Journal of Environmental Protection
Vol.4 No.11A(2013), Article ID:40252,10 pages DOI:10.4236/jep.2013.411A007
Trophic Status of Shallow Lakes of La Pampa (Argentina) and Its Relation with the Land Use in the Basin and Nutrient Internal Load
![]()
Departamento de Ciencias Biológicas, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de La Pampa, Santa Rosa, Argentina.
Email: sechaniz@cpenet.com.ar, santiagoechaniz@exactas.unlpam.edu.ar
Copyright © 2013 Santiago A. Echaniz, Alicia M. Vignatti. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received September 18th, 2013; revised October 17th, 2013; accepted November 15th, 2013
Keywords: Eutrophication; Trophic State; Total Phosphorus; Shallow Lakes; Internal Load; Land Use
ABSTRACT
Phosphorus and nitrogen are essential nutrients for living organisms. Their concentration in the water of an aquatic ecosystem is one of the factors responsible for the trophic status of the lake and is related to the soils of the region and to the human activities carried out in their basins. These nutrients are also found in the bottom sediments, where they can either be retained or re-enter the water column. Since the information about the concentrations of nutrients in the water of some lakes of La Pampa (Argentina) is fragmentary, the aim of this study is to describe the trophic status of some shallow lakes of the semiarid center of Argentina and analyze its relation with the human activities in their basins, the concentrations of nutrients and organic matter and particle size distribution of sediments. To this end, we studied ten shallow lakes subjected to different anthropogenic influences (agriculture, agriculture and livestock and impacted by cities). All were hypertrophic and the concentrations of total phosphorus and total nitrogen were among the highest reported globally. Since some lakes had no fish, cladoceran grazing (top-down effect) led them have reduced concentrations of phytoplankton chlorophyll-a and high water transparency. This relativizes the use of these parameters to determine the trophic status. The sediments of seven of the studied lakes were predominated by fine sands, whereas three were predominated by silts. Nutrient and organic matter content were high, with higher concentrations in lakes with prevalence of fine particles. The reduced adsorption capacity of sediments, the resuspension by wind, the anthropogenic input and the accumulation favored by the arheic character of the basins would explain the high concentrations of nutrients in the water of these Pampean environments.
1. Introduction
Phosphorus and nitrogen are essential nutrients for living organisms because they are part of substances of high biological importance such as nucleic acids and proteins. In aquatic ecosystems, these nutrients are taken up by primary producers, either directly from the water in the case of phytoplankton or floating macrophytes or from sediments in the case of benthic algae or rooted macrophytes, where they pass to the rest of the trophic network.
The concentration of nutrients in the water is very variable and phosphorus is one of the factors responsible for the trophic status of an environment. Low concentrations determine oligotrophic ecosystems, with low biological productivity and high water transparency. Conversely, high concentrations determine eutrophic environments, in which the high biological productivity determines low water transparency [1,2]. Thus, as it can be concluded from the above, the trophic status of a water body can also be determined through the analysis of water transparency and the concentration of chlorophyll-a; however, the concentration of phosphorus is one of the strongest predictors of the characteristics of an aquatic ecosystem [3].
The abundance of phosphorus and nitrogen in an aquatic ecosystem is related to the type of soil in the catchment basin, because it tends to be higher in lakes located in sedimentary basins than in those located in areas with predominance of rocks of volcanic origin [4]. Nutrient concentrations also tend to be higher in environments influenced by human activities that produce discharges, either punctual, as the industrial and domestic ones, or diffuse, as agricultural ones [5-9]. These contributions, which alter the natural cycles, can dramatically change the characteristics of a water body [10,11], and may cause an increase in the biological production, in a process known as eutrophication [1,12].
These nutrients, especially phosphorus, are not only in the water column but also in the bottom sediments, where they can be either retained, forming the internal load [13-15], or resolubilized to re-enter the water column [16-18].
In Argentina, the trophic status of aquatic ecosystems has been studied in lakes at different locations and with contrasting characteristics, such as the deep oligotrophic Patagonian lakes located in the Andes mountain range, south of the 39˚ or, the hypertrophic shallow lakes of the fertile landscapes of the Pampa Plains, in the province of Buenos Aires, frequently associated with fluvial systems [4,19,20]. Given the high fertility of the soil of this region and the intense agricultural activities carried out in the lake basins, the nutrient levels in these lakes are among the highest cited in previous reports [19,21,22].
In the province of La Pampa, in the semiarid central region of Argentina, there are a large number of lakes and lagoons, very different from those mentioned above. Their most remarkable characteristics are their scarce depth, for which they can be considered shallow lakes, their temporality and their relatively high and variable salinity. They are located in arheic basins, in a region where rainfalls are overcome by evapotranspiration [23]. It has been determined that the concentrations of nutrients in the water of some of these shallow lakes are very high [24-31], which allows classifying them as hypertrophic [32]. The concentrations of nutrients in the sediments are also very high [27].
Since the information regarding the trophic status of these lakes is partial and fragmentary, the aim of this work is to describe the concentrations of nitrogen and phosphorus in the water and sediments of ten shallow lakes of La Pampa at different locations and under different anthropogenic influence and analyze their relation with the human activities carried out in their basins and with particle size and amount of organic matter in the sediments.
2. Material and Methods
2.1. Study Area
We studied ten water bodies located in different regions of the province of La Pampa (Table 1 and Figure 1).
Six of them, Estancia San José (ESJ), Chadilauquen (Cha), Estancia Pey-Ma (EPM), Ojo de agua Padre Buodo (OaPB), Utracán (Ut) and El Carancho (EC) are semi-permanent lakes subjected to large fluctuations in their surface area and water level. ESJ is located in the northeast of the province, in the region of the Pampa Plains [33] and is influenced mainly by agricultural activities such as cultivation of cereals and oilseeds and livestock rearing. Cha and EPM are located in the Ecotone between the Pampa Plains and the Thorny Forest [33] and are also influenced mainly by agricultural activities. OaPB, Ut, and EC are located in dune areas of the Thorny Forest, where the dominant activity is cattle breeding.
