Journal of Environmental Protection
Vol.09 No.05(2018), Article ID:84931,14 pages
10.4236/jep.2018.95034
Is the Remediation at Parys Mountain Successfully Reducing Acid Mine Drainage?
Niall Marsay
School of Chemistry, Bangor University, Bangor, UK

Copyright © 2018 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: March 16, 2018; Accepted: May 27, 2018; Published: May 30, 2018
ABSTRACT
Metal ion concentrations and acidity were used as indicators of acid mine drainage (AMD) at Parys Mountain, a large abandoned copper mine on Anglesey, Wales. Water samples were collected in two sessions and taken from a linear stream flowing from the northern side of the mine, and a stream flowing from the south side of the mine that has two settling ponds and long stretches of wetland along its path. pH measurements were taken to measure acidity levels and metal ions (Fe, Al, Zn, Cu, Mn, and Pb) were quantified by inductively coupled plasma (ICP-OES) spectrometry. The pH values at the settling ponds and northern stream were between 2 and 3 while the wetlands had pH values of 5 - 6 implying that it was the wetlands that reduced acidity, and not the distance downstream. Both streams showed a reduction in concentrations of all elements with distance downstream. The decrease was linear for the northern stream and exponential for the southern stream, suggesting that the reed beds and settling ponds were successful at removing metal ions; potentially, through slower flow rates allowing more time for redox reactions to occur, thus precipitating metal hydroxides and pure metals and removing them from solution. In November, the northern stream had substantially higher concentrations of Fe, Al, Zn, Cu, and Mn, but not Pb (126, 34.0, 29.1, 14.6, 10.4, and 0.064 mg/L respectively) in solution when compared to the southern stream, which had concentrations of 10.2, 12.2, 11.9, 2.43, 6.11, and 0.706 mg/L for Fe, Al, Zn, Cu, Mn, and Pb respectively. However, in January the first sample site had higher concentrations of all elements except Mn; (107, 22.0, 26.1, 10.3, 1.48, and 0.506 mg/L for Fe, Al, Zn, Cu, Mn, and Pb respectively) when compared to the northern stream (55.0, 10.6, 7.55, 6.10, 1.59, and 0.041 mg/L for Fe, Al, Zn, Cu, Mn, and Pb respectively): but by the second sample site, the southern stream concentrations had dropped to concentrations present in the northern stream. This data indicates less AMD was produced on the southern side during low rainfall periods. Remediation was measured by calculating the percentage reduction in concentration (PRC) between sample sites. PRCs were higher in January for most of the sites; possibly due to dilution by surface runoff from surrounding farmland. The northern stream had consistently lower PRCs between 15% - 55%. The settling ponds had higher PRCs but did not maintain consistent levels with a range of 5% - 90%, while the bogs had consistently high PRCs of 40% - 100%. The combination of the high PRC and pH in the bogs at Parys Mountain makes this the most effective area at remediating Parys Mountains AMD.
Keywords:
Acid Mine Drainage, Remediation, Parys Mountain, Anglesey

1. Introduction
Britain has a long history of mining for metals, dating back at least 4000 years, this has produced a vast number of mines, with over 3700 sites identified from studies in Wales, the South West and Northumbria alone [1] . Many of these sites pose no hazard to the environment; but sadly this is not the case for all sites, with the Water Framework Directive identifying 7% of British water bodies as being potentially at risk [1] . With the environment under so much pressure steps need to be taken to identify the most effective methods of remediation.
Once the largest copper mine in Europe, today it lies abandoned, but Parys Mountain remains relevant as it is currently the largest provider of zinc and copper to the Irish Sea, annually discharging of 24 tons of zinc and 10 tons of copper [1] .
This study aims to identify which of the three environments along the outflows leaving the site are most effective at preventing acid mine drainage (AMD) from entering the surrounding environment.
