Advances in Bioscience and Biotechnology
Vol.5 No.4(2014), Article ID:43666,7 pages DOI:10.4236/abb.2014.54041
Rapid, Efficient and High-Performance Protocol for Agrobacterium rhizogenes-Mediated Hairy Root Transformation of the Common Bean Phaseolus vulgaris
Sanghamitra Khandual1*, Pallavolu Maheswara Reddy2
1Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ), Guadalajara, México
2The Energy and Resources Institute, New Delhi, India
Email: *khandual@yahoo.com, Pallavolu.Reddy@teri.res.in
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 9 January 2014; revised 13 February 2014; accepted 4 March 2014
ABSTRACT
A rapid, efficient and high-performance transformation protocol employing Agrobacterium rhizogenes was developed for the common bean Phaseolus vulgaris. In this study, we examined competencies of various protocols to induce and explants that respond to hairy root transformation in bean plants. Utilizing young seedlings with severed radicles/hypocotyls, we developed a highly efficient procedure for achieving hairy root transformation frequencies as high as 100% as visualized by GUS reporter gene expression system. Transgenic hairy roots in these young composite plants were susceptible to nodulation by rhizobia, and form an excellent system for high throughput genomic analysis to study root biology and endosymbiosis in common bean.
Keywords:Phaseolus vulgaris; Agrobacterium rhizogenes; Hairy Root Transformation; GUS Expression

1. Introduction
Leguminous plants are capable of entering into symbiotic association with soil bacteria commonly known as rhizobia. A molecular dialogue between the legume host and its bacterial partner leads to the formation specialized plant organ, the nodule, in which rhizobia reside and carry out nitrogen fixation. Two legumes, Lotus japonicus and Medicago truncatula, have been extensively used as model plants to advance our understanding of the molecular dialogue between plants and rhizobia, and the signaling pathway that mediates the development of symbiosis.
Common bean, Phaseolus vulgaris, is a very important crop legume, which originated in Latin America. This crop plant is widely cultivated throughout the tropical regions of the world, including south Asia, as it is the most consumed grain legume by the humankind. It is a source of more than 50% of dietary protein for poor people living in Latin America, Africa and Asia [1] . P. vulgaris is a diploid plant with relatively a small genome (650 Mb), and hence forms an excellent model plant among crop legumes to study nodulation and nitrogen fixation. Yet it has lagged behind the model legumes L. japonicus and M. truncatula in becoming an ideal system to study root symbiosis because of its recalcitrance to genetic transformation. This lack of appropriate genetic transformation technology formed a greatest impediment to the functional analysis of genes in bean. To our knowledge, thus far, the only method for obtaining P. vulgaris transgenic plants, albeit with a very low efficiency, has been reported by Aragão et al. [2] . However, Estrada-Navarrete et al. [3] developed an Agrobacterium rhizogenes-mediated hairy root transformation protocol for common bean, and this paved way to further molecular studies on nodule development in this plant species. Thus far, this is the most widely used hairy root transformation protocol for bean. However, the efficiency of hairy root transformation by this method is highly variable, which ranged between 20% and 80%. Hence, the present investigation was undertaken to develop a simple, rapid and high performance alternative technique to achieve 100% hairy root transformation efficiencies in bean. For achieving this, we examined the effectiveness of various protocols, explants and introduced appropriate amendments to boost hairy root transformation efficiencies to 100%, thus making it a suitable system for high-throughput functional genomic studies in bean.
2. Materials and General Methods
2.1. Development of Agrobacterium rhizogenes Strains Carrying Binary Vectors and Inoculum Preparation
Binary vectors used for bean transformation, pBI101.1 (control vector; Clontech), pBI101-35S:GUS or pIG121 (containing 35S:IntGUS; [4] ) were introduced into Agrobacterium rhizogenes K599 by electroporation [5] . A. rhizogenes strains harboring the transformation vectors were grown at 30˚C in Luria-Bertani (LB) plates supplemented with 50 µg·ml−1 kanamycin. The bacterial cells from a culture grown overnight from a single plate were harvested and re-suspended in 5 ml sterile distilled water (OD600—0.3), and used for infecting bean seedlings/ cotyledons for the induction of genetically transformed hairy roots employing different protocols (see below).