Other three environments Don Tomás (DT), La Arocena (LAr), and Bajo de Giuliani (BG) are located close
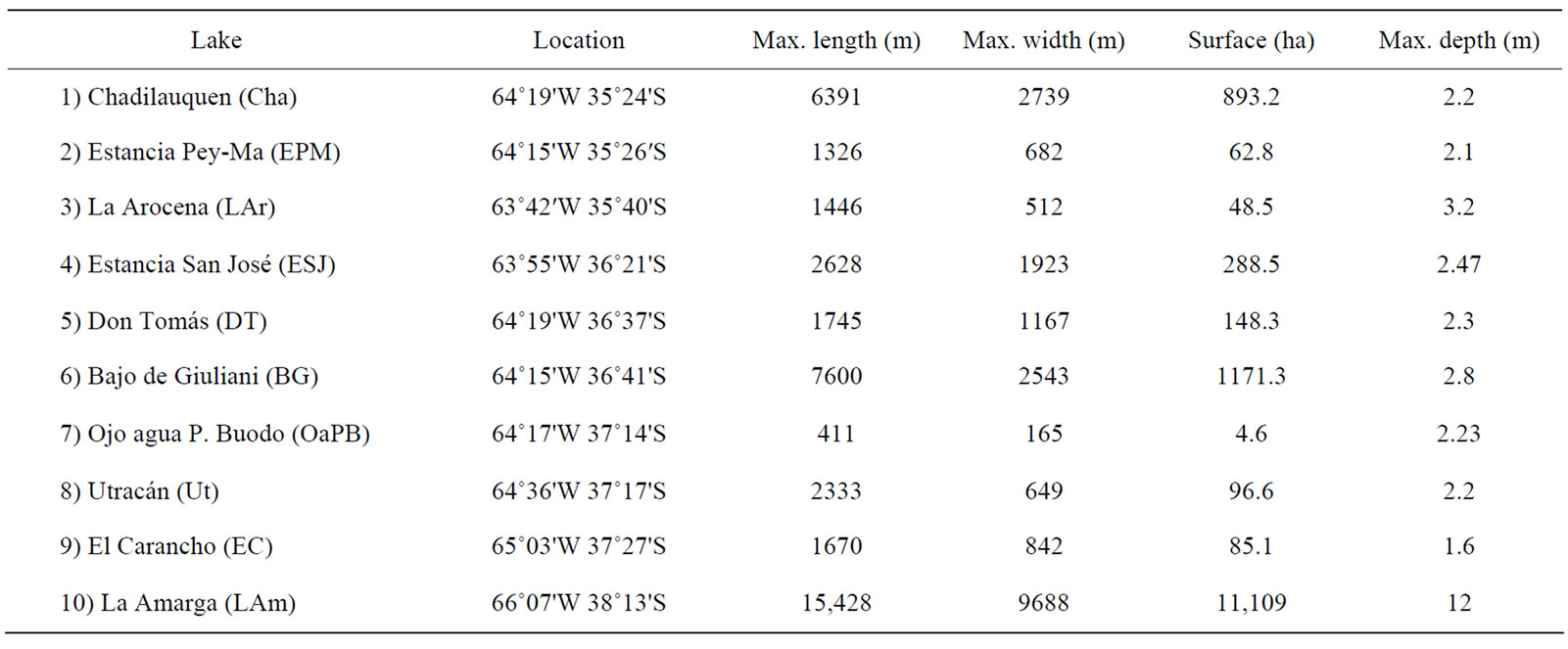
Table 1. Name, abbreviation, location and main morphometric parameters of the lakes studied.
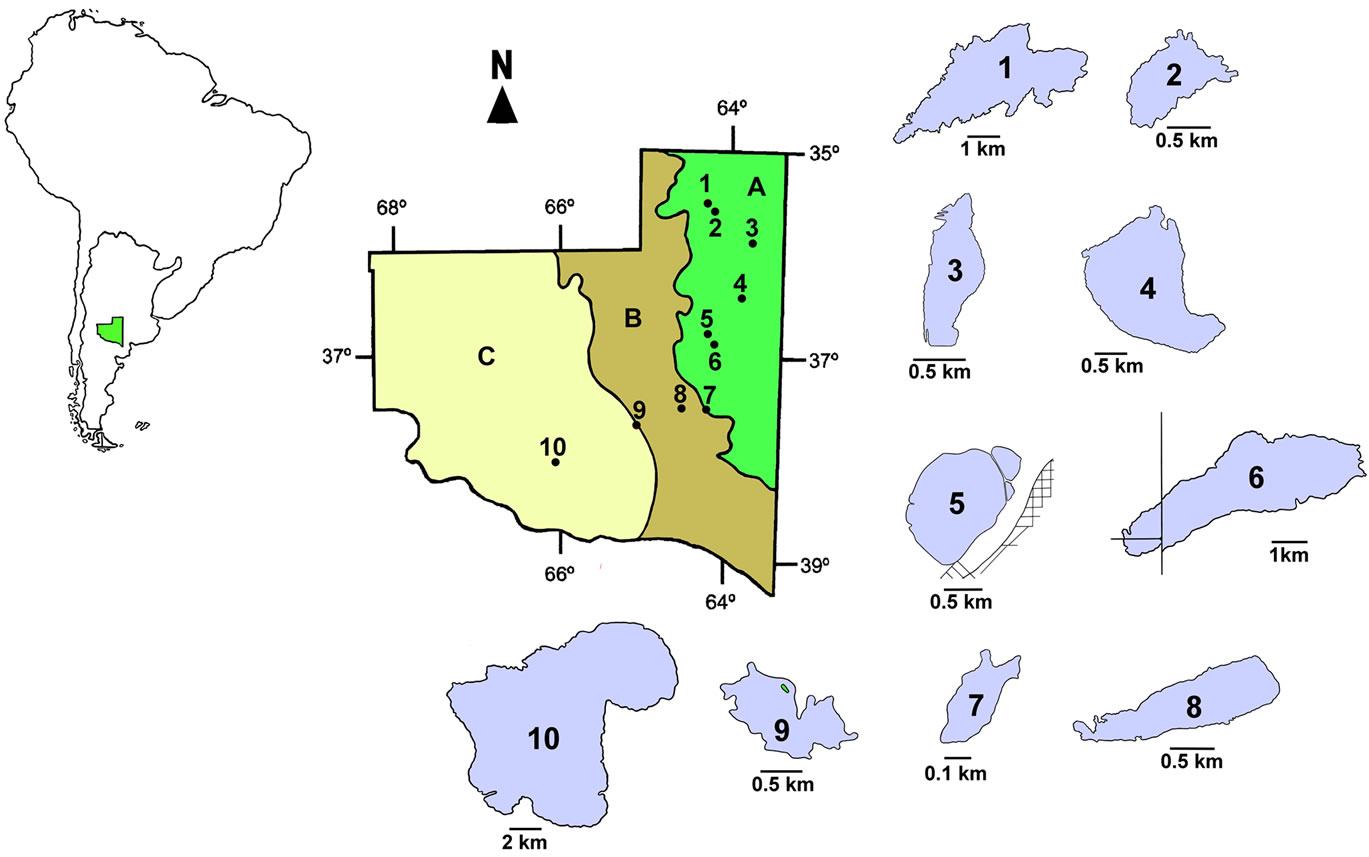
Figure 1. Geographic location of the studied lakes. 1: Chadilauquen; 2: Estancia Pey—Ma; 3: La Arocena; 4: Estancia San José; 5: Don Tomás; 6: Bajo de Giuliani; 7: Ojo de agua Padre Buodo; 8: Utracán; 9: El Carancho y 10: La Amarga. A, B and C: Phytogeographic regions of Pampa Plains, Thorny Forest and Monte respectively (Cabrera, 1976).
to cities and are recipients of storm drains and/or sewage. Due to this significant anthropogenic influence, these originally temporary lakes have become permanent ecosystems. All are shallow lakes with recreational and touristic importance; DT and LAr are adjacent to the cities of Santa Rosa and General Pico, respectively, whereas BG is located 10 km south of Santa Rosa, the capital city of the province of La Pampa.