1.1. What Is Acid Mine Drainage?
AMD is the product of water coming into contact with sulfide minerals and being exposed to the atmosphere; it occurs at almost all mines that have sulfide deposits. The same process occurs as a natural process on natural outcrops, this is known as acid rock drainage (ARD). AMD usually occurs in greater quantities than ARD because mining increases the surface area of sulfide minerals exposed to the environment.
AMD has many effects on the environment. Firstly, it lowers the pH: at Parys mountain water samples taken from the streams leading into Afon Goch were found to have a pH of 2.8 or lower; this was maintained along a 1 km stretch of the stream [2] . This lower pH makes the environment uninhabitable for many flora and fauna but those that can survive the lower pH are threatened by toxic metals, which are more bio-available in low pH environments [3] .
1.2. How Is Acid Mine Drainage Generated?
The generation of AMD involves multiple reactions and follows different chemical routes depending on the pH of the environment. The first step (Equation (1)) is the oxidation of pyrite to aqueous iron and sulphuric acid [4] ; this reaction lowers the pH.
 (1)
(1)
If the environment is sufficiently oxidising, the second step will occur, (Equation (2)) [4] .
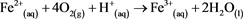 (2)
(2)
At this stage, the reaction depends on the pH of the environment; if it is above 2.3 then (reaction 3) [4] will dominate, but if the pH is lower then (reaction 4) [4] will dominate.
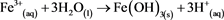 (3)
(3)
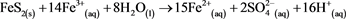 (4)
(4)
Based on (Equations (1)-(3)), where the final product is Fe(OH)3, it is possible to show the whole process as (Equation (5)) [5] . This umbrella equation is agreed by most of the scientific community to be an accurate representation of AMD, but it ignores the fact that in the more acidic conditions Fe3+ is the primary oxidant of pyrite as opposed to oxygen as shown in (Equation (4)). (Equation (6)) [5] better shows this and also has 8.5 moles of H+ per mole of pyrite, as opposed to 4 moles H+ per mole of pyrite.
At Parys Mountain Equation (2) will be closer to the truth on site; but downstream of Parys Mountain the acidity reduces, (reaction 3) will be able to occur and hence (Equation (5)) will be more accurate. In reality neither of these umbrella equations show the overall reaction as both ignore part of the process to create a balanced equation [6] ; the equations also ignore other minerals involved in the generation of AMD, such as chalcopyrite (CuFeS2) and chalcocite (Cu2S) [5] , as well as ignoring the increased rates that iron bacteria, such as Thiobacillus Ferro-oxidans and Leptospirillum Ferro-oxidans, can provide, which have both been isolated at Parys Mountain [2] .
 (5)
(5)
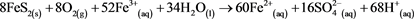 (6)
(6)
Several primary factors have been identified to determine the rate of AMD formation [5] , which are: pH; temperature; [O2] in the gas phase, in areas with poor airflow e.g. deep mines; [O2] in the water phase; [Fe2+] within water; surface area of exposed metal sulfide e.g. [FeS2]; bacterial activity. Controlling these factors is key to successful remediation, including remediation at Parys Mountain.
1.3. AMD at Parys Mountain
Despite Parys Mountains historical significance and visible impact on the environment, (Figure 1) there is a limited supply of peer-reviewed research available
Figure 1. An Ordnance Survey map highlighting surface water systems and showing sample site locations and appearance [11] .
on levels of AMD surrounding the site; the research that does exists was carried out before the controlled breaking of an underground dam in 2003 [1] , [7] , which altered the flow of AMD leaving the site.
Table 1 shows the mean range of metal concentrations and pH collected by Walton et al. [2] across a period of 3 years in the 1980’s and 90’s. His samples focused on the stream that flows from the centre of Parys Mountain through the central ponds to the southern stream over a distance of 900 m. The results showed a decrease in concentration with distance downstream but no other visible trends.
Walton’s study shows that iron, zinc, lead and arsenic all exceed their environmental quality standards of 0.73, 0.0005, 0.005 and 0.005 mg/L respectively, as set out by the Water Framework Directive [8] [9] [10] .
