Journal of Environmental Protection
Vol.4 No.8(2013), Article ID:35463,7 pages DOI:10.4236/jep.2013.48093
Selected Heavy Metals in Water and Sediments and Their Bioconcentrations in Plant (Polygonum pulchrum) in Sosiani River, Uasin Gishu County, Kenya
![]()
1Department of Environmental Biology and Health, School of Environmental Studies, University of Eldoret, Eldoret, Kenya; 2Department of Chemistry, School of Science, University of Eldoret, Eldoret, Kenya.
Email: judithkeny@gmail.com, *gelasmuse@yahoo.com
Copyright © 2013 Judith K. Jepkoech et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 23rd, 2013; revised June 25th, 2013; accepted July 2nd, 2013
Keywords: Bioaccumulation; Bioconcentration factor; food chain; exposure risks
ABSTRACT
Heavy metals’ availability and accumulation along the food chain pose public health risks. Water, sediment and plant samples were collected from selected sampling sites along Sosiani River, Uasin Gishu County, Kenya. The sediment and plant samples were dried in the oven at 50˚C to constant weight and digested in a mixture of acids according to standard procedures. The water samples, sediments and plants digests were analyzed for selected heavy metals using Atomic Absorption Spectrophotometer (model AAS Variant 200). The site near the Moi Teaching and Referral Hospital (MTRH) had the highest total heavy metals concentration in water: Cu (0.18 ± 0.04 ppm); Pb (0.46 ± 0.09 ppm) and Zn (0.70 ± 0.22 ppm) and sediments: Cu (1.62 ± 0.14 ppm); Pb (1.27 ± 0.17 ppm) and Zn (6.73 ± 0.88 ppm) respectively. Fractionation of heavy metals in sediments showed low percentage solubility (Cu 9.3%; Pb 8.5%; Zn 4.2%). Concentration of zinc in studied plants was highest (3.60 ± 0.63 ppm), with a bioconcentration factor of 15.1 based on soluble zinc fraction. This indicates that conditions in the study area show preferential zinc metal uptake in plants and may lead to accumulation in exposed plants posing Zn exposure risks along the food chain. Suggestions are made for monitoring of heavy metals in food crops and aquatic organisms such as fish in the study area.
1. Introduction
Water is essential to life and because of its importance; the pattern of human settlement throughout history has often been determined by its availability [1]. Water is exposed to innumerous natural and/or anthropogenic influence in the form of pollutants including toxic metals such as lead, cadmium and chromium. Rivers are the dominant pathway for metals transport [2]. The behaviour of metals in natural waters is a function of the substrate sediment composition, the suspended sediment composition, and the water chemistry [3]. During their transports, the trace metals undergo numerous changes in their speciations due to dissolution, precipitation, sorption and complexation phenomena [4,5] which affect their bioavailabilities [6]. Thus, contamination of rivers with heavy metals may cause devastating effects on the ecological balance of the aquatic environment and the diversity of the aquatic organisms [7].
Heavy metals are bioconcentrated or bioaccumulated in one or several compartments across food webs [8,9]. Metal bioaccumulation can be of importance from the public health point of view, especially for human at the end of the food chain. An important link in the transfer of heavy metals from soil/sediment to man is plants [10].
The distribution of heavy metals in sediments can provide an evidence of the anthropogenic impact on aquatic ecosystems and therefore aid in assessing the risks associated with discharged waste [11]. According to LópezGonzáles et al. [12], knowledge of metal fractions and their chemical forms in sediment is of great significance in determining remobilization potential of metals in the environment. Studies on the distribution and forms of heavy metals in sediments can provide the actual environmental impact and bioavailability. This study was aimed to assess the bioconcentration of heavy metals (lead, zinc, and copper) in plant (Polygonum pulchrum) in River Sosiani.
2. Materials and Methods
2.1. Study Area
Figure 1 shows the study area along River Sosiani in Uasin Gishu County, Kenya. The socio-economic activities in the study area entail wheat, maize and animal farming in the upper part (Zone 1). The urban set up in the lower part (Zones 2 and 3) comprises the Central Business District (CBD), road networks, garages and vehicle repair sheds, industries and factories, and hospitals including MTRH that discharge their effluents in to the River Sosian.
