Natural Science
Vol.6 No.9(2014), Article ID:46404,9 pages DOI:10.4236/ns.2014.69067
Antidermatophytic Activity of Essential Oils against Locally Isolated Microsporum canis—Gaza Strip
Emad Khalil Abou Elkhair
Biology Department, Faculty of Science, Al Azhar University-Gaza, Gaza Strip, Palestine
Email: e.khahir@alazhar.edu.ps, eabouelkhair@gmail.com
Copyright © 2014 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 28 March 2014; revised 28 April 2014; accepted 4 May 2014
ABSTRACT
There is a need for new antimicrobial drugs due to the continuous development of resistance. Natural antimicrobials are of utmost importance due to safety issue and availability. The antifungal activity of four essential oils (Mentha piperta, Thymus vulgaris, Cymbopogon citratus, and Oreganum majoranum oils) against locally isolated Microsporum canis was determined by disc diffusion method and minimum inhibitory concentration was determined using broth dilution method. Mentha piperta oil showed the highest antifungal activity among tested oils in this study. The total inhibition attached when 0.046% was used with M. piperta, the total inhibition obtained with the 0.133% of C. citratus oil, which is accounted as the second essential oils, also T. vulgaris and O. majoranum oils achieved reductions at 0.133%. The present work has revealed that all oils have been used in low concentrations and produced promising results in comparison to the positive control (Clotrimazole).
Keywords:Antifungal, Microsporum canis, Mentha piperta, Essential Oils, Gaza Strip

1. Introduction
The medicinal plants are reliable sources for the treatment of many health problems. Man has depended a lot on herbs in the past, and even at present the use of plants as medicine is popular. For future health challenges, plants are reasonably prepared to serve man. The only need is to develop the isolation and purification techniques. The medicinal value of plants is due to the phytochemical constituents they produce, which exhibit certain physiological actions on human body [1] .
Majority of phytochemicals have been known to have valuable therapeutic activities like insecticidal, antifungal, antibacterial, anticonstipative and spasmolytic effect [2] -[4] .
The dermatophytes are a group of closely related fungi that have the ability to invade the stratum corneum of the epidermis and keratinized tissues derived from it, such as skin, nail, and hair of humans and animals [5] . Tinea capitis (scalp ringworm) is a highly communicable infection of the scalp and hair caused by dermatophyte fungi. It occurs in all age groups, but predominately children. It is endemic in some of the poorest countries [6] . Microsporum canis is a dermatophyte, which causes ringworm of the scalp and skin in children and has been occasionally reported as the cause of nail infection [7] .
Nowadays, multiple drug resistance has been developing due to the indiscriminate use of commercial antimicrobial drugs used in the treatment of infectious diseases [1] . There is a constant need for new and effective therapeutic agents; therefore, there is a need to develop alternative antimicrobial drugs for the treatment of infectious diseases from medicinal plants or others sources [8] [9] .
Reports are available on the use of several plant byproducts, which possesses antimicrobial properties on several pathogenic bacteria and fungi [10] . Therefore, researchers are increasingly turning their attention to herbal medicines looking for new leads to develop better drugs against microbial infections. All these facts shows that plant based antimicrobial agents possess vast untapped sources for drugs and have enormous therapeutic potential [11] .
The aim of this work is to examine the antifungal activity of Mentha piperta, Oreganum majoranum, Cymbopogon citratus and Thymus vulgaris oils against clinical isolates of Microsporum canis.
2. Materials and Methods
2.1. Materials
Sigma Company kindly provided the four investigated oils (Table 1). Mentha piperta, Oreganum majoranum, Cymbopogon citratus and Thymus vulgaris oils were send from the department of microbiology of Pamplona University in Spain (The oils in study were European Pharmacopeia grade).
2.2. Methods
2.2.1. Samples Selection
M. canis was isolated from clinically diagnosed children came to primary health centers of North Gaza district (Jabalia clinic in north Gaza Strip). Isolation was done before any local or systemic antifungal treatment. Identification of the isolate was accomplished at Microbiology laboratory at Biology Department, Al-Azhar University-Gaza.
2.2.2. Antifungal Activity of Tested Essential Oils
1) Screening methods
Disc diffusion method
The isolated pathogenic fungi Microsporum canis were tested by the disc diffusion method [12] , in the appropriate culture medium (Sabouraud’s dextrose agar).