These nine water bodies are in arheic basins, fed mainly by precipitation and to a lesser extent by phreatic contributions. Water losses occur mainly through evaporation or infiltration.
Finally, we also included La Amarga (LAm) (Figure 1), which differs from the rest by its extent and depth, both of which are considerably higher than those of the rest of the water bodies studied (Table 1), and by its feeding, given mainly by very sporadic contributions from the Chadileuvú River. Given that LAm is located in the phytogeographic province of the Monte [33]; in the most arid region of the province, human activity in the surrounding area is very scarce, restricted to ovine and caprine rearing for subsistence.
2.2. Field and Laboratory Work
Samplings were carried out seasonally during January, April, July and October 2007. Water transparency (Secchi disk) was determined in situ. Water samples were collected at 0.5 m depth with plastic bottles previously conditioned, and refrigerated for physical and chemical analysis and determination of nutrients and chlorophyll-a concentrations.
Sediment samples were collected in November 2007, with an Ekman-type dredge 15 × 15 cm (225 cm2). Samples were placed in polyethylene bags previously conditioned, and kept refrigerated until processing in the laboratory.
The concentration of total Kjeldahl nitrogen (TKN) in water was determined using the method of Kjeldahl and total phosphorus (TP) concentration was determined by digestion of the sample with potassium persulfate in acidic medium and spectrophotometry (Metrolab 1700 spectrophotometer) [34]. The concentration of nutrients in sediments was determined by drying at 55˚C - 60˚C, after acid digestion, neutralization and spectrophotometric reading with color development by reaction with molybdate in acid medium and reduction with ascorbic acid in the case of TP and with Nessler reagent in the case of total nitrogen.
Chlorophyll-a concentration was estimated by extraction with aqueous acetone with Microclar FFG047WPH filters and spectrophotometry (Metrolab 1700 spectrophotometer) [34,35].
We consider the trophic status classification proposed by OECD [32] (Table 2).
The granulometry of the sediments was determined using a Malvern Mastersizer 2000 particle counter. Car
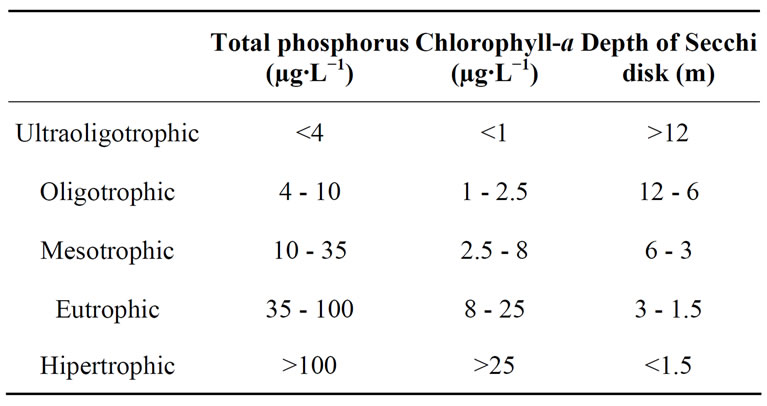
Table 2. Categories and ranges of trophic status considered.
bonates were removed with acetic acid 6% and organic matter was removed with 100-volume hydrogen peroxide. The material was dispersed with sodium hexametaphosphate 1% and application of ultrasound for 30 seconds. To identify the different fractions of the sediment we used the classification shown in Table 3.
A nonparametric Kruskal-Wallis test (H) was applied to determine significant differences between the parameters measured and Spearman correlation coefficients (rs) [36,37] were calculated to examine relationships between environmental factors. To detect possible clustering of lakes, we carried out paired groups and Ward’s method cluster analysis. InfoStat [38] and Past [39] software were used for analyses.
3. Results
Water transparency ranged relatively widely (Figure 2 and Table 4) and was different in the ten lakes studied (H = 33.53; p = 0.0001). We recognized two clusters (Figure 3): one including lakes with water transparency greater than 0.7 m and the other including lakes with significantly lower water transparency (Figure 3). Water transparency indicated that the lakes are hypertrophic, with the exception of LAm, which was in the limit of the eutrophy-hypertrophy intervals (Table 2).
The concentration of nutrients in the water of all the lakes was high and since we verified no seasonal pattern, we considered mean values. Regarding TP, EPM showed the highest concentration and was the only environment where the annual mean concentration was higher than 14 mg∙L−1 (Figure 4 and Table 4). The rest of the lakes showed TP concentrations lower than 10 mg∙L−1 and OaPB showed the lowest (close to 4 mg∙L−1) (Figure 4 and Table 4). However, the concentration of TP in water was not different between lakes (H = 12.53; p = 0.1851).
Since TP concentrations exceeded widely the lower limit proposed for the category (100 µg∙L−1), all the water bodies are framed in the category of hypertrophic (Table 2). Regarding TKN, the highest concentration, which exceeded 34 mg∙L−1, was also recorded in EPM and the lowest, which was close to 8 mg∙L−1, was re
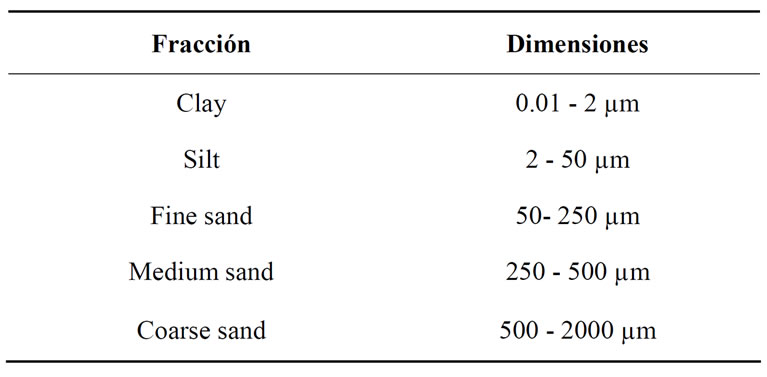
Table 3. Dimensions of the particles and size classes.
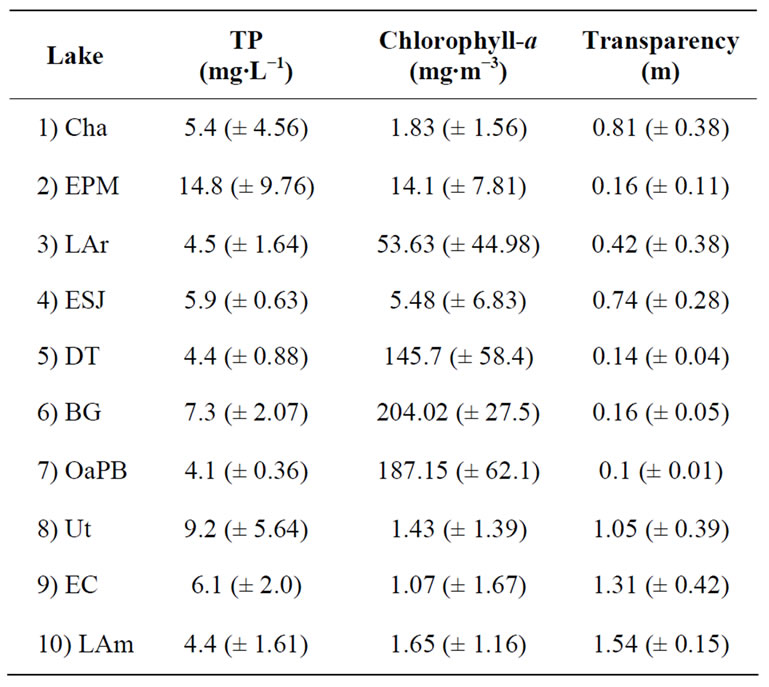
Table 4. Parameters determined in the 10 studied lakes (means and standard deviations).