2.2. Chemicals, Reagents and Materials
The reagents consisted of concentrated nitric acid (HNO3) (Analar, England), hydrogen peroxide (H2O2) -30% (reagent grade, Mumbai, India), concentrated hydrochlo ric acid (HCl) (Analar, MERCK Darmstadt, Germany). Others were concentrated sulphuric acid (Analar, MERCK Damstadt, Germany), distilled water, and standard solution. Materials consisted of Filter papers (Whatman No. 42), beakers, conical flasks, volumetric flask, pestle and mortar, weighing machine and atomic-
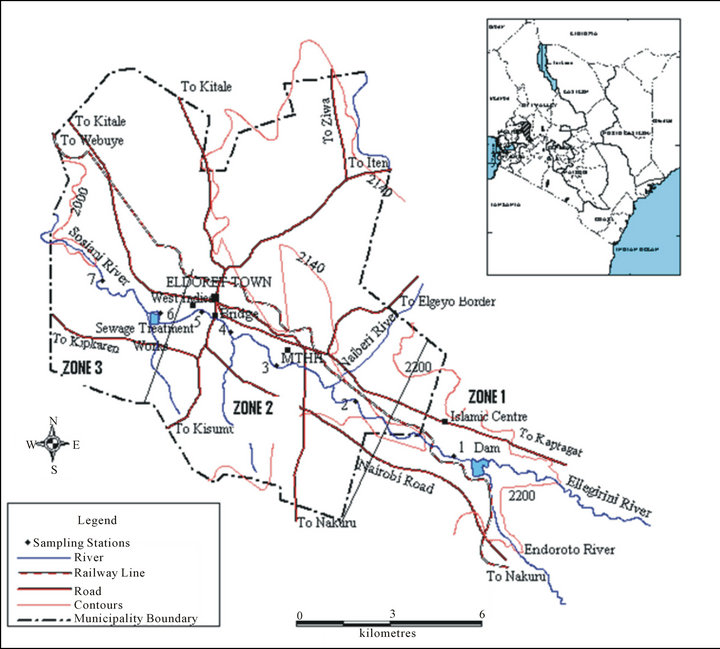
Figure 1. Zonation and location of study area and sampling sites along Sosiani River (zone 1: Kaptagat forest, Islamic center where farming activities like wheat and maize and also animal husbandry is carried out; zone 2: includes Central Business District (CBD), garages industries and hospitals like MTRH and zone 3: estates such as Huruma and sewerage treatment plants).
absorption spectrometer (AAS Variant 200).
3. Field Sampling
3.1. Water Sampling
Water samples were collected at the identified sampling sites using pre-cleaned 0.5 L plastic bottles. During sampling, the bottles were rinsed 3 times with river water before the samples were collected. The water samples were preserved by adding a 2 ml concentrated nitric acid [13]. In each case, temperature and pH of water was measured in-situ by use of pH meter model (Jenway 370).
3.2. Sediment Sampling
Sediment samples were collected from same locations as water samples. Deposited top layer of the bottom sediment samples from the river were collected by scooping, using a trowel at a depth of 5 cm. The samples were kept separately in labeled polythene bags placed in ice-box and transported to Moi University for treatment and metal analyses.
3.3. Plant Samples
Plant samples were collected as close as possible from same sites as water and sediment samples. The leaves and stem of Polygonum pulchrum plant were collected and put in polythene bags placed in iced box and then taken to the laboratory for treatment and analysis.