1.4. Hydrology
Parys Mountain has a complicated surface hydrology (Figure 1) with two catchments, one heading north and one heading south, these collect water from the north and south-east sides of Parys Mountain respectively. There are also many settling ponds that appear to have no outflows, presumably draining underground. There is a large network of underground mine shafts [7] , of which the details are not publicly available.
During the sampling exercise, the northern stream was fast-flowing, narrow and had no active methods of remediation in place; whereas the southern stream had a slow flow rate; large settling ponds and marshland in its path. The streams, therefore, provide an interesting contrast in remediation potential.
2. Methodology
To investigate the remediation capabilities of the two main outflows 10 sample sites were selected (Figure 1, Table 2); sites A, B, C, D and E were placed along the southern stream and sites F, G, and H were on the northern stream. All site locations were selected based on ease of access; southern sites were selected to observe the effects of settling ponds and marshes on AMD levels; whereas the northern stream was selected to be at even distances downstream as no interesting features are present on the northern stream. Sampling trips were carried out on 3rd of November 2015 and 26th of January 2016 to investigate variance with rainfall.
Table 1. Average ranges for elements analysed by Walton et al. along a 900 m stretch of streams flowing from the centre of Parys Mountain and converging with the southern stream [2] .
Table 2. Location of sample sites with distance downstream form highest site on stream, and description of local environment.
November water samples were collected in clean Fisher Scientific HDPE 500 mL and January samples were collected in triplicate in 125 mL sample bottles. The sample bottles were flushed twice with the sample then filled completely, sealed and labeled.
The pH of the samples was measured on return to the lab with a Jenway 3510 pH meter. To preserve samples in storage they were then gravity filtered with no. 52 Watman paper to remove particulates; followed by the addition of Nitric acid, (HNO3 70% trace metals basis ≥ 99.999%) 1 drop per 100 ml, to stabilise samples, and stored at room temperature.
The concentration of elements was analysed by Varian 710-ES ICP-OES; samples were diluted 1:9 with deionized water before analysis.
Calibration was carried out by ICP Expert II Software using one method blank and one standard (ICP multi-element standard solution XIII from Merck Millipore) diluted 1:19 with deionized water to provide the concentrations in Table 3. The wavelengths (Table 3) used were selected to avoid interference from elements expected to be present.
Calculation of Percentage Reduction in Concentration
To measure the remediation of elements in the water column, percentage reduction in concentration (PRC) was calculated. This was done by calculating the amount of an element that remained in the lower site as a percentage of the upper site. This value was then subtracted from 100 to give the percentage that had been removed between the first and second site. This is shown in (Equation (7)).
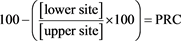 (7)
(7)
3. Results
3.1. pH and ICP
pH values recorded had a range of 2.3 - 6.2 (Figure 2). Unlike element concentrations
Table 3. A list of all elements analysed using ICP-OES, their associated wavelengths used for characterization and concentrations for the standard.
Figure 2. The pH measurements in November and January which shows the distinct difference between the wetland and other environments.
there is no correlation between distance downstream and an increase in pH, there is a difference when results are plotted with the environments. The settling ponds and southern streams had pH’s of 2 - 3 whereas the bogs had a pH range of 5 - 6; excluding the anomaly at site D in November with a pH of 3.2 (Figure 2).
Across all sites the most abundant element analysed was Iron, with a maximum concentration measured of 126.1 mg/L, (Figure 3) followed by in descending order Aluminum, Zinc, Copper, Manganese, and Lead, with respective maximum values of 34.03, 29.05, 14.61, 10.37, and 0.706 mg/L (Figures 4-8). All other elements characterised were below 0.01 mg/L and Arsenic, Mercury and Vanadium were not detected by the ICP at any concentration.
When all sample sites are plotted against distance from the Parys Mountain no trends are visible; but when the two catchment zones (Figure 1) are treated separately, distinct trends are shown. Both show a decrease in concentration of metal ions in both months, but the rates at which the metal ions decrease in concentration varies depending on the month and stream.