2.2. Surface Sterilization of Bean Seeds and Germination
All operations for surface sterilization of seeds were performed at room temperature. Bean (Phaseolus vulgaris var. Negro jamapa) seeds were washed with sterile distilled water and immersed in 70% ethanol for 1 - 2 min. Subsequently, the seeds were immediately washed with sterile distilled water (3 × 10 min) and incubated in 10% (v/v) commercial bleach (sodium hypochlorite) for 10 min. Following this treatment, the seeds were washed repeatedly in excess amounts of sterile distilled water for 6 - 8 h on a shaker before seeding them in petri dishes, magenta boxes or steel trays containing filter paper/paper towels moistened with sterile distilled water and germinated in a plant growth incubator at 28˚C. With this method, more than 90% of the seeds germinated. The 3-day to 8-day seedlings devoid of any contamination was used in the experiments.
2.3. Histochemical Visualization of GUS Expression and Microscopy
GUS staining was essentially the same as that described by Reddy et al. [6] . Briefly, hairy roots were excised from the transformed plants, washed twice with 0.1 M potassium phosphate buffer (pH 7.0), immersed in the GUS substrate solution containing 1 mM X-gluc (5-bromo-4-chloro-3-indolyl glucuronide, sodium salt; Biosynth AG, Switzerland), 10 mM EDTA, 0.1% Triton X-100, 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide, and 100 mM potassium phosphate buffer (pH 7.0), and incubated in the dark at 30˚C for 12 - 24 h. Subsequently, stained tissues were rinsed in phosphate buffer, fixed for more than 4 h in a solution containing 3.7% formaldehyde, 5% acetic acid, and 50% ethanol, examined as whole specimens, and photographed under a stereomicroscope (Carl Zeiss Stemi 2000-C, Germany).
3. Results
In this study we have used different types of plant explants, employed various methods and introduced several modifications in order to develop a rapid and high performance protocol for hairy root transformation in common bean, P. vulgaris.
3.1. Hairy Root Transformation in P. vulgaris Using Rock Wool Support
This protocol is essentially based on the method developed by Collier et al. [7] , with minor modifications to suite bean plants. Bean seeds were surface sterilized and transferred to trays containing a wet paper towel. Trays were covered with aluminum foil and held at 28˚C until seed germination (approximately 3 days). Then the sprouts were transferred to small plastic pots containing sterile vermiculite, irrigated with Summerfield nutrient solution [8] and grown in a greenhouse maintained at 28˚C under normal light for 3 weeks. The apical stem portions (twigs) needed for the induction of hairy roots was derived by excising approximately 3 cm long stem sections. Subsequently, the apical meristems were severed out and discarded, leaving one or two auxiliary buds in each stem section. The twigs thus generated were utilized as explants for the induction of composite plants from these twigs.
Supporting medium/material for growing the twigs was prepared out of rock wool (Grodan, Denmark; http://www.rockwool.com). Rockwool plugs were cut into sections of approximately 3 cm3 cubes, a hole was poked on top of each plug (approximately 3/4th of the thickness of the plug) with a pipette tip, and autoclaved. Two plug sections were placed into a Petri dish bottom and one apical stem section, prepared as described above, was inserted into the hole of each rock wool plug (Figure 1). Subsequently, 5 ml of re-suspended A. rhizogenes culture carrying pBI101.1 or pIG121 (prepared as above) was inoculated carefully with a pipette tip on to the cut end of the twig in each plug placed in open petri dishes within plant growth trays, covered with plastic domes and incubated in an incubator at 28˚C in dark. After 24 h the trays were opened and the stem sections were allowed to dry for a few hours, until the leaves are not turgid. Later, the plugs were saturated with Summerfield.
Nutrient solution, covered with clear plastic domes and returned to the growth room maintained at 28˚C and 14/10 h light/dark cycle. Plugs were checked periodically and watered when necessary for the remainder of the induction period.
