Green and Sustainable Chemistry
Vol. 2 No. 2 (2012) , Article ID: 19004 , 5 pages DOI:10.4236/gsc.2012.22012
Aspects on the Mechanism of the 1-Phenyl-1H-pyrazolo[3,4-b]quinoxaline Formation*
1Department of Chemistry, Faculty of Science, Alexandria University, Ibrahimia, Egypt
2Department of Chemistry, Norwegian University of Science and Technology, Trondheim, Norway
Email: #maesallam@yahoo.com
Received January 1, 2012; revised February 15, 2012; accepted February 25, 2012
Keywords: 2-(D-arabino-tetritol-1-yl)quinoxaline; Pyrazolo[3,4-b]quinoxalines; 3-(D-erythro-glycerol-1-yl)-1-phenyl-1H-pyrazolo-[3,4-b]qunoxaline; 4,5-Dichloro-o-phenylenediamine; 6,7-Dichloro-2-(D-arabino-tetritol-1-yl)quinoxaline; 6,7-Dichloro-3-(D-erythro-glycerol-1-yl)-1-phenyl-1H-pyrazolo-[3,4-b]quinoxaline; N,N-Benzylphenylhydrazine hydrochloride
ABSTRACT
Condensation of D-glucose, o-phenylenediamine and N,N-benzylphenylhydrazine hydro chloride (NNBPHH) in a onepot reaction, or condensation of 2-(D-arabino-tetritol-1-yl) quinoxaline and NNBPHH, gave 3-(D-erythro-glycerol-1- yl)-1-phenyl-1H-pyrazolo[3,4-b]quinoxaline. The structure of the latter was determined by 1H NMR spectroscopy and by synthesis using phenylhydrazine hydrochloride instead of NNBPHH. Condensation of D-glucose and 4,5-dichloro-ophenylenediamine gave 6,7-dichloro-2-(D-arabino-tetritol-1-yl)quinoxaline, which upon condensation with NNBPHH gave the corresponding 6,7-dichloro-3-(D-erythro-glycerol-1-yl)-1-phenyl-1H-pyrazolo[3,4-b]quinoxaline. The structure and mechanism of formation of these compounds are discussed.
1. Introduction
Pyrazolo[3,4-b]quinoxalines are compounds of biological interest as antimicrobial agents [2]. Some of them have tuberculostatic activity in vitro, [3] while others show antifungal [4,5], antiviral [6], anti-proliferative [7] antibacterial [8], antihypertensive [9] activities. Saccharidederived 1-phenyl-1H-pyrazolo[3,4-b]quinoxalines are prepared, either by a one-pot reaction of the sugar, o-phenylenediamine, and phenylhydrazine hydrochloride in acidic medium, or by first preparing the saccharide quinoxaline intermediate (from the sugar and o-phenylenediamine) and then condensing the isolated quinoxaline derivative with phenylhydrazine hydrochloride in acidic medium [9] Previously, we have synthesized a series saccharide 1- aryl-1H-pyrazolo[3,4-b]quinoxalines by the one-pot reaction and converted them into C-nucleoside analogs [11]. The success of the synthesis depends on the type of hydrazine derivative used [12]. The role of arylhydrazine in this reaction, is similar to that has in the osazone formation [13]. In addition, to becoming part of the pyrazolo[3,4-b]quinoxaline molecule, the arylhydrazine serves as a condensing agent, being reduced to aniline and ammonia [14]. In this work, the pyrazolo[3,4-b]quinoxaline reaction is studied using the asymmetrically disubstituted N,N-benzylphenylhydrazine hydrochloride (NNBPHH) in order to investigate the role of benzylphenylhydrazine in this reaction.
