Journal of Biophysical Chemistry
Vol.3 No.3(2012), Article ID:21949,3 pages DOI:10.4236/jbpc.2012.33030
Molecular basis of scavenging effect of zonisamide on hydroxyl radical in vitro
![]()
1Department of Clinical Pharmacy, Shujitsu University School of Pharmacy, Nishigawara, Japan
2Physiology and Pharmacology, School of Health and Social Services, Saitama Prefectural University, Sannomiya, Japan; *Corresponding Author: tanaka-ken-ichi@spu.ac.jp
3Department of Bacteriology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Shikatacho, Japan
4Department of Neurology, Saint Louis University School of Medicine, St. Louis, USA
5Department of Brain Science, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Shikatacho, Japan
Received 2 May 2012; revised 5 June 2012; accepted 12 June 2012
Keywords: Zonisamide; Hydroxyl Radicals; LC/MS; ESR
ABSTRACT
Zonisamide (ZNS), a commonly used anticonvulsant, scavenged hydroxyl radicals at a clinically relevant concentration. Reactants of ZNS with hydrogen peroxide and with hydrogen peroxide plus UV irradiation, yielding hydroxyl radicals, were analyzed by the LC/MS technique. Many small fragments were found in the both reactions, suggesting that ZNS was decomposed not only by hydroxyl radicals but also by hydrogen peroxide. Furthermore, mass-fragmentgrams showed that m/z: 213 (ZNS itself) was decreased markedly and m/z: 118 (may be a decomposed product by ring cleavage of ZNS) was detected specifically by treatment with hydroxyl radical. These data suggested that ZNS may react directly with free radicals.
1. INTRODUCTION
Using electron spin resonance (ESR) spectrometry, it was shown that Zonisamide (ZNS) in molar concentrations, scavenged hydroxyl radicals (·OH), nitric oxide radicals and dopamine quinones [1,2]. These data suggested that the mechanism of antiepileptic effect of ZNS may involve protection of neurons from free radical damage along with stabilization of neuronal membranes [3]. In this study, we observed that ZNS scavenge ·OH in a physiologically relevant concentration, and moreover demonstrated by a liquid chromatography/mass-spectrometry (LC/MS) that ZNS reacts directly with ·OH and is decomposed to smaller fragments.
2. MATERIAL AND METHODS
2.1. Sample
ZNS was a kind gift from Dainippon-Sumitomo Pharmaceutical Co. Ltd. (Osaka, Japan).
2.2. ESR Measurements
ESR measurements were carried out using a computerized ESR spectrometer, the Free Radical Monitor (JESFR30, JEOL, Tokyo). Experimental conditions were as follows: magnetic field, 335.6 ± 5 mT, power, 4 mW, modulation frequency, 9.41 GHz, modulation amplitude, 1 × 0.1 mT, response time, 0.1 s, amplitude, 1 × 200 ~ 1000; and sweep time, 2 min. All measurements were performed at 23˚C.
2.3. Estimation of Hydroxyl Radical Scavenging Activity by ESR
Fenton’s reaction was used to generate •OH [4,5]; 50 µl of 1 mM 5,5’-dimethyl-1-pyrroline-N-oxide (DMPO), 50 µl of sample solution, 50 µl of 0.2 mM H2O2, and 50 µl of 0.02 mM FeSO4 (added last) were mixed for 10 sec and transferred into a 200-µl capacity quartz flat cell. Exactly 30 sec after the addition of FeSO4, ESR spectra of the DMPO-OH spin adduct were recorded.
2.4. Analysis of ZNS and Its Decomposed Fragments, Utilizing LC/MS
In the LC/MS experiments, Milli-Q water was used for all procedures, e.g., for solution of ZNS and H2O2. Hydroxyl radicals were generated by a H2O2-ultraviolet-ray (UV) system, using a UV lamp (254 nm, Handy UV lamp SUV-4, ASONE, Osaka); 40 µl of ZNS (1 µg/ml) and 40 µl of H2O2 (20 mM) in a vial were stirred gently, then irradiated with UV from the top for 30 sec. This vial was capped, and 5 µl of each sample was put on the LC auto-sampler. The procedure was performed without UV irradiation as the control.
