Materials Sciences and Applications
Vol.08 No.05(2017), Article ID:76503,13 pages
10.4236/msa.2017.85028
Alkyl Thiophene Vinylene Electropolimerization in C8mimPF6, Potential Use in Solar Cells
Vania Rojas1, Francisco Martínez1, Jean Christian Bernède2, Linda Cattin Guenadez2, Alexander Efimov3, Helge Lemmetyinen3
1Departamento de Ciencia de los Materiales, Facultad de Ciencias Físicas y Matemáticas, Universidad de Chile, Santiago, Chile
2Lamp, Facultè de Sciences et des Tecniques, Université de Nantes, Nantes, France
3Department of Chemistry and Bioengineering, Tampere University of Technology, Hervanta, Finland

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: February 8, 2017; Accepted: May 23, 2017; Published: May 26, 2017
ABSTRACT
We report the electrosynthesis of a novel semiconductor polymer based on alkyl vinylthiophene derivative in the presence of an ionic liquid (IL). The polymerization was performed under galvanostatic conditions and the polymer was studied as potential donor component of a multilayer heterojunction organic solar cell (OSC). The monomer used was (E)-1,2-di-(3-octyl-2- thienyl) vinylene (OTV) and the IL used for the electropolymerization was 1- octyl-3-methylimidazole hexafluorophosphate C8mimPF6. Optical properties, stability and morphology of the polymer were analyzed using FT-IR, UV-vis, Raman and XPS spectroscopy. Voltammetry analysis and scanning electron microscopy (SEM-EDX) were also performed on the polymer. The OSC assembled with the polymer of OTV was used as electro donor and C60 as acceptor. Molybdenum trioxide (MoO3) and bathocuproine (BCP) were used as buffer layer between anode and cathode respectively. I-V curves, in the dark and under AM 1.5 solar simulator were performed to measure its efficiency.
Keywords:
Conducting Polymer, Vinylene Thiophene, Ionic Liquids, Organic Solar Cells

1. Introduction
The excellent combination between the conductive properties of a metal added to the many advantages of plastics such as chemical inertness, processability, low cost and low density doped polymers give important applications in optoelectronic devices [1] including Field Effect Transistors (FET) [2] , Organic Thin Film Transistos (OTFT) [3] , Organic Light Emitting Diodes (OLED) [4] [5] and Organic Solar Cells (OCS) [6] .
The high global demands for energy supply as a result of population growth and industrialization coupled with the imminent depletion of fossil fuel reserves have caused severe damage to the ecosystem. This situation has led to a growing interest in promoting the use of renewable energy sources, environmentally benign alternatives, which allow a sustainable, decentralized and affordable energy supply, thus protecting the environment. The abundance of sunlight and high radiation in vast sectors of the planet can be used to produce electricity through photovoltaic systems, the best way to obtain clean energy in favor of a modern society highly dependent on electricity. In this area, the important technological advance has allowed the development of three types of photovoltaic cells: crystalline silicon [7] , organic solar cells (OSC) [8] and dye sensitized solar cells (DSSC) [9] . These two latest technologies are being considered as the most promising candidates for next generation solar cells because of their low cost, light weight, flexibility and mass production feasibility.
Within the vast range of existing monomer units to polymerize, this work has addressed the study with structures derived from vinylene thiophene. Its structure consists of a vinyl bond between thiophene rings, which can reduce the aromatic character of the ring, with a consequent increase in the delocalization of π electrons and the decrease in the rotational disorder between aromatic rings [10] . In short, vinylene thiophene structures provide the following structural characteristics: planarity, capacity charge delocalization by the presence of five conjugated double bonds and stability, fundamental in obtaining electroactive polymer. The incorporation of alkyl type groups in the β position of the thiophene ring was to improve the degree of solubility of polymers in organic solvents (THF, CHCl3, CH2Cl2, CH3OH) [11] .