2) Determination of minimum inhibitory concentration
The most potent essential oil of the study was determined. Different concentration of this oil (Mentha piperta) in the range of concentration of efficacy and incubation for 8 days determined the minimum inhibitory concentration of this oil using broth dilution method
Table 1. List of essential oils used in the antifungal assay.
3) Determination of lethal and sub lethal concentration
Determination of lethal and sub lethal concentration of Mentha piperta essential oil was done by determination of mycelial weight after mixing different concentrations of oil to the media used for dermatophyte growth.
Flasks containing mycelia were filtered through pre weighed Whitman filter No. 1 and were then washed with distilled water. The mycelia were placed on pre weighed Petri plates and were allowed to dry at 50˚C for 6 h and then at 40˚C overnight. The net dry weight of mycelia was then determined [13] . Determination of mycelial weight after application of oils determines lethal (weight of disc only before incubation), sub lethal (weight of disc plus 10% increase in original weight), and other percentage of growth inhibition for different concentrations. Clotrimazole was used as positive control at the same concentration as the tested oils.
3. Results and Discussion
3.1. Antifungal Activity of Essential Oils
The antifungal activities of essential oils of Mentha piperta, Cymbopogon citratus, Oreganum majoranum, and Thymus vulgaris against M. canis were determined by the disc diffusion and microdilution broth methods. Determination of the minimum inhibitory concentration of the essential oils is required to avoid the caustic effects of essential oils due to their lipophilic character [14] .
3.1.1. Screening Method
The disc diffusion method
Colony diameter of M. canis without applying oil was 50.3 mm in the petri plates (negative control) (Figure 1). As shown in Table 2, results revealed that the essential oils in the study had strong antifungal activity, because all oils in concentration of 40 μl/20 ml (0.2%) completely prevented the growth of tested clinical M. canis isolates. Moreover, our results revealed that M. piperta oil had the strongest antidermatophytic activity even at concentration of 15 μl/20 ml (0.075%), where it prevented the growth not entirely but with high reduction of colony diameter (5 mm), nearly reduced 90% of zone of growth as shown in (Figure 2), while at concentration of 20 μl/20 ml (0.1%) of C. citratus, O. magoranum, and T. vulgaris oils, the mycelium growth diameter were 10.7, 1.23, and 1.28 mm respectively.
The results also showed that the Mentha piperta oil at concentration of (0.1%) completely inhibited the growth of M. canis. These findings indicated that the required dose of the essential oils in this study to inhibit the growth of clinical M. canis isolates was very low and the dose of M. piperta oil was almost half of the other oils tested in this study. As in another studies, O. majoranum, C. citratus, and T. vulgaris were of the best broad-spectrum candidates for inhibition of food-borne pathogens and spoilage organisms. C. citratus and T. vulgaris were all clearly inhibitory towards the fungi [15] [16] , Mentha piperta oil shown high antifungal activity against clinical M. canis isolates.
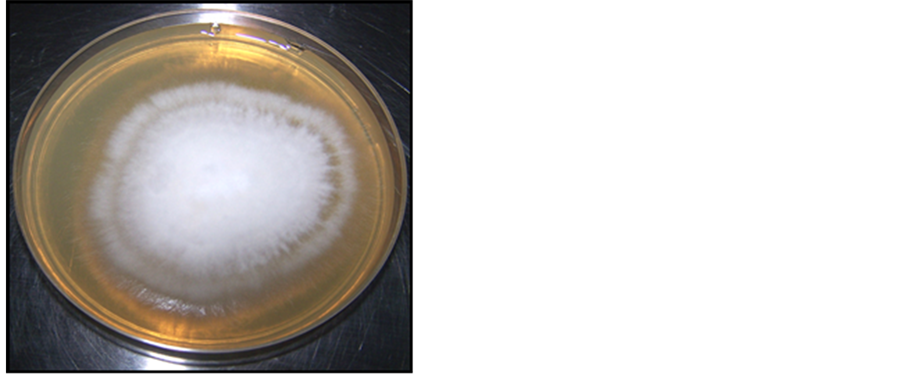
Figure 1. Colony diameter of eight day old (negative control).

Figure 2. Antifungal activity of M. piperta for M. canis 15 μl oil against M. canis.
Table 2. Antimicrobial activities of essential oils against identified M. canis growth inhibition zones (mm).
TI, total inhibition of fungal growth. Each experiment was repeated at least twice.