2. Results and Discussion
The one-pot condensation of D-glucose, o-phenylenediamine (1), and N,N-benzylphenylhydrazine hydrochloride in acidic medium gave a crystalline pale yellow compound which was identified as 3-(D-erythro-glycerol-1-yl)- 1-phenyl-1H-pyrazolo[3,4-b]quinoxaline (3). Compound 3 was also obtained by condensation of 2-(D-arabino-tetritol-1-yl)quinoxaline (2) and NNBPHH in acidic medium. These results indicate that 2 is an intermediate during the formation of 3, which reacts with NNBPHH in acidic medium to give N,N-benzylphenylhydrazone intermediate “A” (Scheme 1). The unisolated intermediate “A” is then cyclized by the excess NNBPHH with elimination of the benzyl group in the form of toluene giving 3. The cyclization of the intermediate “A” takes place by two possible routes: 1) either by removal of the benzyl group
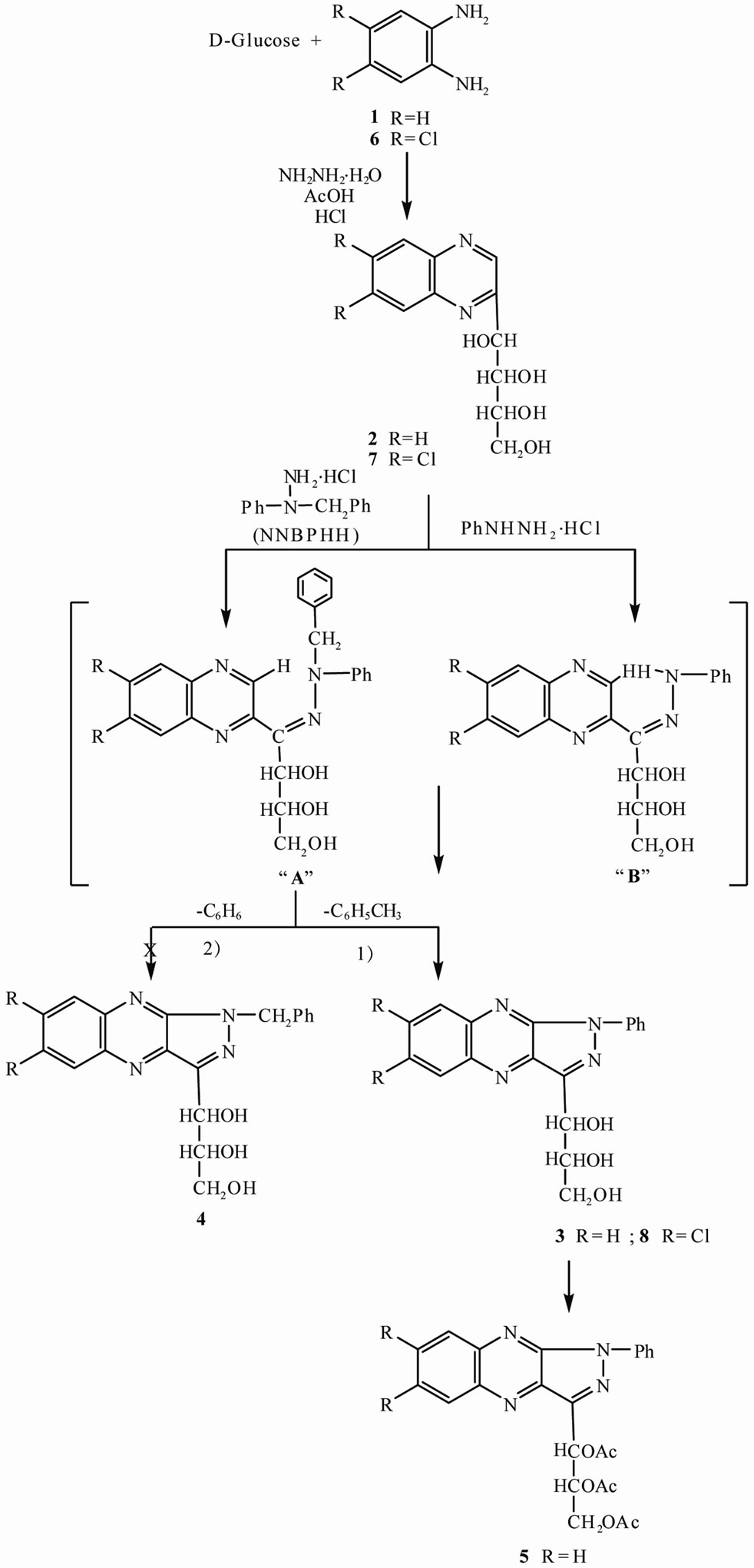
Scheme 1. Synthesis of 3-(D-erythro-glycerol-1-yl)-1-phenyl-1H-pyrazolo[3,4-b]quinoxaline (3) and 6,7-dichloro-3-(D-erythroglycerol-1-yl)-1-phenyl-1H-pyrazolo[3,4-b]quinoxaline (8) by effect of phenylhydrazine hydrochloride and N,N-benzylphenylhydrazine hydrochloride.