Approximately two weeks later, the first hairy roots emerged, and within another 15 - 20 d a composite plant with fully developed root system was established (Figures 1(a) and (a’)). Under N-free nutrient medium regime, these composite plants, when inoculated with Rhizobium tropici CIAT899, showed normal nodulation (Figures 1(a) and (a’)). With this optimized protocol [9] , modified from Collier et al. [7] , even though approximately 70% of explants produced transgenic roots, with an average of 2 - 4 transgenic roots per composite plant, only about 30% of emerging roots showed GUS expression (Figure 1(b), Table 1). Another major drawback of this
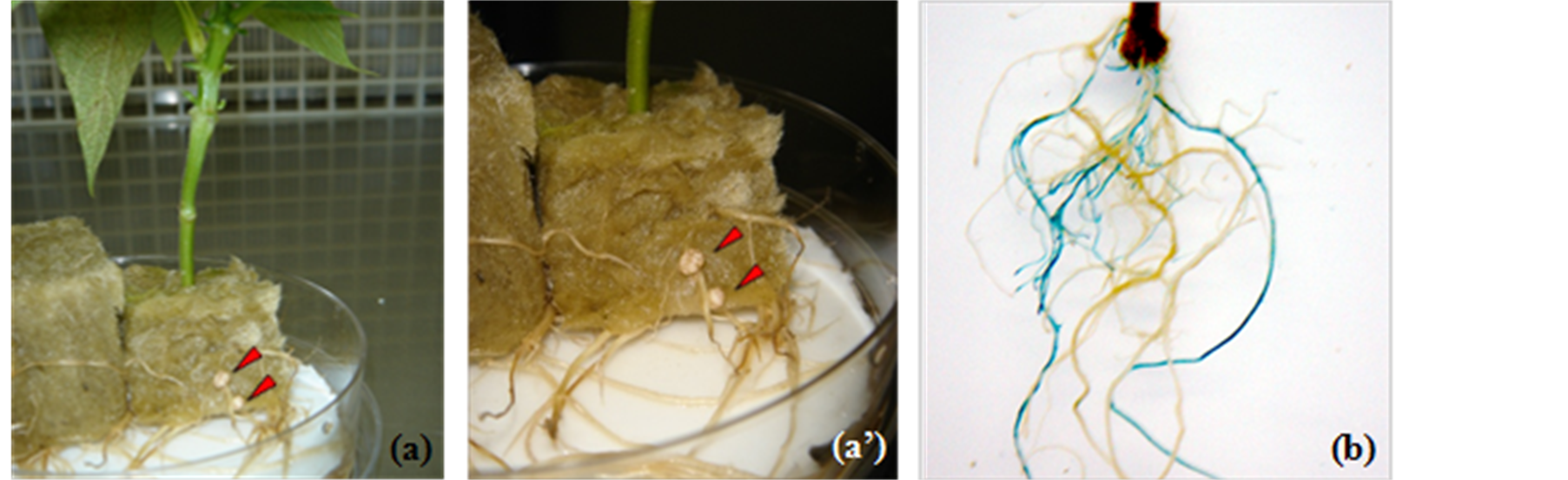
Figure 1. A. rhizogenes-mediated hairy root transformation of the twigs of P. vulgaris inserted in rockwool plugs. (a) Nodulating hairy roots; (a’) Enlarged picture of (a); (b) Transgenic hairy roots expressing GUS. Note that only some roots are stained blue, indicating that only a limited number of the roots are transformed with 35S-IntgusA gene.
Table 1. Efficiency of various methods/types of explants used in promoting hairy root transformation of the common bean Phaseolus vulgaris plants.
protocol is that due to high humid conditions, fungal contamination frequently occurred on the rock wool plugs.
3.2. Hairy Root Transformation in P. vulgaris through Cotyledonary Node Injection
This procedure is fundamentally the one developed by Estrada-Navarrete et al. [3] , with slight variations [10] . Seeds of P. vulgaris were surface sterilized and transferred small plastic pots containing sterile vermiculite impregnated with Summerfield nutrient solution [8] supplemented with 5 mM KNO3, covered with clear plastic bags, and incubated in a growth room (28˚C; 14/10 h light/dark regime).