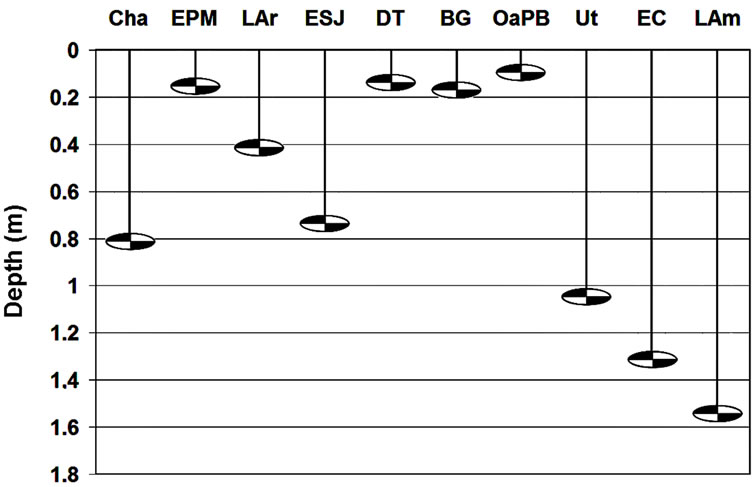
Figure 2. Mean water transparency of the ten studied lakes.
corded in EC (Figure 4 and Table 4). We found significant differences between the lakes (H = 26.27; p = 0.0018) because EPM and LAm showed values higher than 25 mg∙L−1, while the others showed values below 23 mg∙L−1 (Figure 4 and Table 4). We found no correlation between the concentrations of both nutrients in the water (rs = 0.23; p = 0.1553).
Chlorophyll-a concentrations ranged widely, from al-
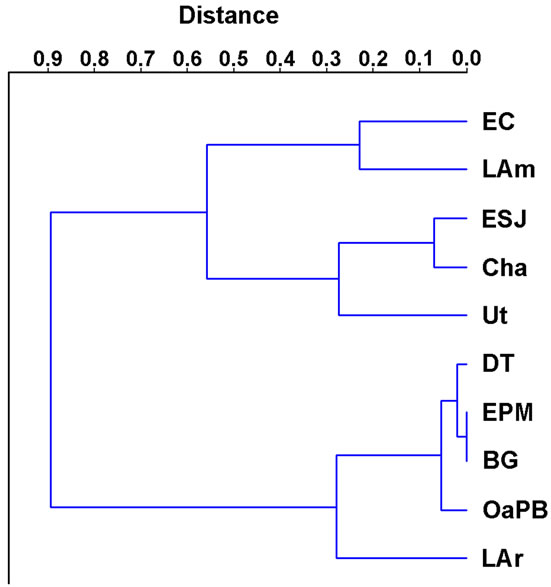
Figure 3. Clustering of lakes according to their transparency.
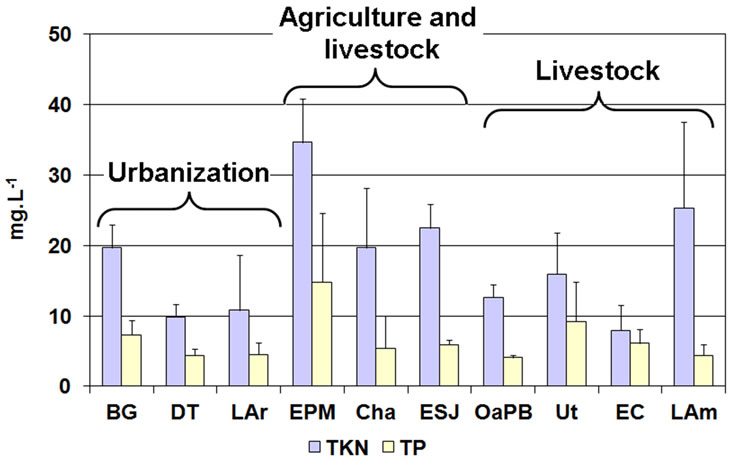
Figure 4. Mean concentration of nutrients in the water of the studied lakes, grouped by prevailing land use.
most 1 mg∙m−3 to more than 200 mg∙m−3, so the differences between lakes were significant (H = 33.33; p = 0.0001) (Table 4). Cluster analysis allowed observing two clusters: one made up of most of the lakes, with chlorophyll-a concentrations lower than 55 mg∙m−3 (Figure 5), and the other, due to the high correlation found between chlorophyll-a concentration and water transparency (rs = −0.84; p = 0.000), matching, to a large extent, the one defined based on water transparency, largely composed of DT, OaPB and BG, with concentrations higher than 140 mg∙m−3 (Figure 5). However, the correlations between chlorophyll-a concentrations and TP (rs = −0.13; p = 0.4207) and TN (rs = −0.10; p = 0.5326) were significant and negative in both cases.
Based on chlorophyll-a concentration, EPM, LAr, DT, BG and OaPB are eutrophic, whereas Cha, ESJ, Ut, EC and LAm were below the limit of 8 mg∙m−3 proposed for this category (Table 2).
The sediments of seven of the lakes studied (DT, LAr, Cha, OaPB, Ut, EC and LAm) were dominated by middle fraction particles, especially fine sand (Figure 6). In
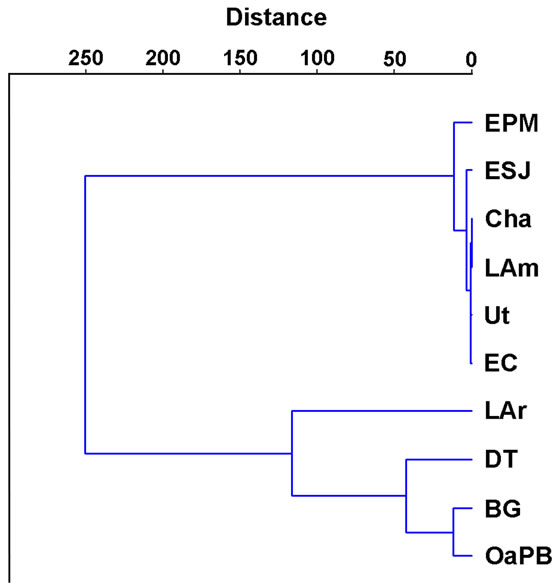
Figure 5. Clustering of lakes according to their phytoplankton chlorophyll-a concentration.