LC/MS analysis was carried out using an Aligent LC/MS system (Aligent 1100 and G1956B, Aligent Technologies Inc., Santa Barbara, CA). The conditions for MS were as follows; column: Inertsil ODS-3 (2.1 × 150 mm; particle diameter: 5 µm) (GL Science, Tokyo); LC eluent: 5 mM HCOONH4/methanol (50:50, v/v); flow rate: 0.2 ml/min, and for MS: ionization mode: AP1-ES (positive mode); fragmentor: 150 V; capillary voltage: 300 V; drying gas flow: 13.0 l/min; drying gas temperature: 300˚C; nebulizer pressure: 42 psig. Data were collected in the scan mode and the selected ion monitoring (SIM) mode, and the content of ZNS was calculated by analyzing the peak area of ZNS (m/z: 213).
2.5. Statistics
All data are expressed as means ± S.E.M. Statistic analysis was carries out using Student’s t-test.
3. RESULTS AND DISCUSSION
DMPO-OH spin adduct showed a typical quarted signal (1:2:2:1) in the ESR recording (hyperfine condition of the DMPO-OH adduct: aN = 1.49 mT, 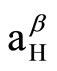 = 1.49 mT). This signal was decreased by addition of ZNS (final concentration, 0.02 mM), and estimated IC50 was approximately 0.9 mM, and even 78 µM of ZNS significantly (77% of control: p < 0.05) scavenged ·OH (Figure 1).
= 1.49 mT). This signal was decreased by addition of ZNS (final concentration, 0.02 mM), and estimated IC50 was approximately 0.9 mM, and even 78 µM of ZNS significantly (77% of control: p < 0.05) scavenged ·OH (Figure 1).
ZNS was treated with H2O2-without UV (Exp. A), or H2O2 with UV, generating ·OH in Exp. B; each reactant was analyzed by LC/MS. In these mass spectrograms, the following fragment peaks were observed except for m/z: 213 (ZNS itself): m/z: 62, 106, 118, 132, 214, 215, 230, 235 and 251. These fragments were analyzed by SIM, and it was found that m/z: 213 (ZNS itself) was markedly decreased and m/z: 118 was detected specifically in the conditions with H2O2 and UV irradiation for 30 sec (Exp. B). No significant difference was found between other fragments compairing Exp. A and Exp. B (Table 1). Meanwhile a fragment peak was not detected by these analyses that would correspond to the hydroxyl radical-adducted molecule.
Mori et al. [1], using ESR spectrometry, first reported that ZNS scavenged •OH. This experiment confirms that this scavenging activity occurs in a clinically relevant concentration of ZNS. Using experimental animals, it has been shown that ZNS is metabolized by direct acetylation, by glucronyl conjugation, and via hydroxylation
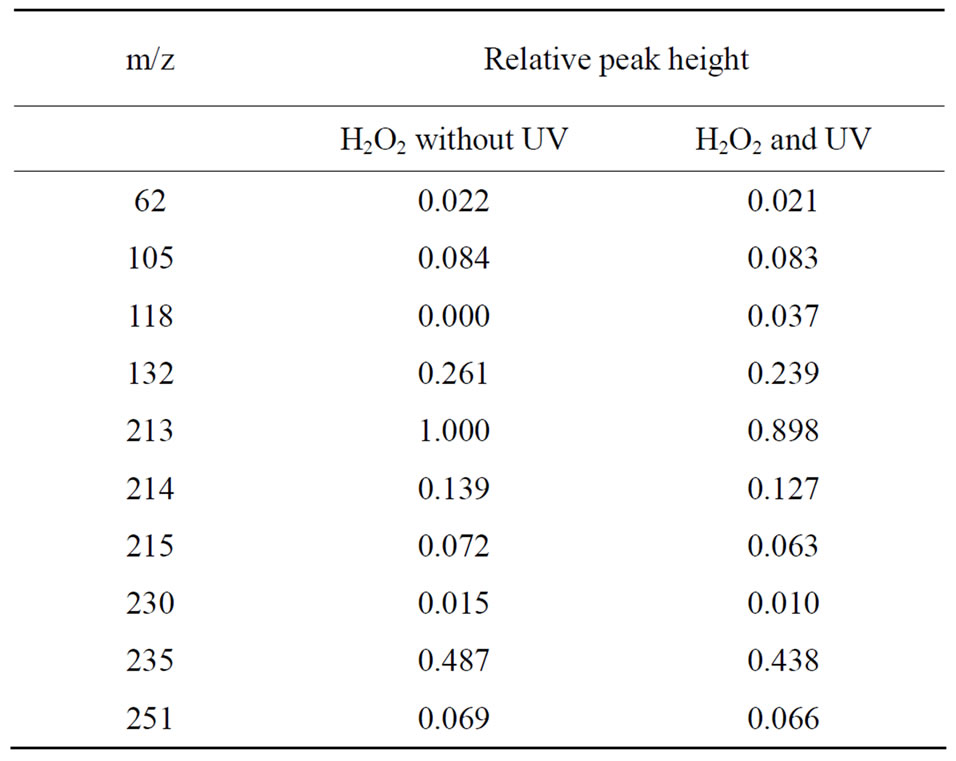
Table 1. Analysis of ZNS and its decomposed fragments by LC/MS.