The present work revealed that the concentration of oil was the most important factor, positively correlated with growth inhibition of fungi. Maximum inhibition growth obtained during the 8th day. Clotrimazole used as a positive control was prepared at concentration of (0.075%) in all experiments. Table 3 showed that the high percentage of zone of inhibition of M. piperta oil at concentration of 0.075% produced 90% growth inhibition, while C. citratus at the same concentration produced 75.75% growth inhibition; moreover, O. magoranum and T. vulgaris produced 72.4% and 71.2% growth inhibition of tested organisms respectively.
The present work concluded that at only 0.1% concentration of the M. piperta oil, it produced 100% effect of growth inhibition, but in 0.2% concentration, all oils used in the study produced total inhibition 100%. Consequently, these results indicated the highly powerful of M. piperta as antidermatophytic agent among tested oils. Also all tested oils showed a significant antidermatophytic activity in comparison to Clotrimazole.
3.1.2. Minimum Inhibitory Concentration
Microdilution broth method
The antifungal activities of M. piperta, C. citratus, O. majoranum and T. vulgaris essential oils on M. canis were determined in Table 4 and Table 5 respectively, tables showed that M. piperta oil had stronger inhibitory effect than the other three oils (T. vulgaris, C. citratus, and O. majoranum oils) in concentrations of 1 μl, 5 μl, 10 μl, 15 μl, 20 μl, 40 μl per 30 ml broth media until 8th days of incubation. M. piperta oil at 5 μl/30 ml (0.0167%) reduced fungal growth to (22.21%) of colony weight while C. citratus, T. vulgaris and O. majoranum oils at the same concentration reduced fungal growth to 67.68%, 67.17%, 68.68% of colony weight respectively; thus, provided the highest degree of growth inhibition of M. canis.
While the total inhibition attached when 0.046% was used with M. piperta, the total inhibition obtained with the 0.133% of C. citratus oil, which is accounted as the second essential oils, also T. vulgaris and O. majoranum oils achieved reductions at 0.133%. As shown in Table 4, T. vulgaris appeared the least potent inhibitor, although both reduction values were higher than those achieved with Clotrimazole as positive control.
Table 3. Percentage of diameters of fungal inhibition zones of M. canis growth by essential oils.
TI, total inhibition of fungal growth. Each experiment was repeated at least twice.
Table 4. Colony weight of dermatophyte after applying different concentration of essential oils.
Weight of colony in mg and concentration of oil per µl.
Table 5. Colony weight percentage of dermatophyte after applying different concentration of essential oils.
The present work showed 0.066% of the oils/disc per Petri plate, M. piperta oil showed the strongest inhibitory effect, there are some reports stating that mentha species exhibit antifungal activity [17] . Terpinene, isomenthone, transcarveol, pipertitinone oxide, and caryophyllene isolated from M. piperita showed in vitro activity against Candida albicans and Escherichia coli [18] . Menthol has been reported to be responsible for antimicrobial property of M. piperita [19] . The essential oil of M. piperita possesses menthol and 1.8 cineole as main components, which also exhibited very good antifungal properties but lower than carvone [20] .
The present study used two growth controls, when the positive control showed adequate growth (8 days for M. canis), the initial results were recorded in relation to the growth present in the control flasks. Final results were recorded after 8 days.
3.1.3. Lethal and Sub Lethal Concentration
According to T-test, the differences among all oils themselves are significant except the difference between T. vulgaris and O. magoranum. However, all tested oils showed significant difference in comparison to the positive control, because oils contain different constituents having different antimicrobial activities.
The antifungal activities of the essential oils of M. piperta, C. citratus, T. vulgaris and O. magoranum, on the isolated M. canis were shown in Table 4 and Table 5. With M. piperta, colony growth was completely inhibited (0% wt. of growing colony) at concentration of 0.066% (the least concentration produced complete inhibition among studied oils). C. citratus oil at concentration of 0.066% reduced mycelium growth significantly (13% weight of growing colony), whereas total inhibition being achieved with 0.133% of oil concentration. T. vulgaris oil reduced fungal growth when used at 0.0167%, although the total inhibition was only achieved when 0.133% oil concentration was used.