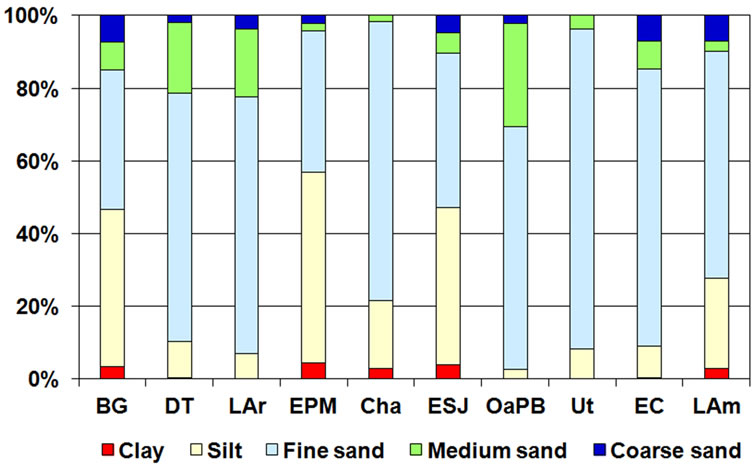
Figure 6. Percentage distribution of the particle size of the sediments of the ten studied lakes.
LAr, DT, OaPB, Ut and EC, this type of sediment exceeded 50% of the total. EPM, ESJ, and BG were dominated by thinner fractions, such as silts and clays, exceeding 3% (Figure 6). Despite the short distance between them, Cha and EPM differed in the composition of sediments: the former was dominated by fine sands whereas the latter, as mentioned above, was dominated by silts.
The content of organic matter in the sediments ranged from 0.47% to 9.61% (Figure 7) and was significantly correlated with particle size, since it was always lower in the lakes dominated by sands (rs = −0.75; p = 0.0116) and much higher in the case of the three water bodies dominated by clays (rs = 0.75; p = 0.0116) and silts (rs = 0.78; p = 0.0079).
The TP content in the sediments was high in all the lakes (Figure 8), ranging between 631.8 and 1606.4 mg∙kg−1. Although it was slightly higher in the lakes whose sediments were dominated by fine particles, we found no significant correlations between both parameters.
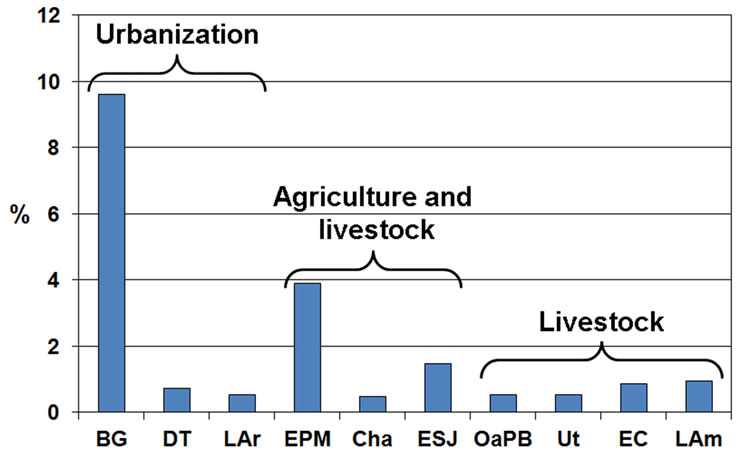
Figure 7. Percent content of organic matter in sediments of the studied lakes, grouped by prevailing land use.
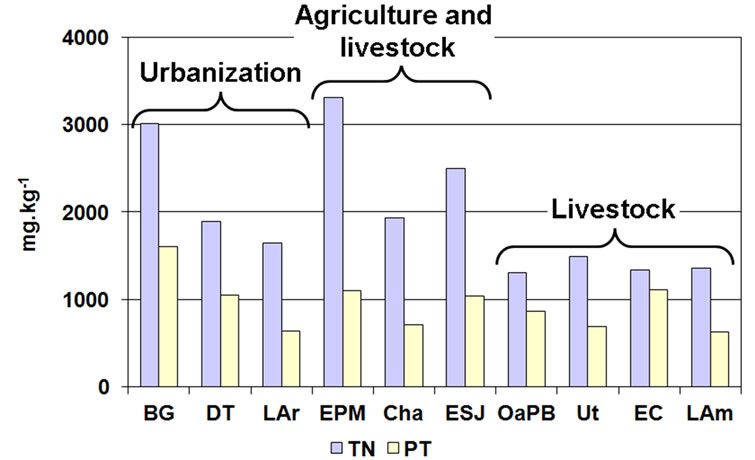
Figure 8. Content of total nitrogen and phosphorus in the sediments of the studied lakes, grouped by predominant land use.
The TN content followed a similar pattern, reaching values above 2500 mg∙kg−1 (Figure 8). We found significant correlations with particle size, since TN content was higher in lakes dominated by clayey (rs = 0.79; p = 0.0061) and silty (rs = 0.81; p = 0.0049) sediments and with higher amount of organic matter. In the lakes dominated by sands, TN was lower than 1900 mg∙kg−1 and was scarce in the lakes located at the center and southwest of the province (OaPB, Ut, EC and LAm).
4. Discussion
The high concentrations of nutrients recorded in the water of the ten shallow Pampean lakes studied in the present work allow categorizing all of them as hypertrophic [32]. TP concentrations were higher than those found by Quirós et al. [21] and Sosnovsky and Quirós [4] in shallow lakes in the upper basin of the Salado River, in the province of Buenos Aires, mentioned as some of the highest recorded in the world literature. In the lakes of the upper basin of the Salado River, which is located in one of Argentina’s most fertile regions, these authors recorded maximum TP concentrations close to 8 mg∙L−1. In contrast, in the lakes of La Pampa studied in the present work, we found that TP concentrations reached nearly two-fold higher values. Regarding TN, Quirós et al. [21] and Sosnovsky and Quirós [4] reported concentrations close to 28 mg∙L−1, whereas in the present study we found TKN values higher than 34 mg∙L−1.
In the province of Buenos Aires, the TP concentration in water is lower in lakes away from urban centers [21]. In contrast, the TP and TKN concentrations in some of the lakes away from urban centers studied in the present work but whose basins are used for agricultural activities tended to be higher than those in lakes influenced by cities, such as DT and LAr. This occurs despite that these lakes receive sewage waste sporadically, and BG permanently receives the waste produced by the nearly 100,000 people living in the city of Santa Rosa. On the other hand, the TP concentration in the water of some Pampean lakes surrounded by crops was found to be slightly lower than that in some lakes located in dune areas surrounded by natural vegetation and whose basins are used for livestock rearing. This could be due to the mechanism of diffuse pollution caused by the dragging of animal feces, especially during a storm, because this is one of the elements with greatest impact on the eutrophication of a water body [5,8], since cattle may excrete between 9 and 16 kg P∙ind∙year−1 [40]. Conversely, TKN concentrations tended to be higher in lakes whose basins are used for agriculture, probably due to the dragging of nitrogen fertilizers.
