Journal of Modern Physics
Vol.08 No.05(2017), Article ID:75832,5 pages
10.4236/jmp.2017.85052
On Nernst’s Theorem and Compressibilities
James R. McNabb III1, Shigeji Fujita1, Akira Suzuki2
1Department of Physics, University at Buffalo, SUNY, Buffalo, USA
2Department of Physics, Tokyo University of Science, Tokyo, Japan

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: March 14, 2017; Accepted: April 25, 2017; Published: April 30, 2017
ABSTRACT
The unattainability of the absolute zero of temperature is proved by using Carnot’s theorem. Hence this unattainability is distinct from the Planck-Fer- mi statement of the Third Law of Thermodynamics that the entropy vanishes at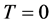 . It is shown that the isothermal compressibility
. It is shown that the isothermal compressibility 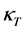 is in general larger than the adiabatic compressibility
is in general larger than the adiabatic compressibility 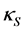 and the difference
and the difference 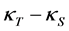 vanishes in the low temperature limit.
vanishes in the low temperature limit.
Keywords:
Nernst’s Theorem, Carnot’s Theorem, Adiabatic Compressibility, Isothermal Compressibility: The Third Law of Thermodynamics

1. Introduction
Fermi in his book [1] stated for the Third Law that the entropy S of any system approaches zero in the zero temperature limit:
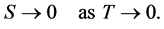 (1)
(1)
This form was proposed earlier by Planck, and will be called Planck-Fermi’s statement. Reif in his book [2] took a view that thermodynamics and statistical mechanics should be studied jointly by introducing Boltzmann’s connection between the entropy S and the number of microstates 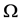 compatible with a set of macroscopic descriptors E, V, and N:
compatible with a set of macroscopic descriptors E, V, and N:
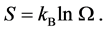 (2)
(2)
Nernst’s theorem (the third law) was expressed as
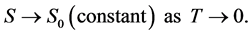 (3)
(3)
The difference between the two statements is due to the zero point motion arising from the Heisenberg’s uncertainty principle. Neither disorder nor dissipation can be generated by the zero-point motion. Quantum statistics will play a roll.
Pauli, in his book [3] , showed that the unattainability of absolute zero can be derived from Planck-Fermi’s statement by considering Carnot’s cycles. We shall show in the present work that the unattainability can be derived by using Carnot’s theorem (the second law).
The heat absorbed by a body is denoted by Q. The heat capacity C is defined by
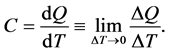 (4)
(4)
This molar heat at constant volume, 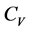 , and that at constant pressure,
, and that at constant pressure, 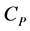 , are defined by
, are defined by
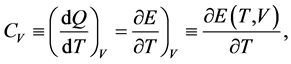 (5)
(5)
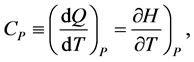 (6)
(6)
where E and 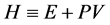 are the internal energy and the enthalpy, respectively. We assume that all thermodynamic functions for a one-component system are analytic within each domain of the gas, liquid and solid (phases). The thermodynamic functions are singular on the phase boundary. The body temperature should rise when heat is supplied. Hence the heat capacity should be non-negative. The body volume should become smaller when a pressure is applied from outside. Hence the compressibility should be positive.
are the internal energy and the enthalpy, respectively. We assume that all thermodynamic functions for a one-component system are analytic within each domain of the gas, liquid and solid (phases). The thermodynamic functions are singular on the phase boundary. The body temperature should rise when heat is supplied. Hence the heat capacity should be non-negative. The body volume should become smaller when a pressure is applied from outside. Hence the compressibility should be positive.
Pauli showed in his book [3] that
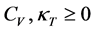 (7)
(7)
by using the increasing entropy principle. The difference between 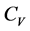 and
and 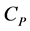 is given by
is given by
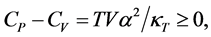 (8)
(8)
where

is the coefficient of thermal expansion and

is the isothermal compressibility.
We see from Equation (8),

The adiabatic compressibility 

Both 


We shall show newly in Section 3 that

2. The Unattainnability of the Absolute Zero
Let us consider a gas. In the Carnot cycle shown in Figure 1 the heat 

The efficiency of the Carnot’s engine is

where W is the work produced. According to Carnot’s theorem, no engine working between two temperatures 




In the Carnot cycle operated in the reverse direction an amount of heat 


This expression indicates that the work W needed to extract a fixed amount of heat 



Figure 1. The Carnot (ideal) cycle: P-V Diagram and a schematic of a Carnot engine operating between hot and cold reservoirs at temperatures 

processes which add an extra heat to the right-hand side of Equation (16).
3. Isothermal and Adiabatic Compressibilities Approach Each Other in the Low Temperature Limit
After straightforward calculations which are outlined in Appendix, we obtain

Using this we obtain

The last inequality follows from inequalities in Equation (8). Hence the iso- thermal compressibility 


Cite this paper
McNabb III, J.R., Fujita, S. and Suzuki, A. (2017) On Nernst’s Theorem and Compressibilities. Journal of Modern Physics, 8, 839-843. https://doi.org/10.4236/jmp.2017.85052
References
Appendix: Derivation of Equation (17)
Pressure P, volume V and temperature T are interrelated by the equation of state. If the variables 

From this we obtain


Using Equations (12) and (22), we obtain

If we regard S as a function of 




Similarly, we obtain

Using Equations (10), (23)-(25), we obtain

Dividing this by 