The total inhibition was also obtained with the 0.133% concentration of O. majoranum oil (no increase of wt of disc), while the 0.0167% and 0.033% concentrations achieved lower reductions, although both reduction values were higher than those achieved with T. vulgaris oil. Only T. vulgaris and O. majoranum showed at the 0.0167% equal reduction of colony growth of M. canis. Table 5 showed that T. vulgaris oil appeared to has good antifungal activity started from concentration of 0.0167% even reached the MIC of T. vulgaris oil for isolated M. canis in concentration of 0.133%. MIC was defined as the lowest concentration, which did not result in any visible growth of the dermatophyte compared with the growth in the control plate [21] [22] . In agreement with earlier publications [23] , essential oil of T. vulgaris showed very strong antifungal activity at low concentrations of 0.05 - 1.0 μl/ml.
The next essential oil in order of its power to inhibit M. canis growth was C. citratus. However, T. vulgaris was the third oil in inhibition of growth while the poorest inhibitor was O. majoranum oil. Table 5 shows the values of percent growth reductions of M. piperta oil, with considerable reduction at concentration 10 μl. M. piperta oil was applied in concentration of 10 μl/30 ml (0.033%), 11 μl/30 ml (0.036%), 12 μl/30 ml (0.04%), 13 μl/30 ml (0.043%), 14 μl/30 ml (0.047%), 15 μl/30 ml (0.5%) and 16 μl/30 ml (0.053%), to determine accurately MIC of M. piperta oil. The weight of disc of freshly colony of clinical M. canis isolates was 198 mg before incubation in the control and the tested samples.
Table 6 clarified that (0.053%) was the minimum inhibitory concentration of M. piperta oil without increase in wt of disc (lethal concentration), and (0.047%) was the sub lethal concentration of oil with 10% increased in wt of disc.
Ketones, aldehydes, and alcohols showed activity but with differing specificity and levels of activity, which is in connection with the functional groups present but also associated with hydrogen-binding parameters in all cases. From our results, it can be seen that MICs is generally lower for all the essential oils and components investigated in disc diffusion method. The low water solubility of the oil and its components limit their diffusion through the agar medium. Only the more water-soluble components, such as carvone or 1.8-cineole diffuse into the agar. The hydrocarbon components either remain on the surface of the medium or evaporate that could be the reason for better results obtained by the broth dilution method [20] . Broth method, carried out in Erlenmeyer flasks, has the advantage of lower workloads for a larger number of replicates and the use of small volumes of the test substance and growth medium. In this method dilution of the oil is better and there is no agar in the medium, which both enable better diffusion through the liquid medium [23] .
Another research [24] reported that, the C. citratus oil reduced the colony development of T. harzianum, Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, Aspergillus alternata, Penicillium citrinum, and Curvularia lunata. Moreover, 0.13% of C. citratus oil employed revealed complete (100%) inhibition on the growth of clinical M. canis isolates. The antifungal activity of C. citratus oil believed to be associated with phytochemical components such as alkaloids, tannins, and cardiac glycoside. Essential oils and related substances act to make the cell membrane of the fungus permeable, causing leakage of the cytoplasmic constituents and consequently can kill the fungal cell [25] .
The present results confirm the antifungal activity of C. citratus oil and it was also superior to Clotrimazole as positive control in the same concentration. Geranial and citral isomers should probably account for the antifungal activity of C. citratus oil [26] .
Table 6. Mycelium weight of M. canis after applying different concentration of M. piperta oil.
O. majoranum oil has been reported to have some promising effect on rats infected with a human dermatophytic fungus, Trichophyton rubrum [2] . O. majoranum oil effectively inhibits in vitro growth of C. albicans. In addition, they have shown that O. majoranum oil directly inhibits germination and filament formation (the two phases required for tissue invasion) by C. albicans [27] .
These observations suggest that the physical and chemical properties (solubility and volatility) can have considerable effect on in vitro antimicrobial activity, but it must be pointed out that the intrinsic activity of a compound is of paramount importance for the evaluation of its efficacy. In this context, the essential oil containing aldehyde or phenol was reported to exhibit a high inhibitory effect, followed by those containing alcohol, ketone and ether [16] [28] . Hence, it is not surprising that T. vulgaris oil proved to be the overall best inhibitors because of phenolic thymol and eugenol as the major contributors to their bioactivity. Although thymol possesses moderate solubility and low volatility, their vapors have been reported to accumulate in great amounts into agar layers [28] .
