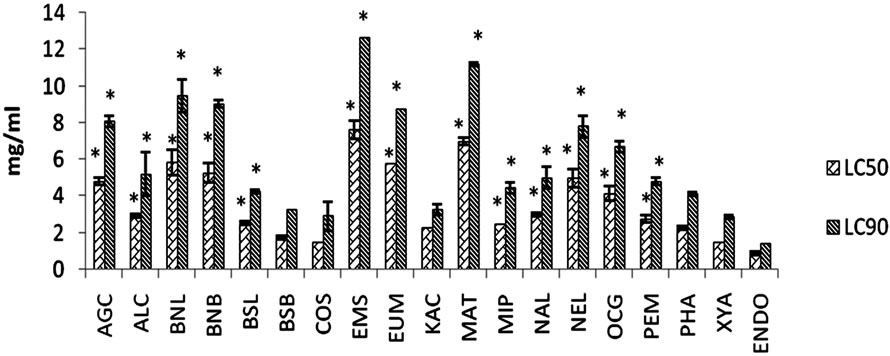
AGC: Ageratum conyzoides whole plant; ALC: Allium cepa bulb; BNL: Baphia nitida leaf; BNB: Baphia nitida bark; BSL: Blighia sapida leaf; BSB: Blighia sapida stem bark; COS: Costus speciosus root; EMS: Emilia sonchifolia whole plant; EUM: Euphorbia macrophylla whole plant; KAC: Kalanchoe crenata leaf; MAT: Markhamia tomentosa stem bark; MIP: Mimosa pudica whole plant; NAL: Nauclea latifolia root; NEL: Newboldia laevis stem bark; OCG: Ocimum gratissimum leaf; PEM: Pentaclethra macrophylla bark; PHA: Phyllanthus amarus whole plant; XYA: Xylopia aethiopica dried seeds; ENDO: Endosulphan (positive control). The larvicidal activities of the extracts were compared with that of Endosulphan (positive control) using one way analysis of variance (ANOVA) followed by Bonferonni post-hoc test. *: p < 0.05 significantly different from Endosulphan. N = 6.
Figure 2. Effects of methanolic extracts of Nigerian medicinal plants against Aedes aegypti at 48 hr.
Based on their activities, the plants could be classified into three groups. The first group consists of extracts of B. sapida stem bark, C. specious root and X. aethiopica seed that demonstrated the highest activities (LC50 < 2.0 mg/ml) against A. aegyptii at 24 and 48 hr, which were comparable (p > 0.05) to that of Endosulphan, the positive drug used (Figures 1 and 2). They were therefore the most active of the extracts tested with the order of activity of C. specious root = X. aethiopica seed > B. sapida stem bark. As shown in Table 1, these plants were either termite resistant [14,15], or used ethnomedically as antimalarial or against fever [16,18,20]. Hence, the high larvicidal activities given by these plant extracts may support the hypothesis that these plants could be excellent sources of plant larvicides for the control of dengue and yellow fevers’ vectors. Efforts are on going to isolate their larvicidal constituents.
The second group includes K. crenata leaf and P. amarus whole plant. Their larvicidal activities (LC50 2.24 and 2.28 mg/ml) at 48 hours were comparable (p > 0.05) to that of the standard drug (Figure 2). Their ethnomedicinal use as anti-malarial or against fever [19,21] may further support this hypothesis of sourcing for plant larvicides. The extracts of B. sapida leaf, P. macrophylla, N. latifolia and M. pudica displayed moderate larvicidal activities (2.0 < LC50 < 4.2 mg/ml) at 24 and 48 hours that were significantly (p < 0.05) less than that of Endosulphan (Figures 1 and 2). The first two are termite resistant while the others are used against fevers [14-16,20]. The moderate LC50 values of A. cepa and O. gratissimum at 48 hours were 2.92 and 4.15 mg/ml (Figure 2), respectively and are used folklorically as febrifuge and mosquito repellent [22,25]. The extracts of N. laevis, A. conyzoides, M. tomentosa, E. sonchifolia, and leaf and stem bark of B. nitida with (LC50 > 4.2 mg/ml) at 48 hours, having the above desired properties and regarded as inactive make up the third group. The LC50 of E. macrophylla at 24 hours could not be determined because all the larvae were alive at the maximum concentration tested. Also, it does not have any of the desired properties and its lack of larvicidal activity may lend credence to this hypothesis. Thus, some plants, with these desired properties, demonstrated high and promising larvicidal activities. Although some (39%) failed, the relatively high 61% of plants that have the desired properties and demonstrated larvicidal activities may confirm these properties as useful in choosing plant larvicides for local use or further investigation.