Five days after planting, plantlets with newly unfolded cotyledons were infected by injection directly into the cotyledonary nodes (4 - 5 times at different positions around the node) with a syringe equipped with 21 G needle delivering approximately 10 - 20 μl of the suspension of A. rhizogenes K599 cells transformed with pBI101.1 or pBI101-35S:GUS vectors (prepared as described above) into the wound. After injection, the plants were securely covered with clear plastic bags (to maintain high internal humidity) and returned to the growth room. Normally, bean plants infected by A. rhizogenes started to show tumors approximately a week after inoculation (Figure 2(a)). Ten to twelve days after infection, plantlets showing profuse hairy root differentiation at the site of infection were selected, primary root removed by severing approximately 1 cm below the cotyledonary node and replanted in fresh pots containing sterile vermiculite. Immediately after transferring to new pots, each of the A. rhizogenes-transformed composite plants were inoculated with 1 - 2 ml R. tropici CIAT899 culture (rhizobial inoculum was prepared by growing CIAT899 strain to a density of 106 cells·ml−1 in peptone-yeast extract (PY) medium supplemented with nalidixic acid, 20 μg·ml−1), and irrigated with Summerfield nutrient solution [8] containing a minimal level (0.385 mM) of KNO3. Subsequent to this, the plants were covered with clear plastic bags to maintain humid conditions, and returned to the plant growth room. After 4 - 5 d of incubation, the plastic bags were perforated to enable the gradual acclimation of transformed plants to the ambient environment for a few days, before they were transferred to the greenhouse maintained at 25˚C. The plants were harvested 3 - 4 weeks after rhizobia inoculation, and roots were analyzed for GUS activity.
With this protocol, even though about 70% of plants produced hairy roots, the transgenic roots expressing GUS ranged generally between 20% - 60% per plant (Figure 2(b), Table 1). In spite of several trials, we could never achieve more than 70% transformation efficiency.
3.3. Hairy Root Transformation of Radicle Severed, Cotyledon-Bearing Common Bean (Miniature) Seedling/Plumule Explants by A. rhizogenes
Surface sterilized P. vulgaris seeds were germinated in dark on moistened paper towels in a try under sterile conditions in an incubator maintained at 28˚C. At day 3 after germination, when the seedlings were just emerging, seed coats were imbibed by spraying sterile water and carefully removed without injuring the cotyledons. Subsequently, seedlings having a radicle length of about 1 cm were aseptically transferred under the laminar flow hood, to a sterile glass Petri dish containing water to avoid desiccation, and