in the form of toluene giving compound 3 or by 2) removal of the phenyl group in the form of benzene giving compound 4. The route 1) is more favorable by benzylic elimination of toluene. Compound 4 obtained by elimination of benzene was not isolated from the reaction mixture. Compound 3 was synthesized by an alternate route, using phenylhydrazine hydrochloride (instead of NNBPHH) either by the one-pot reaction, or by condensing 2 with phenylhydrazine hydrochloride in acidic medium. This reaction takes place through the formation of N-phenylhydrazone intermediate “B” which is cyclized by the excess phenylhydrazine hydrochloride giving compound 3. The two compounds obtained by using either NNBPHH or phenylhydrazine hydrochloride were identical, having the same melting and mixed melting points. In addition, the 1H NMR spectra were identical. These results confirm that the cyclization of the intermediate “A” takes place with the elimination of the benzyl group in the form of toluene instead of the phenyl group in the form of benzene. Acetylation of 3 gave the tri-Oacetyl derivative 5.
Condensation of D-glucose and 4,5-dichloro-o-phenylenediamine (6) afforded 6,7-dichloro-2-(D-arabino-tetritol-1-yl)quinoxaline (7) which was reacted with NNBPHH in acidic medium, to afford the corresponding 6,7-dichloro-3-(D-erythro-glycerol-1-yl)-1-phenyl-1H-pyrazolo [3,4-b]quinoxaline (8). Similarly, the reaction took place in the same manner through the formation of the corresponding hydrazone intermediate A which is cyclized by elimination of the benzyl group in the form of toluene giving 8. Compound 8 was also obtained from the reaction of D-glucose, 6 and phenylhydrazine hydrochloride (instead of NNBPHH) in a one-pot reaction in acidic medium. This reaction takes place through the formation of the corresponding hydrazone intermediate B which is cyclized by the excess phenylhydrazine hydrochloride to give compound 8. The two compounds obtained from D-glucose and 6 using either NNBPHH or phenylhydrazine hydrochloride were identical, having the same melting and mixed melting points and the same NMR spectral pattern. The 1H NMR spectrum of 8, showed the absence of signals corresponding to H-6 and H-7 present in the spectrum of 3.
3. Conclusion
Condensation of D-glucose, o-phenelenediamine and N, N-benzylphenylhydrazine hydrochloride (NNBPHH) in a one pot reaction gave 3-D-erythro-glycerol-1-yl)-1-phenyl- 1H-pyrazolo[3,4-b]quinoxaline with the elimination of toluene. The same product was obtained by condensation of 2-(D-arabino-tetritol-1-yl)quinoxaline and NNBPHH in acidic medium. The structure of the products was obtained by getting the same product by condensation of Dglucose, o-phenylenediamine and phenylhydrazine hydrochloride in a one pot reaction. Using D-glucose, 4,5- dichloro-o-phenylenediamine and NNBPHH in a one pot reaction gave the corresponding 6,7-dichloro-3-(D-erythroglycerol-1-yl)-1-phenyl-1H-pyrazolo[3,4-b]quinoxaline.
The same product was obtained by condensation of 6,7- dichloro-2-(D-arabino-tetritol-1-yl)quinoxaline and NNBPHH.
4. Experimental
4.1. General Methods
Melting points were determined with a Fisher-Johns melting point apparatus and are uncorrected. Evaporations were performed under diminished pressure below 60˚C. Thin layer chromatography (TLC) was conducted on silica gel (Kiesel gel G, Merck) with solvent A, (10:1 CHCl3-MeOH); solvent B, (3:1 EtOAc-hexane); solvent C, (3:1 C6H6-EtOH); and solvent D, (5:1 CHCl3-EtOH). Compounds were detected under short wavelength UV light at 254 nm. IR absorption spectra were recorded with Perkin-Elmer 1430 instrument. UV absorption spectra were recorded with Perkin-Elmer Lambda 48 instrument. 1H NMR spectra were recorded with Varian FT 80 MHz and JEOL EX 400 MHz spectrometers and chemical shifts were reported in units (ppm) relative to Me4Si. 13C NMR spectra were recorded with JEOL EX 400 instrument at 100.4 MHz. Combustion analyses were performed in the Department of Chemistry, Alexandria University, Alexandria, Egypt.