The LC50 0.091 and 0.113 mg/ml obtained for P. amarus petroleum ether extract against A. aegyptii and Culex quiquefasciatus, respectively at 24 hours [28] were much lower than LC50 2.78 mg/ml given by the methanolic extract in this study (Figure 1). The result of inactivity of methanolic extract of A. conyzoides (Figures 1 and 2) was similar to that given for the same extract against Aedes fluviatilis [29], although a contrary report of activity had been made [29]. The 100% mortality given by the volatile oil (15 μl) of A. conyzoides against Culex species led to its establishment as an insecticide [24]. The reported IC50 257 ppm (0.257 mg/ml) given by n-hexane extract of X. aethiopica against C. p. quiquefasciatus at 96 hours [30], was significantly better than that of the methanolic extract (Figures 1 and 2). These differences may indicate that the active constituents of P. amarus, A. conyzoides and X. aethiopica may be non-polar in nature. Also, the LC50 of 0.054 and 0.057 mg/ml reported for ethylacetate extract of M. pudica leaf at 24 h against Culex gelidus and C. quinquefasciatus, respectively [31] were lower than that of the extract used in this study (Figure 1), suggesting that the active constituents were likely to be moderately polar. Also, LC50 0.010 and 0.038 mg/ml at 24 and 48 h given by the root 80 % ethanolic extract of C. speciosus against A. aegyptii [32] were significantly lower than LC50 1.59 and 1.47 mg/ml obtained in this study(Figures 1 and 2). Hence, all these differences in activity, especially that of C. speciosus, may be due to influence of geographical location on the constituents of these plants (geographical variants) [33].
The demonstrated high larvicidal activity (97.8% mortality) against the larvae of cattle tick Boophiltus microplus by leaf ethanolic extract of K. crenata at 1.14 g/cm2 [34] was similar (p > 0.05) to a 100% mortality by the leaf methanolic extract (5 mg/ml) against A. aegyptii after 48 hr (Figure 2). Aerial parts of E. sonchifolia (0.1 mg/ml) gave a weak 44.4% mortality against A. fluviatilis [29], similar to a 20% mortality at 24 hr by the whole plant extract (4 mg/ml) (Figures 1 and 2). An LC50 5.909 ml/l against A. aegyptii has been reported for essential oil of A. cepa [35] while its methanolic extract had LC50 5.53 and 2.92 mg/ml at 24 and at 48 hr, respectively (Figures 1 and 2). However, the LC50 6.12 mg/ml against A. aegypti at 24 h demonstrated by the methanolic extract of O. gratissimum leaf (Figure 1) was significantly higher than 726.56 ppm (0.73 mg/ml) given for the ethanolic extract against C. quinquefasciatus at the same hour [36]. This may confirm that geographical location influenced the constituents of this plant [33]. Other larvicidal activities (LC50 0.38 µl/ml) against C. pipens for essential oil of A. cepa [37] and LC50 > 0.020 mg/ml of its petroleum ether, methanol and aqueous extracts against C. quinquefasciatus [38] have also been reported.
In conclusion, extracts of B. sapida stem bark, C. specious root and X. aethiopica seed had significant activities that were comparable to Endosulphan and may be used as plant larvicides in the control of dengue and yellow fever. Also, properties of termite resistance or ethnomedical use as insecticide, anti-malarial and febrifuge may be important in selecting candidate plant larvicides.