However, more data will be necessary either to confirm this good in vitro efficacy or to explain the essential oils mechanisms of action. Further studies are required to study the effect and toxicity of these compounds in experimental animals (in vivo) and to establish if they could be safely used as antifungal agents against these fungi. Some essential oils are effective against Scop. brevicaulis and Fus. oxysporum which are often resistant to available antifungal agents, open important perspectives in alternative antifungal therapies.
The extensively used method is agar diffusion [29] -[31] and broth dilution [32] [33] . Unfortunately, results obtained by the different methods using the same oils do not always comparable [34] . Solubility and diffusion rates of the oil compounds in the aqueous agar media are of paramount importance, and can lead to misinterpretations in diffusion tests. Emulsifying agents (Tween 80) are added to ensure uniform mixing in dilution tests [35] .
Growth or absence of growth was monitored visually on the second and fourth day after inoculation. Colony diameter was measured on the seventh day and, if no growth had occurred, on day fourteen, the results considered negative. In vitro testing can help to determine the activities of new drugs and to find the right therapy. However, at present there are no in vitro reference methods for dermatophytes. Data on the use of the disc diffusion method for testing dermatophytes are limited [36] [37] . For example, in the case of itraconazole the influence of the culture medium was observed only for Microsporum canis and Trichophyton mentagrophytes [36] .
The free OH group of phenol and alcohol might be a key to the antimicrobial activity. It has been observed that the oil acts not only as a fungicidal agent but also inhibits sporulation of the pathogens [20] .
4. Conclusion and Recommendations
Some of the tested essential oils in this study were found to be effective against M. canis which are often resistant to available antifungal agents. This opens an important frontier in alternative antifungal therapies. The most effective essential oil as antidermatophytic agent against M. canis was the M. piperta oil with very low MIC and superior to synthetic fungicides such as Clotrimazole.
Further studies should be done to investigate the in vivo activity of the M. piperta oil as antidermatophytic agent (for treatment of dermatophytosis) due to its in vitro potent effect.
References
- Ullah, M., Khurram, M.; Amin, U.A., Afridi, H.H., Khan, F.A., Umar Khayam, S.M., Ullah, S. Najeeb, U., Hussain, J. and Asif Khan, M. (2011) Comparison of Phytochemical Constituents and Antimicrobial Activities of Mentha spicata from Four Northern Districts of Khyber Pakhtunkhwa. Journal of Applied Pharmaceutical Science, 1, 81-84.
- Adam, K., Sivropoulou, A., Kokkini, S., Lanaras, T. and Arsenakis, M. (1998) Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia and Salvia fruticosa Essential Oils against Human Pathogenic Fungi. Journal of Agricultural and Food Chemistry, 46, 1738-1745. http://dx.doi.org/10.1021/jf9708296
- Edeogo, H.O., Okwn, D.F. and Mbaebie, B.O. (2005) Phytochemical Constituents of Some Nigerian Medicinal Plants. African Journal of Biotechnology, 4, 685-688. http://dx.doi.org/10.5897/AJB2005.000-3127
- Bisset, N.G. and Wichl, M. (1994) Herbal Drugs and Phytopharmaceuticals. CRC Press, Boca Raton.
- Dobrowolska, A., Staczek, P., Kaszuba, A. and Kozłowska, M. (2004) PCR-RFLP Analysis of the Dermatophytes Isolated from Patients in Central Poland. Journal of Dermatological Science, 42, 71-74. http://dx.doi.org/10.1016/j.jdermsci.2006.01.001
- Gonzàlez, U., Seaton, T., Bergus, G., Torres, J.M. and Jacobson, J. (1993) Staphylococcal and Streptococcal Pyodermas. Seminars in Dermatology, 12, 331-335.
- Summerbell, R.C., Weitzman, I. and Padhye, A.A. (2007) Trichophyton, Microsporum, Epidermophyton and Agents of Superficial Mycoses. In: Murray, P.R., Baro, E.J., Jorgensen, J.H., Landry, M.L. and Pfaller, M.A., Eds., Manual of Clinical Microbiology, 9th Edition, ASM Press, Washington, 1874-1897.
- Gulcin, I., Kufrevioglu, O.I., Oktay, M. and Buyukokuroglu, M.E. (2004) Antioxidant, Antimicrobial, Antiulcer and Analgesic Activities of Nettle (Urtica dioica L.). Journal of Ethnopharmacology, 90, 205-215. http://dx.doi.org/10.1016/j.jep.2003.09.028
- Senthilkumar, C.S., Suresh Kumar, M. and Rajasekara Pandian, M. (2010) In Vitro Antibacterial Activity of Crude Leaf Extracts from Tecoma stans (L) Juss. et Kunth, Coleus Forskohlii and Pogostemon Patchouli against Human Pathogenic Bacteria. International Journal of PharmTech Research, 2, 438-442.