the hypocotyl was severed, with a horizontal cut, precisely at the lower side of the cotyledonary node (shown by an arrow in Figure 3(a)) with a sterile scalpel holding the root tip with the forceps. After removing the radicle the seedling was held by cotyledons and the sectioned surface (at the node) was coated with A. rhizogenes (transformed with pBI101.1 or pIG121 vectors) cells freshly scrapped from the surface of the culture plate, with the help of a sterile scalpel. The plumule explant with intact cotyledons was then transferred to a magenta box, with sterile Whatman number 1 filter paper with nutrient medium to form a moist surface. The plants were incubated in dark at 25˚C for 3 d. Subsequently, excess agrobacterial growth was avoided with washings with antibiotic (kanamycin, 50
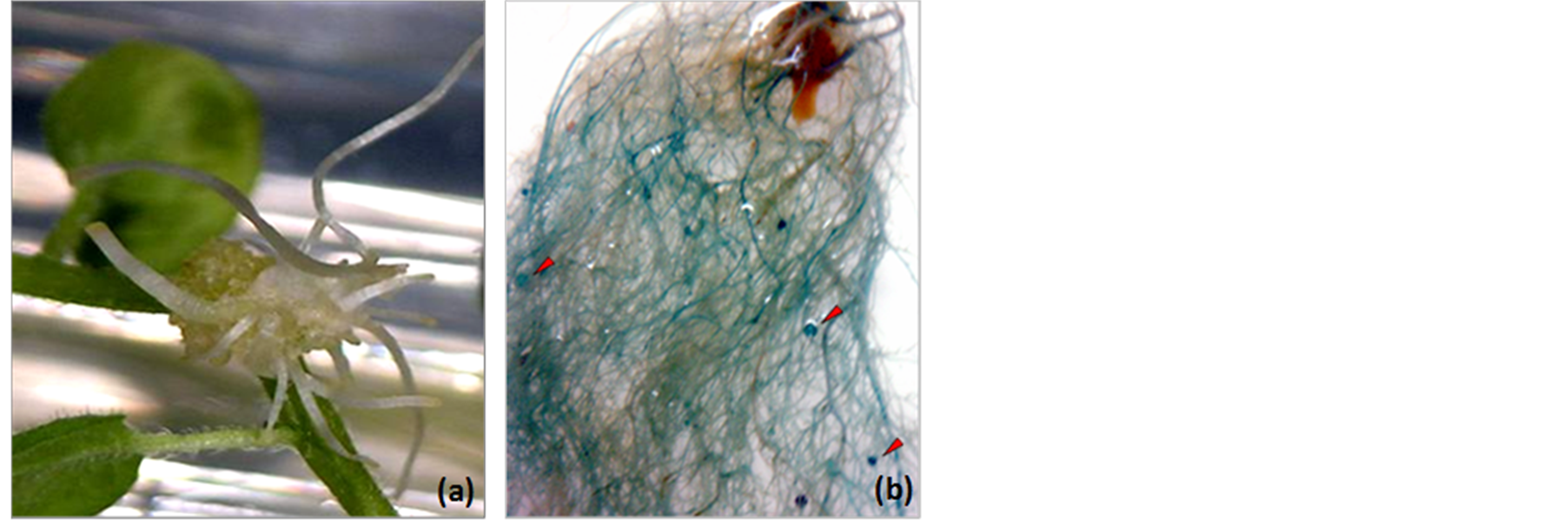
Figure 2. A. rhizogenes-mediated hairy root transformation through infection by injection at the cotyledonary nodes in the seedlings of P. vulgaris. (a) Development of callus and emergence of hairy roots at the infected site; (b) Transgenic hairy roots and nodules (arrowheads) showing GUS expression. Note that several roots are not stained blue indicating that they are not transformed with 35S-gusA gene.
µg·ml−1) solution, and the seedlings were transferred to fresh magenta boxes containing filter paper impregnated with the nutrient solution supplemented with the kanamycin, within a laminar hood. The infected seedlings were allowed to grow in the incubator maintained at a temperature of 28˚C with 14 h light/10 h dark regime for the development of hairy roots (Figure 3(b)).
With this method transformed roots were rapidly induced, normally within 8 - 10 days after infection, and proliferated profusely. The plants were harvested 2 weeks after rhizobia inoculation, and roots were analyzed for GUS activity. With this protocol, 100% of plants produced transgenic roots with 100% of the transgenic roots expressing GUS (Figure 3(c), Table 1). The same protocol was also tested on soybean seedlings with 100% transformation efficiencies (Figures 3(d) and (e)).
Comparison of hairy root transformation methods using various plant parts, the radicle severed (miniature), cotyledon-bearing plumule explants gave 100% transformation efficiency. With this method, composite plants showed good growth with a high degree of root proliferation. This is a less laborious and least time consuming (rapid) method, which results in highest transformation efficiency. The “miniature” plants are also easy to handle/maintain under sterile conditions, and suitable for the labs where space is a constraint. The composite plants generated using this method can also be transferred and grown in vermiculite, and can be used for nodulation studies.