In the mid-1970s, an alternative to replace volatile organic compounds (VOCs) was discovered to perform chemical synthesis and to develop environmentally friendly processes, ionic liquids (ILs). ILs are organic salts which are constituted of a cation and an anion, unlike the common salts have a low tendency to crystallize due to the asymmetric and voluminous structure of the cation [12] , e.g. 1-alkyl-3-methylimidazolium, N-Alkylpyridinium and tetraalkylammonium. The Plechkova and Seddon [13] , researchers have estimated 106 possible combinations of ILs, by the use of the cations and anions became known so far. Among the many advantages of ILs include its intrinsic properties such as low toxicity, non-flammability, high ionic conductivity, high thermal and chemical stability, and ability to dissolve the organic and inorganic compounds in a wide temperature range near room temperature [14] [15] . Use of ILs in the production of conducting polymers has been studied in aromatic systems such as polypyrrole [16] , polythiophene [17] , vinyl benzotriazole [18] and in sys- tems such as methylmethacrylate [19] .
Related vinyl polymerization studies of monomer units in ILs, have shown that occur via radical cation, yielding electroactive polymer material with high conductivity [20] . It is known that the chemical environment during the redox process affects conductivity, morphology, degree and rate of polymerization. In this regard, Woecht et al. [21] reported that ILs strongly influences the propagation rate coefficient depending on the type of monomer and IL used; suggesting that the origin of this phenomenon probably lies in the polarity of the IL.
We report here the electropolymerization of (E)-1,2-di-(3-octyl-2-thienyl) vinylene (OTV) in the IL 1-octyl-3-methylimidazole hexafluorophosphate C8mim- PF6.
2. Methods
2.1. Synthesis of Ionic Liquid
The IL C8mimPF6 was synthesized in one step without the use of solvents, according to the procedure described by Fang et al. [22] , which comprises reacting equimolar amounts of 1-methylimidazole (99%), 1-bromooctane (99%) and potassium hexafluorophosphate salt, KPF6 (98%); all reactive were purchased from Aldrich.
2.2. Synthesis of Monomer Unit (E)-1,2-di-(3-Octyl-2-Thienyl) Vinylene (OTV)
The monomer was synthesized as described by Martinez et al. [23] . First, was synthesized the Grignard reagent, octyl magnesium bromide (Figure 1(a)), a new C-C bond was then generated by Kumada coupling between the octyl group and the thiophene ring through the catalyst NiCl2 (dppp) (Figure 1(b)). Next, formylation, also known as Vilsmeier reaction, was formed in situ from DMF and POCl3 (Figure 1(c)). Finally, (E)-1,2-di-(3-octyl-2-thienyl) vinylene (OTV) was performed by the McMurry reaction using the Mukaiyama reagent (TiCl4/ Zn in THF) [24] [25] [26] (Figure 1).
Figure 1. Synthesis of (E)-1,2-di-(3-octyl-2-thienyl) vinylene (a) octyl magnesium bromide, (b) 3-octylthiophene, (c) 2-fomil-3-octylthiophene) and (d) (E)-1,2-di-(3-octyl-2-thienyl) vinylene.
2.3. Electropolimerization of OTV in IL
The polymer (POTV) was obtained by galvanostatic electrodeposition technique on three types of anode: Pt, ITO glass and PET with a work surface of 2 cm2; before using the electrodes, they were washed with H2O and then with acetone in ultrasonic bath. As counter electrode a graphite bar or Pt sheet was used. The electrochemical cell has two compartments separated by a glass frit, allowing both electrodes in a parallel arranging in each compartment. The polymerization was conducted at a current density of 2 mA/cm2 and at temperature of 30˚C, under nitrogen inert atmosphere. The IL is dried under vacuum at 75˚C prior to use. POTV was obtained from 0.4 mmol mixture IL/CH2Cl2 = 5/2 mL. The polymer was thoroughly washed with methanol, and then dried in vacuum oven at 70˚C for 6h.