- Byika, W., Szaufe-Hajdrych, M., Matalawska, I. and Goslinka, O. (2004) Antimicrobial Activity of Isocytisoside and Extracts of Aquilegia vulgaris L. Letters in Applied Microbiology, 39, 93-97. http://dx.doi.org/10.1111/j.1472-765X.2004.01553.x
- Benkeblia, N. (2004) Antimicrobial Activity of Essential Oil Extracts of various Onions (Allium cepa) and Garlic (Allium satvum). LWT-Food Science and Technology, 37, 263-268. http://dx.doi.org/10.1016/j.lwt.2003.09.001
- Fraternale, D., Giamperi, L. and Ricci, D. (2003) Chemical Composition and Antifungal Activity of Essential Oil Obtained from in Vitro Plants of Thymus Mastichina L. Journal of Essential Oil Research, 15, 278-281. http://dx.doi.org/10.1080/10412905.2003.9712142
- Iraj, R.B., Mohammad, H.F., Davod, Y., Latif, G., Abdolamir, A. and Mohammad, B.R. (2008) Antimycotoxigenic Characteristics of Rosmarinus officinalis and Trachyspermum copticum L. Essential Oils. International Journal of Food Microbiology, 122, 135-139. http://dx.doi.org/10.1016/j.ijfoodmicro.2007.11.048
- Inouye, S., Tsuruoka, T., Watanabe, M., Takeo, K., Akao, M., Nishiyama, Y. and Yamaguchi, H. (2000) Inhibitory Effect of Essential Oils on Spicla Growth of Aspergillus fumigatus by Vapour Contact. Mycoses, 43, 17-23.
- Hammer, K.A., Carson, C.F. and Riley, T.V. (1999) Antimicrobial Activity of Essential Oils and Other Plant Extracts. Journal of Applied Microbiology, 86, 987-990. http://dx.doi.org/10.1046/j.1365-2672.1999.00780.x
- Dorman, H.J.D. and Deans, S.G. (2000) Antimicrobial Agents from Plants: Antibacterial Activity of Plant Volatile Oils. Journal of Applied Microbiology, 88, 308-316. http://dx.doi.org/10.1046/j.1365-2672.2000.00969.x
- Mimica-Dukić, N., Kujundžić, S., Soković, M. and Couladis, M. (2003) Essential Oil Composition and Antifungal Activity of Foeniculum vulgare Mill. Obtained by Different Distillation Conditions. Phytotherapy Research, 17, 368-371. http://dx.doi.org/10.1002/ptr.1159
- Yadegarinia, D., Gachkar, L., Rezaei, M.B., Taghizadeh, M., Astaneh, S.A. and Rasooli, I. (2006) Biochemical Activities of Iranian Mentha piperita L. and Myrtus communis L. Essential Oils. Phytochemistry, 67, 1249-1255. http://dx.doi.org/10.1016/j.phytochem.2006.04.025
- Işcan, G., Kirimer, N., Kürkcüoğlu, M., Başer, K.H.C. and Demirci, F. (2002) Antimicrobial Screening of Mentha piperita Essential Oils. Journal of Agricultural and Food Chemistry, 50, 3943-3946.
- Griffin, G.S., Markham, L.J. and Leach, N.D. (2000) An Agar Dilution Method for the Determination of the Minimum Inhibitory Concentration of Essential Oils. Journal of Essential Oil Research, 12, 149-255.
- Eloff, J.N. (2004) Quantification the Bioactivity of Plant Extracts during Screening and Bioassay Guided Fractionation. Phytomedicine, 11, 370-371. http://dx.doi.org/10.1078/0944711041495218
- Rosatoa, A., Vitalia, C., De Laurentisa, N., Armenisea, D. and Milillob, M.A. (2007) Antibacterial Effect of Some Essential Oils Administered Alone or in Combination with Norfloxacin. Phytomedicine, 14, 727-732. http://dx.doi.org/10.1016/j.phymed.2007.01.005
- Soković, M.D., Vukojević, J.M., Petar, P.D., Brkić, D., Vajs, V. and Van Griensven, L.J.L.D. (2009) Chemical Composition of Essential Oils of Thymus and Mentha Species and Their Antifungal Activities. Molecules, 14, 238-249.