4.2. Synthesis of 3-(D-erythro-glycerol-1-Yl)-1-phenyl-1Hpyrazolo[3,4-b]quinoxaline (3)
4.2.1. By Condensation of D-Glucose, 1 and NNBPHH in a One Pot Reaction
A solution of D-glucose (0.12 g, 0.6 mmol) in water (10 mL) was heated with 1 (0.07 g, 0.6 mmol), NNBPHH (0.8 g, 3 mmol), and AcOH (1.5 mL), in a sealed flask for 8 h in a boiling water bath. The flask was cooled, opened, and the precipitate obtained was filtered off, washed successively with water, 50% EtOH, and Et2O, then dried; yield 0.1 g (45.5%). The crude product was recrystallized from 1-propanol, to give yellow needles of 3, m.p. 216-217C (lit. [10,15] m.p. 218˚C); TLC (solvent A) Rf 0.52. 1H NMR: (80 MHz; Me2SO-d6): 3.71 (dd, 1 H, H-3, J2’,3’ 5.5, J3’,3” 11.3 Hz), 3.93 (dd, 1 H, H-3’, J2’,3’ 3.1 Hz), 4.46 (m, 2 H, 2’-OH, 3’-OH), 4.52 (m, 1 H, H-2’), 5.14 (d, 1 H, H-1`, J1’,2’ 8.7 Hz), 5.77 (d, 1 H, 1’-OH, J1’,OH 5.0 Hz), 7.27 - 7.46 (m, 1 H, H-p), 7.53 - 7.75 (m, 2 H, H-m), 7.77 - 8.15 (m, 2 H, H-o), 8.21 and 8.31 (2 H, H-6 and H-7) and 8.46 (d, 2 H, H-5, H-8, J 8.7 Hz). After addition of CD3CO2D, the three hydroxyl protons disappeared.
4.2.2. By Condensation of 2 and NNBPHH
A suspension of 2 [16] (0.013 g, 0.5 mmol) in water (10 mL) was heated with NNBPHH (0.6 g, 2.5 mol), and AcOH (1 mL), in a sealed flask for 8 h in a boiling water-bath. The flask was cooled, opened, and the precipitate obtained was filtered off, washed successively with water, 50% EtOH, and Et2O, then dried; yield 0.1 g (58.8%). It was recrystallized from 1-propanol, to give yellow needles of 3, m.p. and mixed m.p. (method 4.2.1) 216˚C - 218˚C; TLC (solvent A) showed the same Rf and the same 1H NMR spectral pattern as the product obtained from method 4.2.1.
4.2.3. From D-Glucose, 1 and Phenylhydrazine Hydrochloride in a One Pot Reaction
A solution of D-glucose (1 g, 5 mmol) in water (50 mL) was heated with 1 (0.5 g, 5 mmol), phenylhydrazine hydrochloride (3.6 g, 25 mmol), and AcOH (1.3 mL), in a sealed flask for 6 h in a boiling water-bath. The flask was cooled, opened, and the precipitate obtained was filtered off, washed successively with water, 50% EtOH, and Et2O, then dried; yield 1 g (53.5%). It was recrystallized from 1-propanol, to give yellow needles, m.p. and mixed m.p. with 3 (obtained from methods 4.2.1 and 4.2.2), 216˚C - 217˚C; TLC (solvent A) Rf 0.52.
4.3. 3-(1,2,3-Tri-O-acetyl-D-erythro-glycerol- 1-Yl)-1-phenyl-1H-pyrazolo[3,4-b] quinoxaline (5)
A solution of 3 (0.1 g, 0.3 mmol) in pyridine (2 mL) was treated with Ac2O (2 mL) for 24 h at room temperature; it was poured onto crushed ice, and the acetate obtained was filtered off, washed with water, and dried; yield 0.12 g (87%). It was recrystallized from dilute MeOH to give yellow needles, m.p. 122˚C - 124˚C (lit. [10] m.p. 123˚C - 124˚C); TLC (solvent B) Rf 0.78; 1H NMR: (400 MHz; CDCl3): δ 2.03, 2.06 and 2.26 (three s, 3 H, 3 OAc), 4.52 (q, 1 H, H-3”, J2’,3’ 6.3, J3’,3” 12.2 Hz), 4.67 (dd, 1 H, H-3’, J2’,3’ 2.9 Hz), 6.09 - 6.13 (m, 1 H, H-2’), 6.81 (d, 1 H, H-1’, J1’,2’ 5.9 Hz), 7.33 (dd 1 H, H-p), 7.57 (dd, 2 H, H-m,), 7.72-7.76 (m, 2 H, H-o), 8.17 and 8.27 (1 H each, H-6 and H-7) and 8.44 (2 H, H-5 and H-8). Assignments were verified by 2D NMR. 13C NMR: (100.4 MHz; CDCl3): 20.74, 20.92, 21.07 (three O-acetyl CH3), 62.02 (C-3’), 67.87 (C-1’), 71.03 (C-2’), 120.08 (C-5, C-8), 126.11 (C-p), 128.53 (C-o), 129.06 (C-7), 129.22 (C-m,) 130.56 (C-6), 131.36 (C-o), 135.97, 139.06, 141.42 (double intensity) (four quaternary carbons; *C-13, *C-12, *C-11, *C-10), 141.59 (C-a), 142.41 (C-3), 169.86, 169.91, and 170.59 (three O-acetyl C=O). Assignments were verified by 1H-13C NMR Correlation Spectroscopy (COSY).