followed by oxidation of the methylen carbon of the sulfamoyl group, finally resulting loss of the sulfamoyl group. In addition, there is N-O bound cleavage of the isoxazole ring to produce hydroxylation in the benzene ring. Formed ring-cleft metabolites are followed by conjugation with sulfuric acid or glucronic acid. The 1,2-benzisoxazole N-O cleavage product of ZNS, 2-sulphamolacetylphenol (SMAP; m/z: 215) is one of the major metabolites [6,7]. Reduction of ZNS to SMAP is mediated by cytochrome P450 [7,8] and by mammalian intestinal bacteria in vivo [9].
4. CONCLUSIONS
Yoshida and Masuda, from the Research Laboratory of Dainippon Pharmaceutical Co., Ltd. (Yoshida & Masuda, 1997, on the reaction of ZNS with •OH radical. Personal communication to A.M.) treated ZNS with •OH radicals induced by Fenton’s reaction, and using HPLC, observed decreased level of ZNS. Using GC/MS, they analyzed the possible decomposed fragments from ZNS, but did not identify the specific fragments that originated from ZNS. In this LC/MS study, UV-H2O2 system, but not the Fenton’s reaction, was used for •OH generation. Moreover, UV irradiation was used only for 30 sec, and the decomposing reaction for ZNS was already in progress with the peak height of m/z for ZNS slightly but significantly decreased.
In the analysis by SIM mode, many signals of m/z were observed both in the Exp. A (H2O2 without UV) and B (H2O2 with UV). The common signals may be due to oxidative reaction by H2O2 itself. Some higher m/z signals, e.g., m/z: 230, 235, 251, may be due to secondary reactions among fragments by the oxidation reaction by H2O2. Meanwhile, m/z: 118 in the Exp. B was significantly increased comparing with in Exp. A, which could
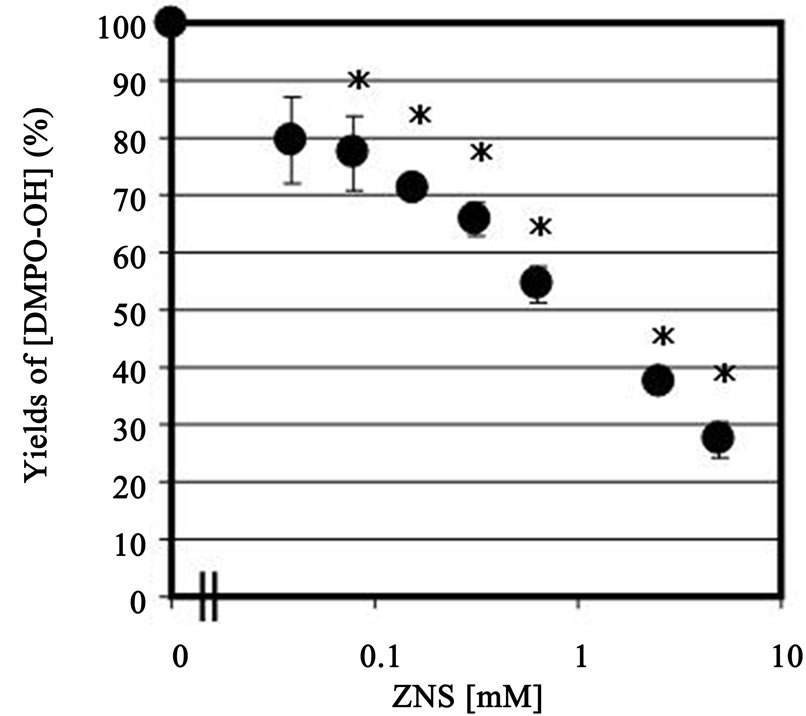
Figure 1. Dose-dependent of •OH scavenging activity by ZNS (39 µM - 5 mM). Values are expressed as mean ± SEM (n=3 - 4). *p < 0.05 compared with control (ZNS = 0).