- Mahanta, J.J, Chutia, M., BordoloiM., Pathak, M.G, Adhikary, R.K., Sarma, T.C. (2007) Cymbopogon citratus L. Essential Oil as a Potential Antifungal Agent against Key Weed Moulds of Pleurotus spp. Spawns. Flavor and Fragrance Journal, 22, 525-530.
- Piper, P., Calderon, C.O., Hatzixanthis, K. and Mollapour, M. (2001) Weak Acid Adaptation: The Stress Response That Confers Yeasts with Resistance to Organic Acid Food Preservatives. Microbiology, 147, 2635-2642.
- Abe, S., Sato,Y., Inoue, S., Ishibashi, H., Maruyama, N., Takizawa, T., Oshima, H., et al. (2003) Anti-Candida Albicans Activity of Essential Oils Including Lemongrass (Cymbopogon citratus) Oil and Its Component, Citral. Japanese Journal of Medical Mycology, 44, 285-291. http://dx.doi.org/10.3314/jjmm.44.285
- Manohar, V., Ingram, C., Gray, J., Talpur, N.A., Echard, B.W., Bagchi, D. and Preuss, H.G. (2001) Antifungal Activities of Origanum Oil against Candida albicans. Molecular and Cellular Biochemistry, 228, 111-117. http://dx.doi.org/10.1023/A:1013311632207
- Inouye, S., Takizawa, T. and Yamaguchi, H. (2001) Antibacterial Activity of Essential Oils and Their Major Constituents against Respiratory Tract Pathogens by Gaseous Contact. Journal of Antimicrobial Chemotherapy, 47, 565-573. http://dx.doi.org/10.1093/jac/47.5.565
- Zaika, L.L. (1988) Spices and Herbs: Their Antimicrobial Activity and Its Determination. Journal of Food Safety, 9, 97-118.
- Sivropoulou, A., Papanikolaou, E., Nikolaou, C., Kokkini, S., Lanaras, T. and Arsenakis, M. (1996) Antimicrobial and Cytotoxic Activities of Origanum Essential Oils. Journal of Agricultural and Food Chemistry, 44, 1202-1205.
- Mangena, T. and Muyima, N.Y.O. (1999) Comparative Evaluation of the Antimicrobial Activities of Essential Oils of Artemisia afra, Pteronia incana and Rosmarinus officinalis on Selected Bacteria and Yeast Strains. Letters in Applied Microbiology, 28, 291-296. http://dx.doi.org/10.1046/j.1365-2672.1999.00525.x
- Consentino, S., Tuberosa, C.I.G., Satta, M., Mascia, V., Arzedi, E., Palmas, F. and Pisano, B. (1999) In-Vitro Antimicrobial Activity and Chemical Composition of Sardinian Thymus Essential Oils. Letters in Applied Microbiology, 29, 130-135. http://dx.doi.org/10.1046/j.1472-765X.1999.00605.x
- Özcan, M. and Boyraz, N. (2000) Antifungal Properties of Some Herb Decoctions. European Food Research and Technology, 212, 86-88. http://dx.doi.org/10.1007/s002170000249
- Hili, P., Evans, C.S. and Veness, R.G. (1997) Antimicrobial Action of Essential Oils: The Effect of Dimethylsulphoxide on the Activity of Cinnamon Oil. Letters in Applied Microbiology, 24, 269-275. http://dx.doi.org/10.1046/j.1472-765X.1997.00073.x
- Smith, M.D. and Navilliat, P.L. (1997) A New Protocol for Antimicrobial Testing of Oils. Journal of Microbiological Methods, 28, 21-24. http://dx.doi.org/10.1016/S0167-7012(96)00958-X
- Fernández-Torres, B., Carrillo-Muñoz, A., Ortoneda, M., Pujol, I., Pastor, F.J. and Guarro, J. (2003) Interlaboratory Evaluation of the E. Test for Antifungal Susceptibility Testing of Dermatophytes. Medical Mycology, 41,125-130.
- Esteban, A., Abarca, M.L. and Cabañes, F.J. (2005) Comparison of Disk Diffusion Method and Broth Microdilution Method for Antifungal Susceptibility Testing of Dermatophytes. Medical Mycology, 43, 61-66. http://dx.doi.org/10.1080/13693780410001711972