In some cases, especially in temporary lakes, chlorophyll-a concentrations or water transparency were lower than the limit values proposed by OECD [32] for the category of hypertrophic environments. However, the TP concentrations recorded in the water exceeded that limit widely, which led to negative relations. Thus, in La Pampa, both parameters should be interpreted with caution. This could be due to the presence of zooplanktivorous fish in lakes where there were higher concentrations of chlorophyll-a and therefore lower transparencies (DT, LAr, BG and OaPB). The presence of fish fauna, mainly of the zooplanktivorous silverside (Odontesthes bonariensis), is favored by the change from temporary to permanent lakes. Because of the presence of this predator, most of the species found in the zooplankton (especially among cladocerans) are small [19,21,22,28,41-43], a fact that has a great influence on the water quality of the lake. The model of alternative states of shallow lakes indicates that the presence of planktivorous fish in the ecosystem causes a trophic cascade or top-down effect [42,44-46]. The predation of this fish on zooplankton of greater size and therefore greater filtration efficiency leads phytoplankton chlorophyll-a concentrations to increase and water transparency to decrease, leading to the state known as turbid [42,44-47].
The bottom sediments of most of the lakes studied were dominated by sands, which is the material that covers most of the surface of the provincial territory [48,49]. The finest sediments were only more abundant in three water bodies, with no regional pattern, given the distance between them and the relatively different landscapes in which they are located.
The sediments of the Pampean lakes have high amounts of nutrients. In some of them, TP concentrations exceeded 1100 mg of P∙kg−1, twice more than that verified in hypertrophic lakes of the USA [50] or Spain [51], but were relatively similar to those recorded in shallow lakes in the middle and lower basin of the Yangtzé River, in China [52].
The contents of organic matter, TP and TN reported in sediments were higher in the three water bodies that showed highest proportion of silt and clay (BG, EPM and ESJ) and lowest in the lakes dominated by sandy sediments, given the lower adsorption capacity of larger particles [14,53]. Both the nutrients and organic matter of sediments were much more abundant in BG, probably due to the continuous sewage discharges from the city of Santa Rosa received by this lake. The lowest TN concentrations were recorded in the four lakes that are in dune areas or surrounded by native vegetation and where activities in the basin are limited to livestock raising (OaPB, Ut, EC and LAm).
In addition to the influence of the activities carried out in the basins, the high concentrations of nutrients in the waters of the Pampean lakes studied could also be due to a process of internal eutrophication caused by the resolubilization of phosphorus from the sediments and its reentry into the water column [15,54], favored by chemical factors, such as alkalinity [15,54,55] and presence of sulfates [2], physical factors, such as diffusion, bubbling or resuspension of sediments, or biological factors, such as bioturbation [1,17,56]. Among the above-mentioned agents, the resuspension of sediments by the effect of wind is particularly important in shallow lakes, since shallowness favors polymixis [13,16,18,57-59]. It has been verified that, in these lakes, the amount of phosphorus that is resuspended is 5 to 10 times higher than that resuspended in deep lakes [2,60].
5. Conclusions
All the shallow lakes studied in La Pampa were hypertrophic.
The nutrient concentrations in some of the lakes whose basins are used for agricultural activities tended to be higher than those influenced by cities, despite that these lakes receive sewage waste sporadically or permanently. This could be due to the mechanism of diffuse pollution caused by the dragging of animal feces or nitrogen fertilizers.
In some temporary lakes, chlorophyll-a concentrations and water transparency were lower than the limit for the category of hypertrophic environments, probably due to the absence of planktivorous fish predation, allowing the presence of large zooplankton with high filtration efficiency.
The bottom sediments of most of the lakes studied were dominated by sands and had high amounts of nutrients and organic matter.
A combination of factors such as the reduced absorption capacity of sediments given by the relatively large size of the particles, the frequent resuspension caused by the wind, the continuous income by human activities and the process of accumulation of nutrients favored by the arheic nature of the basin, would explain the high concentrations of phosphorus and nitrogen in the water of the lakes of the province of La Pampa, which are among the highest values reported for lakes in the world.
However, taking into account that some of the lakes studied are temporary and are currently dry because of a period of low rainfall, new studies carried out during different hydroperiods would be useful to compare these results.
REFERENCES
- R. Wetzel, “Limnology. Lake and River Ecosystems,” Academic Press, Elsevier, San Diego, 2001.
- J. Kalff, “Limnology. Inland Water System,” Prentice Hall, New Jersey, 2002.
- M. Brett and M. Benjamin, “A Review and Reassessment of Lake Phosphorus Retention and the Nutrient Loading Concept,” Freshwater Biology, Vol. 53, No. 1, 2008, pp. 194-211.
- A. Sosnovsky and R. Quirós, “El Estado Trófico de Pequeñas Lagunas Pampeanas, su Relación con la Hidrología y el uso de la Tierra,” Ecología Austral, Vol. 16, No. 2, 2006, pp. 115-124.