4. Laboratory Analysis
4.1. Heavy Metals in Water Samples
The water sample from each sampling bottle was mixed thoroughly by shaking. A 50 ml aliquot of water sample was pipette into a digestion flask. Digestion was done using a mixture of concentrated nitric acid and sulphuric acid [14]. A 3 ml volume of concentrated nitric acid was added and brought to boil slowly on a hot plate controlling the temperature at 70C evaporating it to about 15 ml, followed by addition of 3 ml concentrated nitric acid and 5 ml concentrated sulphuric acid while continuing heating until the solution cleared and brown fumes were no longer evident. On cooling digested samples were filtered through 0.45 um filter paper then topped to the mark with de-ionized water. The digest was analyzed for total copper, zinc and lead using Atomic Absorption Spectrophotometer (AAS Variant 200).
4.2. Heavy Metals in Sediment Samples
Sediment samples were first air dried and later oven dried at 50˚C for 24 hours to a constant weight. The sediment were crushed with pestle and mortar into fine particles then passed through a 2 mm sieve. A 1.0 g of sieved sample was weight accurately into a digestion conical flask. The sample was refluxed with 5 ml of concentrated nitric acid (analar grade) and shook for 2 minutes, and then 2 ml of concentrated hydrochloric acid was added while continuing shaking. The Sample mixture was transferred to a hot plate covered with a watch glass. The mixture was heated for about 2 hours until no more brown fumes evolve controlling temperature at 70˚C. The solution was cooled, filtered into a 50 ml flask. The filtrate was made to the mark with distilled water. Zinc, copper and lead in the filtrate were determined using Atomic Absorption Spectrophotometer as described by Cui et al. [15].
4.3. Heavy Metals in Plant Sample
Leaves and stems of Polygonum pulchrum plant samples were cleaned with de-ionized water, air dried and then oven dried to constant weight at 50˚C. The dried sample was crushed into fine particles in a pestle and mortar and passed through a 2 mm mesh sieve. A 1.0 gram of evenly grounded sample was accurately weighed into a conical flask. A 5 ml volume of concentrated sulphuric acid (Analar grade) was added. The mixture was gently shaken for 2 minutes, and 5 ml of 30% hydrogen peroxide was slowly added and left to react. After reaction had subsided the mixture was heated on a hot plate at 70˚C for 2 hours. A 2 ml concentrated nitric acid portion was added to remove excess sulphuric acid. The heating continued until solution of light straw colour was obtained. The solution was cooled, filtered into a 50 ml flask [16]. The filtrate was made to the mark with distilled water. The analysis for heavy metals copper, zinc and lead were done the same way as in case of water samples.
5. Results and Discussion
5.1. Total Heavy Metals Concentrations
5.1.1. Heavy metal Concentrations in Water Samples
The mean copper, zinc and lead concentrations water for dry and wet season are summarized in Table 1. There was no significant spatial and seasonal variation in mean copper concentration in water samples among sampling sites (df = 1, f = 3.36, p = 0.07). This indicated that there were no significant point sources of copper in the study area or the river water dilution factor was high and therefore the incoming pollution had no significant impacts on copper concentrations in water. However copper concentration was relatively elevated at the MRTH site (0.18 ± 0.04 mg/L) in the dry season samples signifying reduced river dilution factor coupled with appreciate inputs from activities in this catchment area.
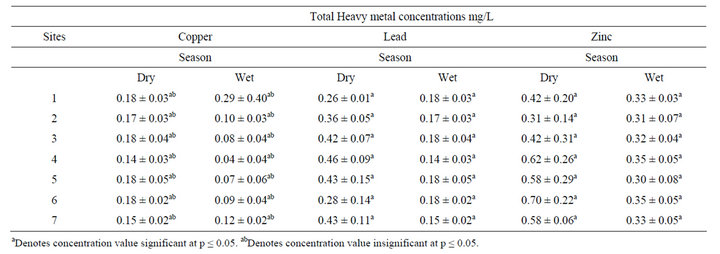
Table 1. Total heavy metal concentrations in water samples (mg/L).
Inputs from textile industries and timber treatment plants upstream are likely to contribute to enhanced copper concentration at this site.