For all elements except Lead and Manganese concentrations were higher in the northern stream compared to the southern stream (Figures 3-6). However, these elements in January at site A had a higher concentration than the northern stream but by site B the concentration had decreased to within the range of the northern stream’s concentrations (Figures 3-6).
Manganese followed the same trend as most other elements in November however in January both the northern and southern streams had similar values of approximately 1 mg/L (Figure 7). In both months Lead had a substantially
Figure 3. The iron concentrations across north and south streams for November and January.
Figure 4. The copper concentrations across north and south streams for November and January.
Figure 5. The zinc concentrations across north and south streams for November and January.
Figure 6. The aluminium concentrations across north and south streams for November and January.
Figure 7. The manganese concentrations across north and south streams for November and January.
Figure 8. The lead concentrations across north and south streams for November and January.
higher concentration in the southern stream when compared to the northern stream (Figure 8).
When comparing the streams separately across the two months, the northern stream had higher concentrations in November for all elements; and the southern stream had higher concentrations in January for Iron and Copper (Figure 3 and Figure 4) but not Zinc Aluminium Manganese or Lead (Figures 5-8).
3.2. Remediation
January’s results (Figure 9) had percentage reduction in concentration (PRC) that are similar for each element excluding Manganese but varied across sites. The southern stream shows higher PRC for all stages except the 2nd settling pond (B to C) when compared to the northern stream.
November’s results (Figure 10) were a lot less consistent; Iron and Manganese specifically did not have similar levels to the other metals and in some cases increased in concentration after passing between sites.
Sites A to B and B to C (the settling ponds) showed higher PRC compared to the northern stream some of the time; but only sites C to D and D to E which covered the bog area had constantly high PRC over both months compared to other sites.
Figure 9. January’s percentage reduction in concentration highlighting the difference in remediation across the different environments at Parys Mountain.
Figure 10. November’s percentage reduction in concentration highlighting the difference in remediation across the different environments at Parys Mountain.
4. Discussion
4.1. Order of Magnitude
Out of all the elements analysed Iron had the highest Concentration; this is almost certainly due to the fact that FeS2 is the predominant sulphide mineral at Parys Mountain [12] ; also, iron has a higher affinity for the sulphate ions in solution.
The order of element concentration correlates with the work of Walton et al. [2] who found that the element he analysed had the decreasing order of Iron, Aluminium, Zinc, Copper and Lead. Walton et al. did find Arsenic to have a maximum concentration of 1.6 mg/L where this study found no trace of Arsenic, this matches up as all elements Walton analysed were substantially higher than the values this study found e.g. 550 mg/L for Iron by Walton et al. compared to a maximum value of 126 mg/L found by this study.
Walton et al. study looked at the southern stream alone; the highest result from this study for the southern stream showed a maximum value of 106 and 34 mg/L for Iron and Aluminium respectively compared to 550 and 90 mg/L from Walton et al.; this was to be expected as the de-watering project in 2003 [7] changed the northern stream to the primary outlet for Parys Mountains AMD [1] .
4.2. Variance with Weather
The two sampling trips were carried out in very different weather conditions; in November the weather was wet but mild, the Met Office recorded 59.4 mm [13] of rainfall in the month preceding sampling; this is in sharp contrast to January; when the Met Office recorded 146.4 mm [13] of rainfall. This made a substantial difference to the visible level of the streams with parts being up to approximately 15 - 20 cm higher.
This difference in rainfall is the most likely the factor in the difference seen across the 2 sampling trips. In the northern catchment zone, there is more bedrock containing Sulphide minerals [14] in addition to this the majority of the underground workings drain into the northern catchment zone [1] . Both of these sources gain there O2 and H2O from groundwater. As groundwater levels are not affected by seasonal rain there will be a constant level of AMD being generated, but the high rainfall in January would dilute the AMD in the stream hence the lower concentrations observed in January.