2.4. Organic Solar Cell Construction
The cell is based on superposed multilayer structured on ITO-glass according to the following layer order: ITO/MoO3/POTV/C60/BCP/Al/Se (BCP: bathocuproine). Two types of cells were made, depending on the deposition techniques: spin coating and vacuum evaporation. By the technique of spin coating a polymer solution of 10 mg/mL was used. 50 uL of solution is deposited on the substrate (glass ITO/MoO3) at a speed of 1500 rpm for 30 seconds. The other components were deposited under vacuum at 10−4 Pa, the thin film deposition rate and thickness were estimated in situ on a quartz monitor: MoO3 (2 nm), POTV (Deposited by stin coating), C60 (40 nm), BCP (5 nm), Al (3.4 nm) and Se (10 nm). By the technique of vacuum deposition, in a vacuum chamber at 10-4 Pa was placed the ITO-glass substrate and then was deposited the following components, layer by layer: MoO3 (2 nm), POTV (10/20 nm), C60 (40 nm), BCP (5 nm), Al (3.4 nm) and Se (10 nm). The use of buffer layer (MoO3, BCP) is very important in these devices, because this layer produces good work function adjustment, surface defects passivation, and ITO surface smoothing [27] . The devices were measured under conditions of darkness and light (AM 1.5 solar simulator of 100 mW/cm2) through the ITO glass. I-V graphs were obtained which were performed at room temperature without an inert atmosphere.
2.5. Characterization Methods
NMR, IR and UV-Vis. confirmed precursor and monomer purities and structures. The ILs were characterized by NMR, IR, cyclic voltammetry and refractive index. The POTV was analyzed using mass spectroscopy, UV-vis, IR, XPS, Raman, morphological analysis SEM-EDX and differential pulse voltammetry (DPV).
3. Results and Discussion
3.1. Electrochemical Polymerization
Obtaining polymers using as solvent ILs was feasible without the need to add support electrolyte to the process. However, even having a structural similarity between the monomer unit and the cation of IL, i.e. both have a planar aromatic structure and side alkyl chain it was not possible to solubilize the monomer in pure IL. This situation may be associated with a faint solvation by the IL, due to the strong interactions between cation-anion of IL, being at a disadvantage the interactions cation-solute and anion-solute. Therefore CH2Cl2 was used only in the necessary amount to solubilize the monomer unit in the required volume of IL. In turn, this process reduces slightly the viscosity of IL.
While the electropolymerization was a quick process that after the first few seconds of was started the current path, the coating was observed at the anode, the polymers obtained on anode of ITO glass and ITO-PET had poor adhesion on the electrodes. Rather important part diffused into the solution and avoided the formation of homogeneous polymeric films on the anode surface, appearing as islands. The Table 1 summarizes the experimental conditions of the electropolymerization.
3.2. Spectroscopic Analice of POTV: Uv-vis, FTIR, Raman and XPS
The peaks absorption of UV-vis (Figure 2) was associated with the transitions of the aromatic rings π − π*. It was observed that there are no significant variations in the displacement of the bands by the use either platinum electrodes or ITO glass; the maximum peaks only differ in 7 nm.
Figure 3 shows the FTIR spectrum for POTV, which were measured through the attenuated total reflection (ATR). The blue spectrum corresponds to the polymer obtained on the electrode ITO-PET, while green spectrum corresponds to POTV obtained on Pt. The absorption bands in the range of 2900 - 2800 cm−1 are assigned to C-H stretching of alkyl chain (octyl group). To The 1557 cm−1 stretching vibration is assigned to C = C. The vibrations of the ring corresponding to C-C and C-H appear to 1268 and 1129 cm−1, respectively. P-F vibration binding of the counterion appears in 828 cm−1. Finally, C-H deformation (out of plane) corresponds to the thiophene ring in the 2,5-disubstituted α-α’ [17] link to 709 cm−1.