With regard to lead concentrations, there was a significant variation in mean lead concentration in water samples among sampling stations and between seasons (df = 1, F = 85.4, P < 0.05) respectively. The relatively high dry season mean lead concentrations could be attributed to evaporative concentrations and point and diffused inputs from industrial, farms and urban associated activities like garages and car wash respectively. In 1998, Muhalulukhu [17] reported high levels of lead in water, fish and sediment samples from the Winam Gulf, which was attributed to industrial and geological activities in the lake basin. Similarly, zinc concentrations varied significantly (df = 1, F = 18.05, P < 0.05) seasonally and among sampling stations.
5.1.2. Heavy Metal Concentrations in Sediment Samples
Total concentrations of copper, lead and zinc in sediment samples collected during the wet and try seasons are summarized in Table 2. Unlike in water samples, there was a marked seasonal variation of sediment copper concentrations. The reduced water flow in the dry season tended to favour deposition of copper in sediments. Apparently the referral hospital site (MRTH) had the highest sediment copper concentration (1.62 ± 0.14 mg/kg), corresponded with site specific elevated water copper concentrations. A study by Simiyu and Tole [18] has shown the same trend where copper in water column corresponded with elevated copper in sediments.
The same trend was observed in mean lead sediment concentration, whereby it varied significantly seasonally and among sampling stations (df = 1, F = 12.3, P < 0.05). This is attributed to gravitation settling of lead in sediments and geochemistry of the soils.
There was no significant variation in mean zinc concentration in sediments between sampling stations in the two seasons of dry and wet seasons (Df = 1, F = 3.73, P = 0.059) as shown in Table 3.1. The zinc sediment concentrations were generally elevated ranging from 2.79 ± 0.67 mg/kg to 6.73 ± 1.11 mg/kg. This is typical of most soil lead concentration due its ubiquitous nature in the environment. This could also be attributable to anthropogenic activities including animal husbandry and farming practices that entail use of pesticides and insecticides.
5.1.3. Heavy Metals Bioconcentration in Plant (Polygonum pulchrum)
Bioconcentration factor (BCF) was used to determine the quantity of heavy metals absorbed by the plant from the sediment [19]. The BCF is an index of the ability of the plant to accumulate a particular metal with respect to its concentration in the sediment and was based on formula [20] shown in Equation (1):
BCF = Cp/Cs (1)
where Cp = Metal concentration in the whole plant; Cs = Metal concentration in sediment.
As is evidenced in Table 3, the BCF ranged from 0.6 (copper) to 15.1 (Zinc) at site MTRH. Sediment pH tended to influence plant heavy metal uptake as evidenced in the correlation coefficients of sediment pH versus plant heavy metal concentrations. Zinc with the highest BCF showed relatively high positive correlation (R2 = 0.894) with sediment pH. Figure 2 shows that zinc tended to be available to plant uptake as pH increased from acidic to neutral. The reverse was shown for copper (R2 = −0.460). The higher the BCF value, the higher the risk posed to the organisms along the food chain. BCF values >2 are regarded as high values [20] and it apparent that except for copper at MTRH, most BCF values were above the optimum BCF value of 2. The pH plays a
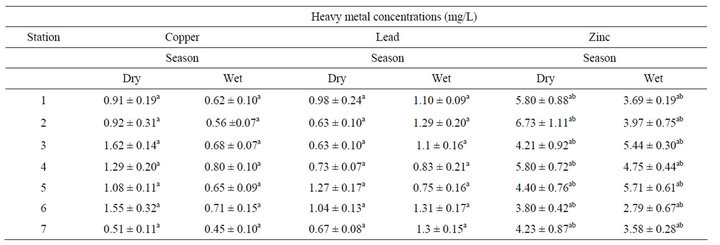
Table 2. Total heavy metal concentrations in sediment samples.
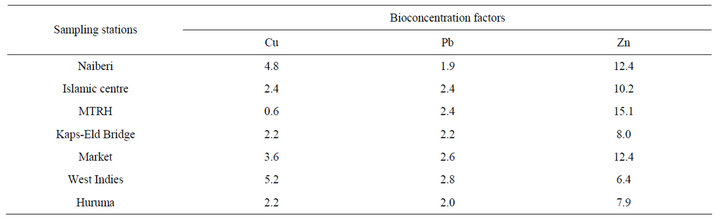
Table 3. Heavy metal bioconcentration factors in plant (Polygonum pulchrum).