In the southern catchment zone, there are a large number of spoil heaps these spoil heaps could potentially contribute to the increase in element concentration in the southern stream in two ways. Spoil heaps have large surface areas but the majority will not be in contact with H2O (a requirement for AMD generation) during drier weather hence they generate more AMD when there is more rain. The second factor is pore water inside spoil heaps; there will be pore water that will allow AMD to be created but it is stagnant in dry weather. When large rainfall occurs it flushes out pore water and hence an increase in element concentrations is observed in the southern stream.
4.3. pH
Unlike element concentrations pH did not decrease with distance downstream (Figure 2 this could be due to (Equation (3)) the reaction of ferric iron with water producing iron oxide and hydrogen ions, which occurs at pH’s above 2.3, this reaction produces hydrogen ions which in turn lower the pH acting as a pH buffer preventing the pH from rising.
Sites D and E were the only sites to have pH’s higher than 3, as these sites were both bogs this is the most likely cause of the increased pH.
4.4. Remediation
To identify if the streams were successfully remediating the AMD leaving Parys Mountain, the percentage reduction in concentration (PRC) was calculated for each element (calculations are shown in methodology).
PRC was similar for each element at sample sites in January (Figure 9) but in November (Figure 10) this was not the case; this could be due to the complex interplay of redox reactions favouring the removal of certain elements over others, for example (Equation (8)) reacts iron hydroxide with copper ions in acid to produce solid copper and ferric iron ions and water, removing copper from the water column but adding iron back in. but if this was the case then the inconsistent PRCs should be visible in January. A more likely explanation is that Novembers results were anomalous but as samples were not collected in triplicate this cannot be verified.

As well as reduction levels being varied in November, iron increased in concentration between sites A to B and D to E, and manganese increased across sites A to B. in addition to this iron had very low PRC, these results could be due to the effects of redox reactions like (Equation (8)); but there is also the possibility that the areas around these sites contain large amounts of iron and manganese sulphates that are submerged in groundwater and drain in to the stream between sample sites. This would explain why these results were not seen in January and why they do not occur at all sites.
In January all sites except B to C had higher PRCs; one theory for this is that the increased rainfall meant water sources (small streams and surface runoff) from surrounding farmland that did not contain AMD were not active in November but were active in January due to the increased rainfall; which diluted the elements between sample sites. B to C could have decreased in PRC again because of water sources not active in November but these water sources came from Parys Mountain adding AMD to the stream masking the true PRC across sites B to C
There are several reasons that could explain why the removal efficiency is higher in the southern steam. The first is that between sites A-B and B-C there are large settling ponds in which the water moves slowly; allowing a longer time for the (reaction 3) to occur converting Ferric iron into iron hydroxide, which would leave the water column through sedimentation. In a fast-flowing stream, AMD would be able to travel further before oxidising and settling out. Then between sites C-D and D-E are the wetlands with the raised pH; this allows for (reaction 3) at a higher rate, in addition to this the large areas of mud act as filters with AMD binding to the soil as the water percolates through.
5. Conclusions
In conclusion, element concentrations decreased with distance downstream, but the rate at which they decreased varied depending on the environment of the stream. Furthermore, the level of rainfall in the preceding month correlated with the element concentrations at Parys Mountain. However, pH did not vary with rainfall or distance downstream and instead varied with the environmental state of the stream i.e. settling pond, bog, or fast flowing stream.
Of the elements analysed, Iron was the most common element followed by Aluminium, Zinc, Copper, Manganese, and Lead. The other elements analysed were all below 0.1 mg/L with arsenic mercury and vanadium not being detected at any concentration.
In November, all elements except Lead were higher in concentration in the northern stream. But in January, all elements except Manganese were the highest in concentration at site A, but by site B, the concentration had dropped to similar levels as seen in the northern stream. The Lead concentrations were higher in the southern stream in both months and manganese was approximately 1 mg/L across all sample sites in January.