In the resonance Raman spectrum (Figure 4) 1601 cm−1 signal was assigned to the vinyl bond stretch between C = C. The signals at 1542 and 1421 cm−1 corresponds to the asymmetrical and symmetrical stretching of the C = C of the thiophene ring, respectively. Finally, vinyl bond C-H deformation was observed at 1298 cm−1 and C-C stretching at 1196 cm−1.
Table 1. Conditions of electropolimerization of OTV in C8mimPF6.
a. 4 electrodes of ITO-PET were submerged c/1h.
Figure 2. Absorption spectrum of POTV on Pt electrode and ITO glass doped with PF6 measured in CHCl3 at room temperature.
Figure 3. Infrared spectra of the polymers. Blue: POTV on ITO-PET; Red: POTV on Pt.
In the XPS spectrum, Figure 5, the elements present in the sample were: C1s, S2p, F1s, O1s y P2p [28] . The prominent mark was assigned to F1s located at 686.3 eV. This signal together with signal P2p clearly confirm the incorporation of the dopant anion and the polymer matrix [29] .
Figure 4. RAMAN spectrum of POTV on ITO-PET.
Figure 5. RAMAN spectrum of POTV on ITO-PET.
3.3. Morphological Analysis
The morphology of the different polymers was determined by SEM (Figure 6). The image for POTV on ITO-PET (picture on the left of Figure 6) shows a no- dular morphology, whose nuclei have the appearance of a cauliflower, coincident with those reported in literature [30] [31] . A similar situation was observed for POTV on Pt (picture on the right of Figure 6), in whose morphology it is possible to appreciate granular and coral-like growths. These small differences may be associated with the nucleation and growth type of the polymer in the electropolymerization process on the different substrates. Also can be attributed to the influence of possible links in positions α, β in the POTV. Observing morphology can be deduced that the process was determined by the type of nucleation in 3D [32] [33] .
From the EDX (Figure 6) the incorporation of the anion PF6− (part of IL) was confirmed in the polymer.
3.4. Voltammetric Analysis
Differential pulse voltammetry (DPV) (Figure 7) was performed in benzonitrile using TBAPF6 (0.1 M) as supporting electrolyte. A platinum wire as the counter electrode, the pseudo reference electrode Ag/AgCl and platinum as working electrode. Ferrocene was used as internal reference. From the graph were obtained the oxidation and reduction potential of the polymer. The band gap for
Figure 6. SEM images of POTV on ITO-PET (left) and on Pt (rigth). Graph of EDX for POTV on ITO-PET (down).
Figure 7. Differential Pulse Voltammetry (DPV) for POTV. It was performed in ben- zonitrile, using 0.1M TBAPF6 as carrier electrolyte. WE: Pt, pseudo RE: Ag/AgCl and CE: Pt wire; internal reference: ferroceno.
Table 2. Photovoltaic characterization of OSC, using different deposition techniques, under light AM 1.5 (100 mWcm−2) and in darkness.
the polymer was calculated from the HOMO-LUMO voltammetric measurements, giving values of 5.33eV and 3.43 for HOMO and LUMO respectively, thus the calculated band gap energy was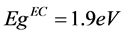 .
.
3.5. Bilayer/Multilayer Heterojunction Solar Cell
The cell was based on the following structure: ITO-glass/MoO3/POTV/C60/BCP/ Al/Se. Two techniques were employed to place the polymer on the substrate: spin coating and vacuum evaporation. For each cell a total of six I-V graphs were obtained, the average of its parameters allowed us to elucidate the capacity of the OSC. The Table 2 summarizes the values obtained from the I-V charts. Energy conversion efficiencies for cell whose polymeric material was deposited by spin-coating was 2.1 × 10−3% and by evaporation was 2.9 × 10−4%. The Figure 8 shows one of the most representative I-V graphs of each cell.