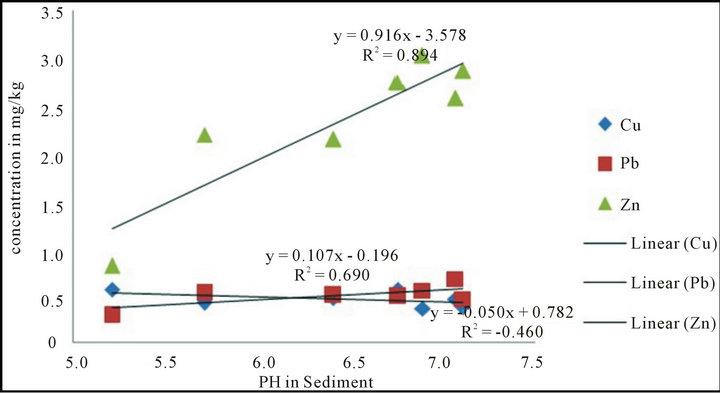
Figure 2. pH in sediment versus concentrations of heavy metals in plants.
critical role in chemistry of river and is an important parameter which affects adsorption of heavy metal on the sediment. Depending on nature of heavy metal, a decrease in pH would increase metal availability lending itself to greater uptake by organisms (plants included) and can cause physiological damage to life [21].
6. Conclusions
Based on the results, it’s concluded that the total heavy metals studied in water and plant samples are within the accepted limits and others are above the WHO limits. Results showed bioconcentration of heavy metals in studied plants with zinc gave a bioconcentration factor of 15 at MTRH which was highest. Zinc is one of the most soluble and mobile metal cations, thus transport from the sediment to roots to shoots of the plants is very high. And because it is easily assimilated by plants, it can be highly phytotoxic. The results have indicated the accumulation along the food chain.
There is a need for controlling point sources that could be contributing to heavy metal pollution along Sosiani River, and this could be done by encouraging farmers to use soil and water conservation measures like terracing, growing of cover crops and also use of organic fertilizer as it does not contain heavy metal. Continuous monitoring of the river pollution should be carried out and appropriate monitoring protocol should be established. Suggestion is made for further research in monitoring of heavy metal bioaccumulation in organisms such as fish in the study area.
7. Acknowledgements
We wish to acknowledge Mr. P. K. Martim, the Chief Technician of the School of Environmental Studies, Biochemistry laboratory, for his support during sample analysis. We also thank Mr. and Mrs. Keny for their encouragements and financial support
REFERENCES
- R. S. Dobson and J. E. Burgess, “Biological Treatment of Precious Metal Refinery Wastewater: A Review,” Mineral Engineering, Vol. 20, No. 6, 2007, pp. 519-532. doi:10.1016/j.mineng.2006.10.011
- C. V. Miller, G. D. Foster and B. F. Majedi, “Base Flow and Storm Flow Metal Fluxes from Two Small Agricultural Catchments in the Coastal Plain of Chesapeake Bay Basin, United States,” Applied Geochemistry, Vol. 18, No. 4, 2003, pp. 483-501.
- D. L. Osmond, D. E. Line, J. A. Gale, R. W. Gannon., C. B. Knott, K. A. Bartenhagen, et al., “Water, Soil and Hydro-Environmental Decision Support System,” 1995. www.water.ncsu.edu/watersheds/info/hmetals.html
- M. Dassenakis, M. Scoullos and A. Gaitis, “Trace Metals Transport and Behaviour in the Mediterranean Estuary of Archeloos River,” Marine Pollution Bulletin, Vol. 34, No. 2, 1997, pp. 103-111.