3.4. Rapid Development of Axenic Hairy Root Cultures from Bean Cotyledons
During the course of our studies, we have also developed an easy and rapid protocol for generating hairy root cultures from isolated cotyledons. This procedure is essentially based on the protocol used for soybean by Subramanian et al. [11] , with minor modifications. Briefly, surface sterilized common bean seeds were germinated on moistened paper towels in steel trays under sterile conditions as described above. On day 3, when the seedlings were just emerging, seed coats were removed after moistening them by spraying sterile distilled water, and the seedlings were allowed to grow for further 3 days. Cotyledons were harvested from the seedlings at day 6 by gently twisting them off the hypocotyls. It is very important that the cotyledons be at the correct developmental stage, neither very young and dense nor old and flaccid. When bent, the ideal cotyledons bow only marginally before breaking into two. Individual cotyledons were surface sterilized again by wiping with an alcohol swab soaked in 70% ethanol. The alcohol swab was rung out slightly before use, so that it was wet but not dripping. The surface sterilized cotyledon was briefly dried, then etched by making shallow cuts/incisions about 2 - 6 mm from the petiole end of the cotyledon. Cuts were made with a razor blade through a 10 - 20 µl droplet of A. rhizogenes K599 (carrying the control vector or pIG121) inoculum such that the bacteria were directly introduced into the wound site. The infected cotyledons were then transferred on to a nutrient solution-impregnated what man number 1 filter paper placed in a Petri plate and covered with a top lid having a layer of filter paper moistened with nutrient solution (i.e., above the cotyledons) to maintain humid conditions. The Petri dishes containing
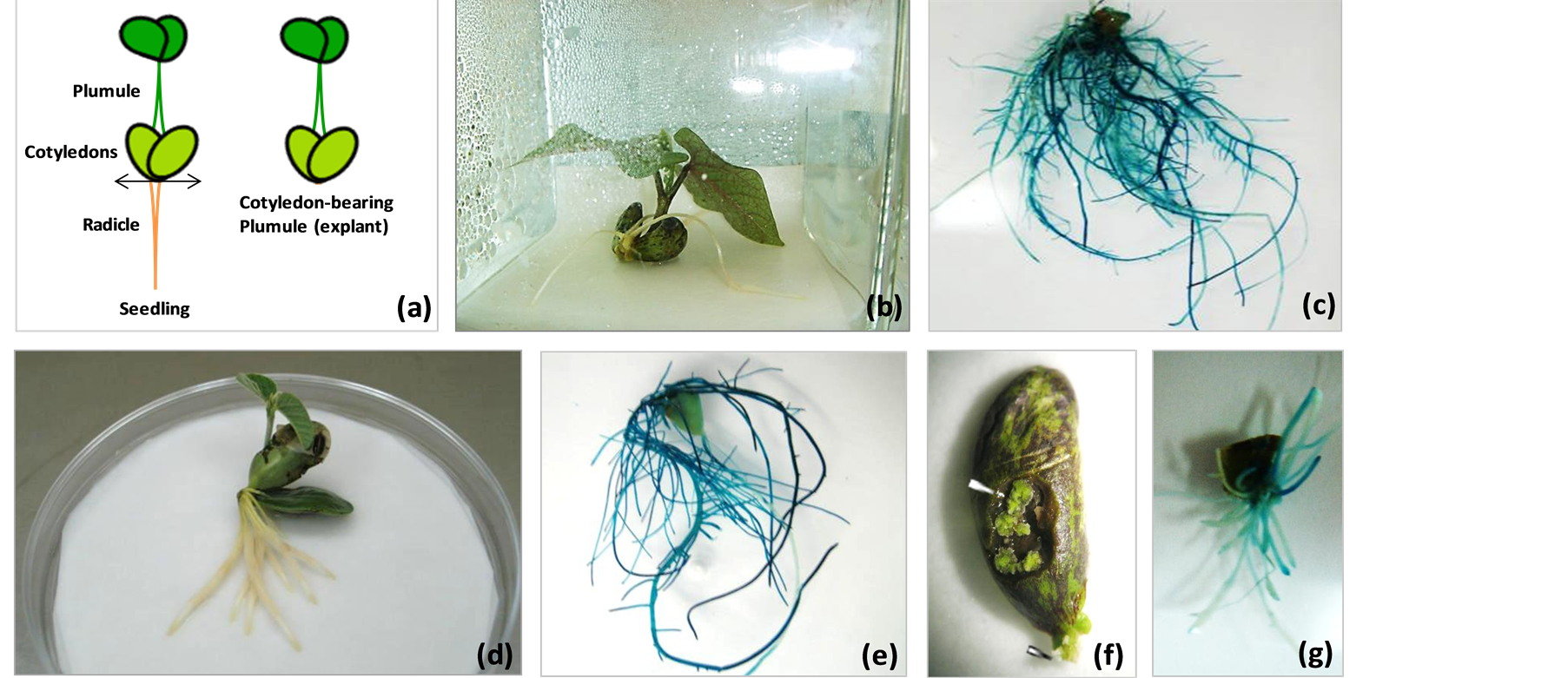
Figure 3. A. rhizogenes-mediated hairy root transformation in the radicle severed, cotyledon-bearing (b) and (c) common bean and (d) and (e) soybean seedling/plumule explants, and (f) and (g) generation of hairy roots from cotyledonary explants. (a) Schematic representation of the bean/ soybean seedling showing the site of cut (arrow, left), and the plumule explant used for transformation (right); (b) Bean explant with emerging hairy roots at the cut site; (c) Hairy roots from bean showing GUS expression; (d) Soybean explant showing hairy root emergence at the cut site; (e) Soybean hairy roots expressing GUS; (f) Bean cotyledonary explant exhibiting calli formation on the surface and initiation of hairy root differentiation (arrowheads, root primordia); (g) Hairy roots developed from the cotyledons showing GUS expression. Note that 100% of the hairy roots that emerged from cotyledon-bearing plumule or cotyledonary explants are expressing GUS, demonstrating that 35S-IntgusA is transferred with extremely high efficiency in these explants.
the infected cotyledons were incubated at 25˚C in dark. After 3 days of infection, the cotyledons were rinsed with kanamycin (50 µg·ml−1) solution to wash off bacterial cells, and then transferred to a new Petri dish with filter papers moistened with nutrient solution (as described above) supplemented with kanamycin (50 µg·ml−1). The plates were incubated in a culture room maintained at a temperature of 28˚C and 14 h/10 h light/dark cycle, and examined for hairy root formation at regular intervals.