It can be observed that the best PCE values are achieved by spin-coating deposition, recording a value of 2.1 × 10−3%. A strong decrease of Rsh and Rs is observed in comparison to evaporation deposits, in the same way the values of Voc, Jsc and FF are better. On the other hand, evaporation leads to the breakdown of polymer chains, resulting in structures with less conjugation, a situation that is reflected in a slightly lower value of PCE of 2.9 × 10−4%. This contributes to an increase of the value Rs in the device which leads to a decrease in the performance of the device, and finally to a low value of the FF.


Figure 8. I-V curves for OSC with polymer deposit by (a) spin-coating and (b) evapo- ration.
4. Conclusion
The OTV monomer was possible to electrochemically polymerize by using an ionic liquid, C8mimPF6 as solvent. The polymer yields were dependant on the anode substrate and pair anode/cathode used in the electropolymerization. The ITO-glass and ITO-PET electrodes significantly reduce the electrodeposition of the monomer. Therefore, it can be said that there was a strong dependence on the nature of the anode surface and the amount and morphology of obtained polymer. From the DPV measurements, the polymer presented a good level HOMO/LUMO, however the bilayer heterojunction solar cells gave deficient energy conversion percentages <10−3, independent of the method by which the polymeric material was deposited, it would be possible to estimate other types of assembly between different substrates which could improve the efficiencies of OCS. Bulk heterojunction for instance, increases the contact surface between the donor and acceptor to interlockable networks. It might also be considered the use of different buffer layers.
Acknowledgements
The authors are grateful to the financial support from AKA-CONICYT-ERNC- 013 Project: “Bulk heterojunction (BHJ) solar cells based on thienylene oligomers” and Doctoral Fellowship CONICYT, Chile; Mobility Doctoral Scholarship, French Embassy in Chile.
Cite this paper
Rojas, V., Martínez, F., Bernède, J.C., Guenadez, L.C., Efimov, A. and Lemmetyinen, H. (2017) Alkyl Thiophene Vinylene Electropolimerization in C8mimPF6, Potential Use in Solar Cells. Materials Sciences and Applications, 8, 405- 417. https://doi.org/10.4236/msa.2017.85028
References
- 1. Galvin, M. (1997) Electrically Active Polymers and Their Application. Journal of the Minerals, Metals and Materials Society, 49, 52-55.
https://doi.org/10.1007/BF02914658 - 2. Horowitz, G., Garnier, F., Yassar, A., Hajlaoui, R. and Kouki, F. (1996) Field-Effect Transistor Made with a Sexithiophene Single Crystal. Advanced Materials, 8, 52-54.
https://doi.org/10.1002/adma.19960080109 - 3. Yan, H., Chen, Z., Zheng, Y., Newman, C., Quinn, J.R., Dotz, F., Kastler, M. and Facchetti, A. (2009) A High-Mobility Electron-Transporting Polymer for Printed Transistors. Nature, 457, 679-686.
https://doi.org/10.1038/nature07727 - 4. Braun, D. (2002) Semiconducting Polymer LEDs. Materials Today, 5, 32-39.
https://doi.org/10.1016/S1369-7021(02)00637-5 - 5. Chamorro, P. (2008) Fundamentos de la tecnología OLED. Digital, M., Ed., Publisher, Valladolid.
- 6. Bernède, J.C. (2008) Organic Photovoltaic Cells: History, Principle and Techniques. Journal of the Chilean Chemical Society, 53, 1549-1564.
https://doi.org/10.4067/S0717-97072008000300001 - 7. Saga, T. (2010) Advances in Crystalline Silicon Solar Cell Technology for Industrial Mass Production. NPG Asia Materials, 2, 96-102.
https://doi.org/10.1038/asiamat.2010.82 - 8. Gratzel, M. (2001) Photoelectrochemical Cells. Nature, 414, 338-344.