- H. Akcay, A. Orcuz and C. Karapire, “Study of Heavy Metal Pollution and Speciation in Buyak Menderes and Gediz River Sediments,” Water Research, Vol. 37, No. 4, 2003, pp. 813-822. doi:10.1016/S0043-1354(02)00392-5
- R. Nicolau, A. Galera-Cunha and Y. Lucas, “Transfer of Nutrients and Labile Nutrients from the Continent to the Sea by Small Mediterranean River,” Chemosphere, Vol. 63, No. 3, 2006, pp. 469-476. doi:10.1016/j.chemosphere.2005.08.025
- Y. Suziki, A. Nogi and T. Fukasawa, “Gall L Protein, an Auxiliary Transcription Activator for Genes Encoding Galactose-Metabolizing Enzymes in Saccharomyces cereuisiae,” Molecular and Cellular Biology, Vol. 8, No. 11, 1988, pp. 4991-4999.
- E. O. Oyewo, “Industrial Sources & Distribution of Heavy Metals in Lagos Lagoon and Their Biological Effects on Estuarine Animals,” Ph.D. Thesis, University of Lagos, Lagos, 1998.
- A. A. Otitoloju and K. N. Don-Pedro, “Integrated Laboratory and Field Assessments of Heavy Metals Accumulation in Edible Periwinkle, Tympanotonus fuscatus var. Radula (L),” Ecotoxicology and Environmental Safety, Vol. 57, No. 3, 2004, pp. 354-362.
- A. Lozak, K. Soltyk, P. Ostapezuk and Z. Fijalek, “Determination of Selected Trace Elements in Herbs and Their Infusions,” Science of the Total Environment, Vol. 14, No. 1-3, 2001, pp. 1-8.
- L. J. Tsai, K. C. Yu, S. F. Chen and P. Y. Kung, “Effect of Temperature on Removal of Heavy Metals from Contaminated River Sediments via Bioleaching,” Water Research, Vol. 37, No. 10, 2003, pp. 2449-2457.
- N. López-González, J. Borrego, J. A. Morales, O. Carro and O. Lozano-Soria, “Metal Fractionation in Oxic Sediments of an Estuary Affected by Acid Mine Drainage (Southwestern Spain),” Estuarine, Coastal and Shelf Science, Vol. 68, No. 3-4, 2006, pp. 297-304.
- APHA, “Standard Methods for the Examination of Water and Waste Water,” 17th Edition, American Public Health Association, Washington DC, 1989.
- APHA, “Standard Methods for the Examination of Water and Waste Water,” 18th Edition, American Public Health Association, Washington DC, 1992.
- Y. J. Cui, Y. G. Zhu, R. H. Zhai, D. Y. Chen, Y. Z. Huang and Y. Qiu, “Transfer of Metals from Soil to Vegetables in an Area near a Smelter in Nanning, China,” International Journal of Environment, Vol. 30, No. 6, 2010, pp 785-791. doi:10.1016/j.envint.2004.01.003
- AOAC, “Official Methods of Analysis,” Journal of AOAC (Association of Official Analytical Chemists) International, 2000.
- J. M. Shitsama and M. P. Tole, “Heavy Metal Contaminants in Fish, Water and Sediments in Lake Victoria, Kenya,” 2003. www.aehms.org/pdf/Tole.pdf
- G. M. Simiyu and M. P. Tole, “pH and Variations of Some Heavy Metals in the Infiltration Ponds at Olkaria, Kenya,” Journal of African Environmental Review, Vol. 1, No. 1, pp. 1-14.
- M. Ghos and S. P. Singh, “A Comparative Study of Cadmium Phytoextraction by Accumulator and Weed Species,” Environment Pollution, Vol. 133, No. 2, 2005, pp. 365-371.
- J. Mellem, H. Baijanth and B. Odhav, “Translocation and Accumulation of Cr, Hg, As, Pb, Cu and Ni by Amaranthus dubius (Amaranthaceae) from Contaminated Sites,” Journal of Environmental Science and Health, Vol. 44, No. 6, 2009, pp. 568-575.
- D. W. Connell and G. Miller, “Chemistry and Ecotoxicology of Pollution,” John Wiley and Sons Press, New York, 1984, pp. 407-412.
NOTES
*Corresponding author.