indicate induction by •OH. This fragment was estimated as decomposed products by cleavage of ZNS from the side chain, methane sulfonamide, and may be related to opening of the ring at -O-Nposition. We compared our mass spectral data of decomposed fragments of ZNS by UV-H2O2 system with the data of ZNS metabolites in the rat, and found no common mass fragmental datum existed including SMAP. On the other hand, m/z: 118, we found, was not observed in metabolites of ZNS in the urine using in vivo experiments [6], we suspect these metabolites may be unstable in vivo. These data suggested that ZNS may react directly with free radicals.
REFERENCES
- Mori, A., Noda, Y. and Packer, L. (1998) The anticonvulsant zonisamide scavenges free radicals. Epilepsy Research, 30, 153-158. doi:10.1016/S0920-1211(97)00097-1
- Asanuma, M., Miyazaki, I., Diaz-Corrales, F.J., Miyoshi, K., Ogawa, N. and Murata, M. (2008) Preventing effects of a novel anti-parkinsonian agent zonisamide on dopamine quinone formation. Neuroscience Research, 60, 106- 113. doi:10.1016/j.neures.2007.10.002
- Mori, A., Noda, Y., Kaneyuki, T. and Packer, L. (2000) Antiepileptic mechanism of zonisamide involves its antioxidant effect in the brain. In: Karanithi, N. and Packer, L., Eds., Microneutrients and Health: Molecular Biological Mechanisms, AOCS Press, Champaign, 236-246.
- Rosen, G.M. and Rauckman, E.J. (1984) Spin trapping of superoxide and hydroxyl radicals. In: Packer, L., Ed., Methods in Enzymology, Oxygen Radicals in Biological Systems, Academic Press, San Diego, 198-209. doi:10.1016/S0076-6879(84)05026-6
- Janzen, E.G. (1984) Spin trapping. In: Packer, L., Ed., Methods in Enzymology Oxygen Radicals in Biological Systems, Academic Press, San Diego, 188-198.
- Ito, T., Yamaguchi, T., Miyazaki, H., Sekine, Y., Shimizu, M., Ishida, S., Yagi, K., Kakegawa, N., Seino, M. and Wada, T. (1982) Pharmacokinetic studies of AD-810, a new antiepileptic compound. Phase I trials. Arzneinmittelforsch, 32, 1581-1586.
- Nakasa, H., Komiya, M., Ohmori, S., Rikihisa, T., Tiuchi, M. and Kitada, M. (1993) Characterization of human liver microsomal cytochrome P450 involved in the reductive metabolism of zonisamide. Molecular Pharmacology, 44, 216-221.
- Nakasa, H., Ohmori, S. and Kitada, M. (1996) Formation of 2-sulphamoylphenol from zonisamide under aerobic conditions in rat liver microsomes. Xenobiotica, 26, 495- 501. doi:10.3109/00498259609046727
- Kitamura, S., Sugihara, K., Kuwasako, M. and Tatsumi, K. (1997) The role of mammalian intestinal bacteria in the reductive metabolism of zonisamide. Journal of Pharmacy and Pharmacology, 49, 253-256. doi:10.1111/j.2042-7158.1997.tb06790.x