https://doi.org/10.1038/35104607 - 9. Ghann, W., Rahman, A., Rahman, A. and Uddin, J. (2016) Interaction of Sensitizing Dyes with Nanostructured TiO2 Film in Dye-Sensitized Solar Cells Using Terahertz Spectroscopy. Scientific Reports, 6, 30140.
https://doi.org/10.1038/srep30140 - 10. Neculqueo, G., Rojas Fuentes, V., López, A., Matute, R., Vásquez, S.O. and Martínez, F. (2012) Electronic Properties of Thienylene Vinylene Oligomers: Synthesis and Theoretical Study. Structural Chemistry, 23, 1751-1760.
https://doi.org/10.1007/s11224-012-9979-0 - 11. Xu, J.M., Chan, H.S.O., Ng, S.C. and Chung, T.S. (2002) Polymers Synthesized from (3-Alkylthio)thiophenes by the FeCl3 Oxidation Method. Synthetic Metals, 132, 63-69.
https://doi.org/10.1016/S0379-6779(02)00214-X - 12. Mallakpour, S. and Dinari, M. (2012) Ionic Liquids as Green Solvents: Progress and Prospects Green Solvents II. In: Mohammad, A. and Inamuddin, D., Eds., Springer, Netherlands, 1-32.
- 13. Plechkova, N.V. and Seddon, K.R. (2008) Applications of Ionic Liquids in the Chemical Industry. Chemical Society Reviews, 37, 123-150.
https://doi.org/10.1039/B006677J - 14. Welton, T. (1999) Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chemical Reviews, 99, 2071-2084.
https://doi.org/10.1021/cr980032t - 15. Zhao, J., Guo, W., Li, J., Chu, H. and Tu, Y. (2012) Study of the Electrochemically Generated Chemiluminescence of Reactive Oxygen Species on Indium Tin Oxide Glass. Electrochimica Acta, 61, 118-123.
https://doi.org/10.1016/j.electacta.2011.11.109 - 16. Ding, J., Zhou, D., Spinks, G., Wallace, G., Forsyth, S., Forsyth, M. and MacFarlane, D. (2003) Use of Ionic Liquids as Electrolytes in Electromechanical Actuator Systems Based on Inherently Conducting Polymers. Chemistry of Materials, 15, 2392-2398. https://doi.org/10.1021/cm020918k
- 17. Torres, S., Neculqueo, G. and Martínez, F. (2007) Morphological and Structural Characterization of Poly(3-decylthiophene) Prepared by Electropolymerization Using 1-Butyl-3-Methyl-Imidazoliumtetrafluorborate as Solvent. Journal of the Chilean Chemical Society, 52, 1235-1236.
https://doi.org/10.4067/S0717-97072007000300008 - 18. Zhou, Z., He, D.L., Yang, R.H., Guo, Y.N., Zhong, J.F. and Li, G.X. (2008) Electropolymerization of Benzotriazole in Room Temperature Ionic Liquid [bmim]PF-6. Journal of Applied Electrochemistry, 38, 1757-1761.
https://doi.org/10.1007/s10800-008-9627-y - 19. Jelicic, A., Beuermann, S. and García, N. (2009) Influence of Ionic Liquid Structure on the Propagation Kinetics of Methyl Methacrylate. Macromolecules, 42, 5062-5072.
https://doi.org/10.1021/ma900774e - 20. Noda, A. and Watanabe, M. (2000) Highly Conductive Polymer Electrolytes Prepared by In Situ Polymerization of Vinyl Monomers in Room Temperature Molten Salts. Electrochimica Acta, 45, 1265-1270.
https://doi.org/10.1016/S0013-4686(99)00330-8 - 21. Woecht, I., Schmidt-Naake, G., Beuermann, S., Buback, M. and García, N. (2008) Propagation Kinetics of Free-Radical Polymerizations in Ionic Liquids. Journal of Polymer Science Part A: Polymer Chemistry, 46, 1460-1469.