5. Acknowledgements
A. C. A. and G. F. F. wish to acknowledge OAU for the research grant 1425RN.
REFERENCES
- G. N. Grantz, “What Must We Do to Effectively Control Aedes aegypti?” Journal of Tropical Medicine, Vol. 35, No. 4, 1993, pp. 243-251.
- V. Prajapati, A. K. Tripathi, K. K. Aggarwal and S. P. S. Khanuja, “Insecticidal, Repellent and Oviposition-Deterrent Activity of Selected Essential Oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus,” Bioresource Technology, Vol. 96, No. 16, 2005, pp. 1749-1757. doi:10.1016/j.biortech.2005.01.007
- L. Donno, “Malaria: Prevalence and Distribution,” Malaria, Montedison Group, Farmitalia Carlo Erba, Milan, 1984, pp. 7-13.
- J. A. Rozendaal, “Mosquitoes and other Biting Diptera,” In: Vector Control, World Health Organization, Geneva, 1997, pp. 5-177.
- J. E. Casida and G. B. Quistad, “Insecticide Targets: Learning to Keep up with Resistance and Changing Concepts of Safety,” Agricultural Chemistry and Biotechnology, Vol. 43, No. 4, 2000, pp. 185-191.
- J. B. Lima, M. P. Da-Cunha, R. C. Da Silva, A. K. Galardo, S. S. Soares, I. A. Braga, et al., “Resistance of Aedes aegypti to Organophosphates in Several Municipalities in the State of Rio de Janeiro and Espírito Santo, Brazil,” American Journal of Tropical Medicine and Hygiene, Vol. 68, No. 3, 2003, pp. 329-333.
- F. Chandre, F. Darriet, M. Darder, A. Cuany, J. M. C. Doannio, N. Pasteur, et al., “Pyrethroid Resistance in Culex quinquefasciatus from West Africa,” Medical and Veterinary Entomology, Vol. 12, No. 4, 1998, pp. 359-366. doi:10.1046/j.1365-2915.1998.00120.x
- R. P. Penilla, A. D. Rodriguez, J. Hemingway, J. L. Torres, J. I. Arredondo-Jimenez and M. H. Rodriguez, “Resistance Management Strategies in Malaria Vector Mosquito Control. Baseline Data for a Large-Scale Field Trial against Anopheles albimanus in Mexico,” Medical and Veterinary Entomology, Vol. 12, No. 3, 1998, pp. 217- 233. doi:10.1046/j.1365-2915.1998.00123.x
- T. Su and M. S. Mulla, “Ovicidal Activity of Neem Products (Azadirachtin) against Culex tarsalis and Culex quinquefasciatus (Diptera: Culicidae),” Journal of American Mosquito Control Association, Vol. 14, No. 2, 1998, pp. 204-209.
- W. Choochote, D. Kanjanapothi, A. Panthong, T. Taesotikul, A. Jitpakdi, U. Chaithong, et al., “Larvicidal, Adulticidal and Repellent Effects of Kaempferia galangal,” Southeast Asian Journal of Tropical Medicine and Public Health, Vol. 30, No. 3, 1999, pp. 470-476.
- A. M. Arriaga, J. Q. Lima, J. N. e Vasconcelos, M. C. de Oliveira, T. L. Lemos, A. M. Fonseca, et al., “Antioxidant and Larvicidal Activities of Tephrosia egregia Sandw against Aedes aegypti,” Natural Products Communications, Vol. 4, No. 4, 2009, pp. 529-530.
- S.-S. Cheng, H.-T. Chang, C.-Y. Lin, P.-S. Chen, C.-G. Huang, W.-J. Chen, et al., “Insecticidal Activities of Leaf and Twig Essential Oils from Clausena excavata against Aedes aegypti and Aedes albopictus Larvae,” Pest Management Science, Vol. 65, No. 3, 2008, pp. 339-343. doi:10.1002/ps.1693
- L. C. Ming, “Ageratum conyzoides: A Tropical Source of Medicinal and Agricultural Products,” In: J. Janick, Ed., Perspectives on New Crops and New Uses, ASHS Press, Alexandria, 1999, pp. 469-473.