- S. Carpenter, N. Caraco, D. Correll, R. Howarth, A. Sharpley and V. Smith, “Nonpoint Pollution of Surface Waters with Phosphorus and Nitrogen,” Ecological Applications, Vol. 8, No. 3, 1998, pp. 559-568. http://dx.doi.org/10.1890/1051-0761(1998)008[0559:NPOSWW]2.0.CO;2
- K. Arbuckle and J. Downing, “The Influence of Watershed Land Use on Lake N: P in a Predominantly Agricultural Landscape,” Limnology and Oceanography, Vol. 46, No. 4, 2001, pp. 970-975. http://dx.doi.org/10.4319/lo.2001.46.4.0970
- K. Krogerus and P. Ekholm, “Phosphorus in Settling Matter and Bottom Sediments in Lakes Loaded by Agriculture,” Hydrobiologia, Vol. 429, 2003, pp. 15-28. http://dx.doi.org/10.1023/A:1024879309946
- M. Bremigan, P. Soranno, M. González, D. Bunnell, K. Arend, W. Renwick, R. Stein and M. Vanni, “Hydrogeomorphic Features Mediate the Effects of Land Use/Cover on Reservoir Productivity and Food Webs,” Limnology and Oceanography, Vol. 53, No. 4, 2008, pp. 1420-1433. http://dx.doi.org/10.4319/lo.2008.53.4.1420
- C. Zhou, Y. Zhou, X. Chen, Y. Li, X. Cao and C. Song, “Linkage between Land Use Patterns and Sediment Phosphorus Sorption Behaviours along Shoreline of Chinese Large Shallow Lake (Lake Chaohu),” Knowledge and Management of Aquatic Ecosystems, No. 403, 2011, pp. 6-14. http://dx.doi.org/10.1051/kmae/2011067
- A. Hargeby, I. Blindow and L. Hansson, “Shifts between Clear and Turbid States in a Shallow Lake: Multi-Causal Stress from Climate, Nutrients and Biotic Interactions,” Archives fur Hydrobiologie, Vol. 161, No. 4, 2004, pp. 433-454. http://dx.doi.org/10.1127/0003-9136/2004/0161-0433
- M. Genkai-Kato, “Regime Shifts: Catastrophic Responses of Ecosystems to Human Impacts,” Ecological Research, Vol. 22, No. 2, 2007, pp. 214-219. http://dx.doi.org/10.1007/s11284-006-0304-5
- W. K. Dodds, “Trophic State, Eutrophication and Nutrient Criteria in Streams,” Trends in Ecology and Evolution, Vol. 22, No. 12, 2007, pp. 669-676. http://dx.doi.org/10.1016/j.tree.2007.07.010
- M. Søndergaard, J. Jensen and E. Jeppesen, “Role of Sediment and Internal Loading of Phosphorus in Shallow Lakes,” Hydrobiologia, Vol. 506-509, No. 1-3, 2003, pp. 135-145. http://dx.doi.org/10.1023/B:HYDR.0000008611.12704.dd
- K. Łukawska-Matuszewska, R. Vogt and R. Xie, “Phosphorus Pools and Internal Loading in a Eutrophic Lake with Gradients in Sediment Geochemistry Created by Land Use in the Watershed,” Hydrobiologia, Vol. 713, No. 1, 2013, pp. 183-197. http://dx.doi.org/10.1007/s10750-013-1506-9
- E. Liu, J. Shen, H. Yuan, E. Zhang and C. Du, “The Spatio-Temporal Variations of Sedimentary Phosphorus in Taihu Lake and the Implications for Internal Loading Change and Recent Eutrophication,” Hydrobiologia, Vol. 711, No. 1, 2013, pp. 87-98. http://dx.doi.org/10.1007/s10750-013-1465-1
- I. de Vicente, V. Amores and L. Cruz-Pizarro, “Inestability of Shallow Lakes: A Matter of the Complexity of Factors Involved in Sediment and Water Interaction?” Limnética, Vol. 25, No. 1-2, 2006, pp. 253-270.
- M. Hupfer and J. Lewandowski, “Oxygen Controls the Phosphorus Release from Lake Sediments—A LongLasting Paradigm in Limnology,” International Review of Hydrobiology, Vol. 93, No. 4-5, 2008, pp. 415-432. http://dx.doi.org/10.1002/iroh.200711054
- Y. Kang, X. Song and Z. Liu, “Sediment Resuspension Dampens the Effect of Nutrient Inputs on the Phytoplankton Community: A Mesocosm Experiment Study,” Hydrobiologia, Vol. 710, No. 1, 2012, pp. 117-127. http://dx.doi.org/10.1007/s10750-012-1221-y
- M. Boveri and R. Quirós, “Trophic Interactions in Pampean Shallow Lakes: Evaluation of Silverside Predatory Effects in Mesocosms Experiments,” Verhandlungen des Internationalen Verein Limnologie, No. 28, 2002, pp. 1-5.
- A. Torremorell, J. Bustingorry, R. Escaray and H. Zagarese, “Seasonal Dynamics of a Large, Shallow Lake, Laguna Chascomús: The Role of Light Limitation and Other Physical Variables,” Limnologica, Vol. 37, No. 1, 2007, pp. 100-108. http://dx.doi.org/10.1016/j.limno.2006.09.002
- R. Quirós, A. Rennella, M. Boveri, J. J. Rosso and A. Sosnovsky, “Factores que Afectan la Estructura y el Funcionamiento de las Lagunas Pampeanas,” Ecología Austral, Vol. 12, 2002, pp. 175-185.
- M. Boveri and R. Quirós, “Cascading Trophic Effects in Pampean Shallow Lakes: Results of a Mesocosm Experiment Using Two Coexisting Fish Species with Different Feeding Strategies,” Hydrobiologia, Vol. 584, No. 1, 2007, pp. 215-222. http://dx.doi.org/10.1007/s10750-007-0581-1
- E. Ponce de León, “Evapotranspiración,” In: F. Chadileuvú Ed., El agua en La Pampa, Fondo Editorial Pampeano, Santa Rosa, 1998.
- S. Echaniz, A. Vignatti and P. Bunino, “El Zooplancton de un Lago Somero Hipereutrófico de la Región Central de Argentina. Cambios Después de una Década,” Biota Neotropica, Vol. 8, No. 4, 2008, pp. 63-71. http://dx.doi.org/10.1590/S1676-06032008000400005
- S. Echaniz, A. Vignatti and C. Cabrera. “Características Limnológicas de una Laguna Turbia Orgánica de la Provincia de La Pampa y Variación Estacional del Zooplancton,” Biología Acuática, No. 26, 2009, pp. 71-82.
- S. Echaniz, A. Vignatti, S. B. José de Paggi and G. Cabrera, “El Modelo de Estados Alternativos de Lagos Someros en La Pampa: Comparación de Bajo de Giuliani y El Carancho,” Libro de Trabajos del 3° Congreso Pampeano del Agua, 2010, pp. 45-53.
- S. Echaniz, A. Vignatti, S. B. José de Paggi and J. C. Paggi, “Los Nutrientes en los Sedimentos de Lagunas de La Pampa. Relación con la Granulometría y uso de la Tierra,” Libro de Trabajos del 3° Congreso Pampeano del Agua, 2010, pp. 23-31.
- S. Echaniz, A. Vignatti, G. Cabrera and S. B. José de Paggi, “Zooplankton Richness, Abundance and Biomass of Two Hypertrophic Shallow Lakes with Different Salinity,” Biota Neotropica, Vol. 12, No. 2, 2012, pp. 37- 44. http://dx.doi.org/10.1590/S1676-06032012000200005
- S. Echaniz, G. Cabrera, C. Rodríguez and A. Vignatti, “Do Temporary Lakes Vary from Year to Year? A Comparison of Limnological Parameters and Zooplankton from Two Consecutive Annual Cycles in an Argentine Temporary Saline Lake,” International Journal of Aquatic Science, Vol. 4, No. 1, 2013, pp. 44-61.
- S. Echaniz and A. Vignatti, “Seasonal Variation and Influence of Turbidity and Salinity on the Zooplankton of a Saline Lake in Central Argentina,” Latin American Journal of Aquatic Research, Vol. 39, No. 2, 2011, pp. 306-315. http://dx.doi.org/10.3856/vol39-issue2-fulltext-12
- A. Vignatti, G. Cabrera and S. Echaniz, “Changes in the Zooplankton and Limnological Variables of a Temporary Hypo-Mesosaline Wetland of the Central Region of Argentina during the Drying,” Pan American Journal of Aquatic Sciences, Vol. 7, No. 2, 2012, pp. 93-106.
- OECD (Organization for Economic Cooperation and Development), “Eutrophication of Waters. Monitoring, Assesment and Control. Final Report,” París, 1982.
- A. Cabrera, “Regiones Fitogeográficas Argentinas,” Fascículo 1, Enciclopedia Argentina de Agricultura y jardinería. Acme, Buenos Aires, 1976.