With the above described method, profuse calli developed at the sites of infection (incisions) in cotyledons within 7 - 8 d (Figure 3(f)), and hairy roots started appearing by 12 - 15 d after infection. Histochemical staining of the hairy roots by x-glu revealed that 100% of the hairy roots generated from cotyledons were positive for GUS (Figure 3(g)), indicating very high transformation efficiency. The isolated transgenic hairy roots could be grown and maintained for extended periods on hormone-free MS salts medium supplemented with vitamins.
4. Discussion
The common bean P. vulgaris is a most widely cultivated and consumed crop legume in the developing world. It is a diploid plant with relatively a small genome (650 Mb), and consumed crop legume in the developing world. It is a diploid plant with relatively a small genome (650 Mb), hence potentially an ideal model plant from among crop legumes to study genetic basis of plant development including root nodule symbiosis. However, its recalcitrance to genetic transformation has formed a great stumbling block to undertake extensive molecular analysis of cellular processes in this crop legume plant. Thus far, the only method for obtaining P. vulgaris transgenic plants, albeit with a very low efficiency, has been reported by Aragão et al. [2] using biolistic approach. Nevertheless, in several recalcitrant plant species including common bean, Agrobacterium rhizogenes-mediated transformation was used as an alternative method to obtain transgenic roots to study root biology. By the method of A. rhizogenes-mediated transformation, “composite plants” can be produced having transgenic hairy root system attached to non-transformed shoots and leaves. Recently, Estrada-Navarrete et al. [3] developed an A. rhizogenes-mediated hairy root transformation protocol for P. vulgaris plants, which paved way at least to take forward the molecular studies of root biology, particularly the processes involved in nodule development and function in this plant species. However, the efficiency of hairy root transformation by this method is highly variable. Using the conventional procedure developed by Estrada-Navarrete et al. [3] , we could never improve transformation efficiencies beyond 70% (see above). Hence, the present investigation was undertaken to develop a rapid and high performance alternative technique to achieve high hairy root transformation efficiency in bean. For achieving this we examined the effectiveness of various protocols, explants and introduced appropriate amendments to boost hairy root transformation efficiencies to 100%. By adopting a new protocol we were able to develop a more efficient A. rhizogenes-mediated transformation procedure for bean plants. In the new method, we have utilized radicle severed, cotyledon-bearing common bean (miniature) young seedlings as explants for A. rhizogenes-mediated transformation. With these young explants we were able to achieve 100% efficiency consistently (Figures 3(a)-(c)), thus making this procedure a suitable system for high-throughput functional genomic studies in bean. The major advantage of this new approach is the rapidity and efficiency of A. rhizogenes-mediated transformation of common bean plants. Indeed, the same protocol was also found to be equally effective in soybean as well (Figures 3(a), (d) and (e)).