https://doi.org/10.1002/pola.22485 - 22. Fang, D., Cheng, J., Gong, K., Shi, Q.-R., Zhou, X.-L. and Liu, Z.-L. (2008) A Green and Novel Procedure for the Preparation of Ionic Liquid. Journal of Fluorine Chemistry, 129, 108-111.
https://doi.org/10.1016/j.jfluchem.2007.09.004 - 23. Martinez, F. and Neculqueo, G. (1999) Synthesis and Polymerization of 3-Octyl-substituted Thiophene, Bithiophene and Terthiophene. International Journal of Polymeric Materials and Polymeric Biomaterials, 44, 265-274.
https://doi.org/10.1080/00914039908009698 - 24. Cooke, A.W. and Wagener, K.B. (1991) An Investigation of Polymerization via Reductive Coupling of Carbonyls. Macromolecules, 24, 1404-1407.
https://doi.org/10.1021/ma00006a029 - 25. Ephritikhine, M. and Villiers, C. (2004) The McMurry Coupling and Related Reactions. In: Modern Carbonyl Olefination, Wiley-VCH Verlag GmbH & Co. KGaA, Hoboken, 223-285.
- 26. Takeda, T. and Tsubouchi, A. (2004) Carbonyl Olefination Utilizing Metal Carbene Complexes. In: Modern Carbonyl Olefination, Wiley-VCH Verlag GmbH & Co. KGaA, Hoboken, 151-199.
- 27. Dahou, F., Cattin, L., Garnier, J., Ouerfelli, J., Morsli, M., Louarn, G., Bouteville, A., Khellil, A. and Bernede, J. (2010) Influence of Anode Roughness and Buffer Layer Nature on Organic Solar Cells Performance. Thin Solid Films, 518, 6117.
https://doi.org/10.1016/j.tsf.2010.06.009 - 28. Nelson, A.J., Glenis, S. and Frank, A.J. (1987) XPS and UPS Investigation of PF6 Doped and Undoped Poly 3-Methyl Thiophene. Journal of Chemical Physics, 87, 5002.
https://doi.org/10.1063/1.452815 - 29. Ahmad, S., Deepa, M. and Singh, S. (2007) Electrochemical Synthesis and Surface Characterization of Poly(3,4-Ethylenedioxythiophene) Films Grown in an Ionic Liquid. Langmuir, 23, 11430-11433.
https://doi.org/10.1021/la702442c - 30. del Valle, M.A., Cury, P. and Schrebler, R. (2002) Solvent Effect on the Nucleation and Growth Mechanisms of Poly(thiophene). Electrochimica Acta, 48, 397-405.
https://doi.org/10.1016/S0013-4686(02)00685-0 - 31. Sekiguchi, K., Atobe, M. and Fuchigami, T. (2003) Electrooxidative Polymerization of Aromatic Compounds in 1-Ethyl-3-Methylimidazolium Trifluoromethanesulfonate Room-Temperature Ionic Liquid. Journal of Electroanalytical Chemistry, 557, 1-7. https://doi.org/10.1016/S0022-0728(03)00344-9
- 32. Schrebler, R., Grez, P., Cury, P., Veas, C., Merino, M., Gómez, H., Córdova, R. and del Valle, M.A. (1997) Nucleation and Growth Mechanisms of Poly(Thiophene) Part 1. Effect of Electrolyte and Monomer Concentration in Dichloromethane. Journal of Electroanalytical Chemistry, 430, 77-90.
https://doi.org/10.1016/S0022-0728(97)00109-5 - 33. Soto, J.P., Díaz, F.R., del Valle, M.A., Vélez, J.H. and East, G.A. (2008) Nucleation and Growth Mechanisms during Electropolymerization of Substituted 3-Alkylthiophenes. Applied Surface Science, 254, 3489-3496.
https://doi.org/10.1016/j.apsusc.2007.11.052