- S. A. Mitchell and M. H. Ahmad, “A Review of Medicinal Plant Research at the University of West Indies, Jamaica,” West Indian Medical Journal, Vol. 55, No. 4, 2001, pp. 243-269.
- C. Orwa, A. Mutua, R. Kindt, R. Jamnadass and S. Anthony, “Agroforestree Database: A Tree Reference and Selection Guide Version 4.0,” World Agroforestry Centre, Kenya, 2009.
- J. O. Igoli, O. G. Ogaji, T. A. Tor-anyiin and N. P. Igoli, “Traditional Medicine Practice amongst the Igede People of Nigeria. Part II,” African Journal of Traditional, Complementary and Alternative Medicine, Vol. 2, No. 2, 2005, pp. 134-152.
- F. Tantangmo, B. N. Lenta, F. F. Boyom, S. Ngouela, M. Kaiser, E. Tsamo, et al., “Antiprotozoal Activities of Some Constituents of Markhamia tomentosa (Bignoniaceae),” Annals of Tropical Medicine and Parasitology, Vol. 104, No. 5, 2010, pp. 391-398. doi:10.1179/136485910X12743554760180
- J. Kayode, “Conservation of Indigenous Medicinal Botanicals in Ekiti State, Nigeria,” Journal of Zhejiang University Science B, Vol. 7, No. 9, 2006, pp. 713-718. doi:10.1631/jzus.2006.B0713
- R. P. Umbare, G. S. Mate1, D. V. Jawalkar, S. M. Patil and S. S. Dongare, “Quality Evaluation of Phyllanthus amarus (Schumach) Leaves Extract for Its Hypolipidemic Activity,” Biology and Medicine, Vol. 1, No. 4, 2009, pp. 28-33.
- H. M. Burkill, “The Useful Plants of West Tropical Africa,” Kew Publishing, Royal Botanical Gardens, Kew, 1985, pp. 11-20.
- A. Sofowora, “Medicinal Plants and Traditional Medicine in Africa,” Spectrum Books Ltd., Nigeria, 1993, pp. 142- 157.
- E. A. Ross, “Medicinal Plants of the World: Chemical Constituents, Traditional and Modern Medicinal Uses,” Humana Press, Totowa, Vol. 2, 2001, p. 487.
- V. M. Couto, F. C. Vilela, D. F. Dias, M. H. Dos Santos, R. Soncini, C. G. Nascimento, et al., “Antinociceptive Effect of Extract of Emilia sonchifolia in Mice,” Journal of Ethnopharmacology, Vol. 134, No. 2, 2011, pp. 348- 353. doi:10.1016/j.jep.2010.12.028
- B. A. Ayinde and F. Odigie, “Larvicidal Properties of the Volatile Oil of Ageratum conyzoides L. (Compositae) against Culex specie Mosquito Larvae,” Nigerian Journal of Applied Science, Vol. 19, 2001, pp. 23-25.
- E. T. Oparaocha, I. Iwu and J. E. Ahanaku, “Preliminary Study on Mosquito Repellent and Mosquitocidal Activities of Ocimum gratissimum (L.) Grown in Eastern Nigeria,” Journal of Vector Borne Diseases, Vol. 47, No. 1, 2010, pp. 45-50.
- WHO, “Guidelines for Laboratory and Field Testing of Mosquito Larvicides,” World Heath Organisation Communicable Disease Control, Prevention and Eradication— WHO Pesticide Evaluation Scheme, 2005, pp. 10-11.