Acknowledgements
We deeply acknowledge Centro de Ciencias de Genomicas, Universidad Autonoma de Mexico, Cuernavaca, Morelos, Mexico for financial and lab support.
References
- Broughton, W.J., Hernandez, G., Blair, M., Beebe, S., Gepts, P. and Vanderleyden, J. (2003) Beans (Phaseolus spp.) Model Food Legumes. Plant Soil, 252, 55-128. http://dx.doi.org/10.1023/A:1024146710611
- Aragão, F.J.L., Vianna, G.R., Albino, M.M.C. and Rech, E.L. (2002) Transgenic Dry Bean Tolerant to the Herbicide Glufosinate Ammonium. Crop Science, 42, 1298-1302. http://dx.doi.org/10.2135/cropsci2002.1298
- Estrada-Navarrete, G., Alvarado-Affantranger, X., Olivares, J.E., Guillén, G., Díaz-Camino, C., Campos, F., Quinto, C., Gresshoff, P.M. and Sánchez, F. (2007) Fast, Efficient and Reproducible Genetic Transformation of Phaseolus spp. by Agrobacterium rhizogenes. Nature Protocols, 2,1819-1824.
- Ohta, S., Mita, S., Hattori, T. and Nakamura, K. (1990) Construction and Expression in Tobacco of a β-Glucuronidase (GUS) Reporter Gene Containing an Intron within the Coding Sequence. Plant and Cell Physiology, 31, 805-813.
- Nagel, R., Elliot, A., Masel, A., Birch, R.G. and Manners, J.M. (1990) Electroporation of Binary Ti Plasmid Vector into Agrobacterium tumefaciens and Agrobacterium rhizogenes. FEMS Microbiology Letters, 67, 325-328. http://dx.doi.org/10.1111/j.1574-6968.1990.tb04041.x
- Reddy, P.M., Ladha, J.K., Ramos, M.S., Maillet, F., Hernandez, R.J., Torrizo, L.B., Oliva, N.P., Datta, S.K. and Datta, K. (1998) Rhizobial Lipochitoligosaccharide Nodulation Factors Activate Expression of the Legume Early Nodulin Gene ENOD12 in Rice. The Plant Journal, 14, 693-702. http://dx.doi.org/10.1046/j.1365-313x.1998.00170.x
- Collier, R., Fuchs, B., Walter, N., Lutke, W.K. and Taylork, C.G. (2005) Ex Vitro Composite Plants: An Inexpensive, Rapid Method for Root Biology. The Plant Journal, 43, 449-457. http://dx.doi.org/10.1111/j.1365-313X.2005.02454.x
- Summerfield, R.J., Huxley, P.A. and Minchin, F.R. (1977) Plant Husbandry and Management Techniques for Growing Grain Legumes under Simulated Tropical Conditions in Controlled Environments. Experimental Agriculture, 13, 81-92. http://dx.doi.org/10.1017/S0014479700007638
- Chabaud, M., Boisson-Dernier, A., Zhang, J., Taylor, C.G., Yu, O. and Barker, D.G. (2006) Agrobacterium rhizogenes-Mediated Root Transformation. Medicago Truncatula Handbook. The Samual Roberts Noble Foundation, USA.
- Silvente, S., Reddy, P.M., Khandual, S., Blanco, L., Alvarado-Affantranger, X., Sanchez, F. and Lara-Flores, M. (2008) Evidence for Sugar Signalling in the Regulation of Asparagine synthetase Gene Expressed in Phaseolus vulgaris Roots and Nodules. Journal of Experimental Botany, 59, 1279-1294. http://dx.doi.org/10.1093/jxb/ern034
NOTES

*Corresponding author.