Rainfall was substantially higher in the month before January’s sample collection when compared to the month before November’s sample collection 146.4 and 59.4 mm respectively [13] . This leads to a visible difference of approximately 15 cm in the height of the streams.
pH values did not vary with month, except for one outlier at site D. pH values for the settling ponds and northern stream ranged between 2 and 3. The bogs had higher pHs of 5 - 6.
Percentage reduction in concentration (PRC) was calculated to show remediation, January had similar PRCs for all elements in each area, but the areas varied when compared to each other. In November, the elements had a larger variance in PRC for each area. Overall the northern stream was less effective at reducing the concentration of elements, the settling ponds were more effective but did not maintain the high PRC across all results. The bogs were the only environment to maintain a high PRC across all elements and months.
Due to the fact that the bogs maintain a high PRC in both sample trips and the increased pH in the bogs, it appears to be the most effective environment at Parys Mountain.
Acknowledgements
The author would like to thank Vera Thoss, Matt Balmforth and his parents for assistance in various aspects of laboratory analysis and report writing. This study was funded by the School of Chemistry at Bangor University.
Cite this paper
Marsay, N. (2018) Is the Remediation at Parys Mountain Successfully Reducing Acid Mine Drainage? Journal of Environmental Protection, 9, 540-553. https://doi.org/10.4236/jep.2018.95034
References
- 1. Johnston, D., Potter, H., Jones, C., Rolley, S., Watson, I. and Pritchard, J. (2008) Abandoned Mines and the Water Environment. Bristol.
- 2. Walton, K.C. and Johnson, D.B. (1992) Microbiological and Chemical Characteristics of an Acidic Stream Draining a Disused Copper Mine. Environmental Pollution, 76, 169-175. https://doi.org/10.1016/0269-7491(92)90105-J
- 3. Riba, I., Garcia-Luque, E., Blasco, J. and DelValls, T.A. (2003) Bioavailability of Heavy Metals Bound to Estuarine. Chemical Speciation & Bioavailability, 15, 101-114. https://doi.org/10.3184/095422903782775163
- 4. Jennings, S.R., Blicker, S., Neuman, P. and Dennis, R. (2008) Acid Mine Drainage and Effects on Fish Health and Ecology: A Review. Reclamation Research Group Publication, Bozeman, MT, 1-26.
- 5. Akcil, A. and Koldas, S. (2006) Acid Mine Drainage (AMD): Causes, Treatment and Case Studies. Journal of Cleaner Production, 14, 1139-1145. https://doi.org/10.1016/j.jclepro.2004.09.006
- 6. Motsamai, T. (2010) Application of Seqwuential Exstraction Procedure for Determination of Copper in an Old Mine Site: Parys Mountain. Bangor Universty, Bangor.
- 7. (2016) Parys Underground Group. http://www.parysmountain.co.uk/
- 8. Peters, A., Merrington, G., Simpson, P. and Crane, M. (2012) Proposed Quality Standards for Iron in Freshwaters Based on Field Evidence. Edinburgh.
- 9. Maycock, D., Peters, A., Merrington, G. and Crane, M. (2012) Proposed EQS for Water Framework Directive Annex VIII Substances?: Zinc. Edinburgh.
- 10. UKTAG (2016) Technical Report on Groundwater Hazardous Substances.
- 11. (2015) OS Map of Parys Mountain. https://www.ordnancesurvey.co.uk/osmaps/#53.38560232817629,-4.348433533135393
- 12. Barrett, T.J., Tennant, S.C. and MacLean, W.H. (1999) Geology and Mineralization of the Parys Mountain Polymetallic Deposit, Wales, United Kingdom. http://www.oresystems.com/global/geominparys.html
- 13. (2017) MIDAS: UK Daily Rainfall Data.
- 14. (2015) Plan Map of Volcanic and Sedimentary Units at the Parys Mountain Polymetallic Sulfide Deposit. http://www.oresystems.com/global/wales.html