- APHA (American Public Health Association), “Standard Methods for the Examination of Water and Wastewater,” 18th Edition, Washington, DC, 1992.
- E. J. Arar, “In Vitro Determination of Chlorophylls a, b, c + c and Pheopigments in Marine and Freshwater Algae by Visible Spectrophotometry,” Method 446.0. US Environmental Protection Agency, 1997.
- R. Sokal and F. Rohlf, “Biometría. Principios y Métodos Estadísticos en la Investigación Biológica,” Blume, Barcelona, 1995.
- J. H. Zar, “Biostatistical Analysis,” Prentice Hall, New Jersey, 1996.
- J. A. Di Rienzo, F. Casanoves, M. G. Balzarini, L. González, M. Tablada and W. Robledo, “InfoStat (Versión 2010),” Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina, 2010.
- Ø. Hammer, D. Harper and P. Ryan, “PAST: Paleontological Statistics Software Package for Education and Data Analysis,” Palaeontologia Electronica, Vol. 4, No. 1, 2001, pp. 1-9.
- M. Russell, D. Weller, T. Jordan, K. Sigwart and K. Sullivan, “Net Anthropogenic Phosphorus Inputs: Spatial and Temporal Variability in the Chesapeake Bay Region,” Biogeochemistry, Vol. 88, No. 3, 2008, pp. 285- 304. http://dx.doi.org/10.1007/s10533-008-9212-9
- J. Brooks and S. Dodson, “Predation, Body Size and Composition of Plankton,” Science, Vol. 150, No. 3692, 1965, pp. 28-35. http://dx.doi.org/10.1126/science.150.3692.28
- M. Scheffer, “Ecology of Shallow Lakes,” Chapman & Hall, London, 1998.
- J. Rosso, “Peces Pampeanos. Guía y Ecología,” L.O.L.A. (Literature of Latin America), Buenos Aires, 2006.
- M. Scheffer and E. Jeppesen, “Regime Shifts in Shallow Lakes,” Ecosystems, Vol. 10, No. 1, 2007, pp. 1-3. http://dx.doi.org/10.1007/s10021-006-9002-y
- E. Jeppesen, M. Søndergaard, M. Meerhoff, T. Lauridsen and J. P. Jensen, “Shallow Lake Restoration by Nutrient Loading Reduction—Some Recent Findings and Challenges Ahead,” Hydrobiologia, Vol. 584, No. 1, 2007, pp. 239-252. http://dx.doi.org/10.1007/s10750-007-0596-7
- E. Jeppesen, M. Meerhoff, B. Jacobsen, R. Hansen, M. Søndergaard, J. Jensen, T. Lauridsen, N. Mazzeo and C. Branco, “Restoration of Shallow Lakes by Nutrient Control and Biomanipulation—The Successful Strategy Varies with Lake Size and Climate,” Hydrobiologia, Vol. 581, No. 1, 2007, pp. 269-285. http://dx.doi.org/10.1007/s10750-006-0507-3
- E. Bakker, J. Sarneel, R. Gulati, Z. Liu and E. van Donk, “Restoring Macrophyte Diversity in Shallow Temperate Lakes: Biotic versus Abiotic Constraints,” Hydrobiologia, Vol. 710, No. 1, 2013, pp. 23-37. http://dx.doi.org/10.1007/s10750-012-1142-9
- E. Cano, “Inventario Integrado de los Recursos Naturales de la Provincia de La Pampa,” Instituto Nacional de Tecnología Agropecuaria (INTA), Provincia de La Pampa y Universidad Nacional de La Pampa, Buenos Aires, 1980.
- A. Calmels and S. Casadío, “Compilación Geológica de la Provincia de La Pampa,” Amerindia, Santa Rosa, 2005.
- A. Pilati, M.Vanni, M. González and A. Gaulke, “Effects of Agricultural Subsidies of Nutrients and Detritus on Fish Andplankton of Shallow-Reservoir Ecosystems,” Ecological Applications, Vol. 19, No. 4, 2009, pp. 942- 960. http://dx.doi.org/10.1890/08-0807.1
- P. López, “Composición del Sedimento en Sistemas Acuáticos del Litoral Mediterráneo Español,” Limnética, Vol. 2, 1986, pp. 11-18.
- S. Wang, X. Jin, H. Zhao and F. Wu, “Phosphorus Fractions and Its Release in the Sediments from the Shallow Lakes in the Middle and Lower Reaches of Yangtze River Area in China,” Colloids and Surfaces A Physicochemical Engineering Aspects, No. 273, 2006, pp. 109-116.
- G. Kapanen, “Phosphorus Fractionation in Lake Sediments,” Estonian Journal of Ecology, Vol. 57, No. 4, 2008, pp. 244-245. http://dx.doi.org/10.3176/eco.2008.4.02
- A. Smolders, L. Lamers, E. Lucassen, G. Van Der Velde and J. Roelofs, “Internal Eutrophication: How It Works and What to Do about It—A Review,” Chemistry and Ecology, Vol. 22, No. 2, 2006, pp. 93-111. http://dx.doi.org/10.1080/02757540600579730
- S. Katsev, I. Tsandev, I. Heureux and D. Rancourt, “Factors Controlling Long-Term Phosphorus Effluxes from Lakes Sediments: Exploratory Reactive-Transport Modeling,” Chemical Geology, Vol. 324, No. 1-2, 2006, pp. 127-147. http://dx.doi.org/10.1016/j.chemgeo.2006.05.001
- K. Havens, K-R. Jin, N. Iricanin and R. James, “Phosphorus Dynamics at Multiple Time Scales in the Pelagic Zone of a Large Shallow Lake In Florida, USA,” Hydrobiologia, Vol. 581, No. 1, 2007, pp. 25-42. http://dx.doi.org/10.1007/s10750-006-0502-8
- E. Nagid, D. Canfield Jr. and M. Hoyer, “Wind-Induced Increases in Trophic State Characteristics of a Large (27 km2), Shallow (1.5 m Mean Depth) Florida Lake,” Hydrobiologia, Vol. 455, No. 1-3, 2001, pp. 97-110. http://dx.doi.org/10.1023/A:1011913200302
- H. Markensten and D. C. Pierson, “A Dynamic Model for Flow and Wind Driven Sediment Resuspension in a Shallow Basin,” Hydrobiologia, Vol. 494, No. 1-3, 2003, pp. 305-311. http://dx.doi.org/10.1023/A:1025459525880
- C. Borell Lövstedt and L. Bengtsson, “The Role of NonPrevailing Wind Direction on Resuspension and Redistribution of Sediments in a Shallow Lake,” Aquatic Sciences, Vol. 70, No. 3, 2008, pp. 304-313. http://dx.doi.org/10.1007/s00027-008-8047-8
- U. Selig and G. Schlungbaum, “Characterisation and Quantification of Phosphorus Release from Profundal Battom Sediments in Two Dimictic Lakes during Summer Stratification,” Journal of Limnology, Vol. 62, No. 2, 2003, pp. 151-162. http://dx.doi.org/10.4081/jlimnol.2003.151