- G. F. Ibikunle, A. C. Adebajo, F. G. Famuyiwa, A. J. Aladesanmi and C. O. Adewunmi, “In Vitro Evaluation of Anti-Trichomonal Activities of Eugenia uniflora Leaf,” African Journal of Traditional, Complementary and Alternative Medicine, Vol. 8, No. 2, 2011, pp. 170-176.
- A. A. Rahuman, G. Gopalakrishnan, P. Venkatesan and K. Geetha, “Larvicidal activity of some Euphorbiaceae plant extracts against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae),” Parasitology Research, Vol. 102, No. 5, 2008, pp. 867-873. doi:10.1007/s00436-007-0839-6
- M. E Macêdo, R. A. G. B. Consoli, T. S. M. Grandi, A. M. G. dos Anjos, A. B. de Oliveira, N. M. Mendes et al., “Screening of Asteraceae (Compositae) Plant Extracts for Larvicidal Activity against Aedes fluviatilis (Diptera: Culicidae),” Mems do Instituto Ostwaldo Cruz, Rio de Janeiro, Vol. 92, No. 4, 1997, pp. 565-570.
- A. A. S. Amusan and O. A. Oke, “The Toxicity of HexaNolic Extract of Xylopia aethiopica to Laboratory Reared Larvae of Cx. p. quiquefasciatus,” Proceedings of the International Conference on Science & National Development, 25-28 October, 2004, College of Natural Sciences Proceedings, Federal University of Agriculture, Abeokuta, Nigeria, pp. 109-112.
- C. Kamaraj, A. A. Rahuman, A. Mahapatra, A. Bagavan and G. Elango, “Insecticidal and Larvicidal Activities of Medicinal Plant Extracts against Mosquitoes,” Parasitology Research, Vol. 107, No. 6, 2010, pp. 1337-1349. doi:10.1007/s00436-010-2006-8
- S. Promsiri, A. Naksathit, M. Kruatrachue and U. Thavara, “Evaluations of Larvicidal Activity of Medicinal Plant Extracts to Aedes aegypti (Diptera: Culicidae) and other Effects on a Non Target Fish,” Insect Science, Vol. 13, 2006, 179-188. doi:10.1111/j.1744-7917.2006.00080.x
- J. Reisch, A. C. Adebajo, A. J. Aladesanmi, S. K. Adesina, D. Bergenthal and U. Meve, “Chemotypes of Murraya koenigii growing in Sri Lanka,” Planta Medica, Vol. 60, No. 3, 1994, pp. 295-296. doi:10.1055/s-2006-959486
- N. Chungsamarnyart, S. Jiwajinda and W. Jansawan, “Larvicidal Effect of Plant Crude-Extracts on the Tropical Cattle Tick (Boophiltus microplus),” Kasetsart Journal: Natural Science, Vol. 25, 1991, pp. 80-89.
- M. R. Dowlathabad, G. SapthaJyothi, R. M. Reddy, M. V. V. Prasad and K. Subramanyam, “Larvicidal Activity of Essential Oils from Indian Medicinal Plants against Aedes aegypti L.,” Journal of Pharmacy Research, Vol. 2, No. 4, 2009, pp. 762-764.
- B. Pitasawat, W. Choocote, D. Kanjanapothi, A. Panthong, A. Jitpakdi and U. Chaithong, “Screening for Larvicidal Activity of Ten Carminative Plants,” Southeast Asian Journal of Tropical Med Public Health, Vol. 29, No. 3, 1998, pp. 660-662.
- S. M. Habeeb, A. H. El-Namaky and M. A. Salama, “Efficiency of Allium. cepa and Commiphora molmol as a Larvicidal Agent against Fourth Stage Larvae of C. pipiens (Diptera: Culicidae),” American-Eurasian Journal of Agriculture & Environmental Science, Vol. 5, No. 2, 2009, pp.196-203.
- S. S. Ranaweera, “Mosquito-Larvicidal Activity of some Sri Lankan Plants,” Journal of the National Science Council of Sri Lanka, Vol. 24, No. 2, 1996, pp. 63-70.
NOTES
*Corresponding author.