4.4. 6,7-Dichloro-2-(D-arabino-tetritol-1- Yl)quinoxaline (7)
A solution of D-glucose (0.4 g, 2 mmol) in water (10 mL) was heated with 6 (0.4 g, 2 mmol), hydrazine hydrate (1 mL), conc. HCl (0.5 mL), and AcOH (0.5 mL) in a sealed flask for 6 h in a boiling water bath. The flask was cooled, opened and the precipitate obtained was filtered off, washed successively with water, 50% EtOH and Et2O, then dried giving colorless needles of 7; yield 0.3 g (42.9%). It was recrystallized from dilute MeOH to give yellow needles (turns pale brown by light), m.p. 179˚C - 181˚C; TLC (solvent A) Rf 0.32; 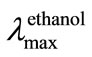 236 and 326 nm (log ε 4.1 and 3.8);
236 and 326 nm (log ε 4.1 and 3.8); ![]() 3432 (OH) and 1587 cm–1 (C=N); 1H NMR: (400 MHz; Me2SO-d6): 3.46 - 3.82 (m, 1 H, H-4), 3.65 - 3.66 (m, 3 H, H-2’, H-3’, H-4’), 4.42 (t, 1 H, 4’-OH), 4.70 (d, 1 H, 3’-OH, J3’,OH 6.8 Hz), 4.75 (d, 1 H, 2’-OH, J2’,OH 4.4 Hz), 5.15 (d, 1 H, H-1’, J1’,2, 4.9 Hz), 5.74 (d 1 H, 1’-OH J1’,OH 5.9 Hz), 8.37 (s, 1 H, H-8), 8.40 (s, 1 H, H-5), and 9.14 (s, 1 H, H-3’); after addition of CD3CO2D, the four hydroxyl protons disappeared: 3.48 (q, 1 H, H-4”, J3’,4’ 5.9, J4’,4” 11.2 Hz), 3.65 - 3.67 (m, 3 H, H-2’, H-3’, H-4’), and 5.15 (d, 1 H, H-1’, J1’,2’ 4.9 Hz); 13C NMR: (100.4 MHz; Me2SO-d6): 63.40 (C-4’), 71.05, 72.55, 74.23 (C-1’, C-2’, C-3’), 129.55, 129.79 (*C-5, *C-8), 131.95, 132.68, 139.83, 139.90 (*C-6, *C-7, *C-9, *C-10), 146.67 (C-2), and 161.26 (C-3). Anal. Calc. for C12H12Cl2N2O4: C, 45.25; H, 3.80; N, 8.60. Found: C, 45.16; H, 3.79; N, 8.78%.
3432 (OH) and 1587 cm–1 (C=N); 1H NMR: (400 MHz; Me2SO-d6): 3.46 - 3.82 (m, 1 H, H-4), 3.65 - 3.66 (m, 3 H, H-2’, H-3’, H-4’), 4.42 (t, 1 H, 4’-OH), 4.70 (d, 1 H, 3’-OH, J3’,OH 6.8 Hz), 4.75 (d, 1 H, 2’-OH, J2’,OH 4.4 Hz), 5.15 (d, 1 H, H-1’, J1’,2, 4.9 Hz), 5.74 (d 1 H, 1’-OH J1’,OH 5.9 Hz), 8.37 (s, 1 H, H-8), 8.40 (s, 1 H, H-5), and 9.14 (s, 1 H, H-3’); after addition of CD3CO2D, the four hydroxyl protons disappeared: 3.48 (q, 1 H, H-4”, J3’,4’ 5.9, J4’,4” 11.2 Hz), 3.65 - 3.67 (m, 3 H, H-2’, H-3’, H-4’), and 5.15 (d, 1 H, H-1’, J1’,2’ 4.9 Hz); 13C NMR: (100.4 MHz; Me2SO-d6): 63.40 (C-4’), 71.05, 72.55, 74.23 (C-1’, C-2’, C-3’), 129.55, 129.79 (*C-5, *C-8), 131.95, 132.68, 139.83, 139.90 (*C-6, *C-7, *C-9, *C-10), 146.67 (C-2), and 161.26 (C-3). Anal. Calc. for C12H12Cl2N2O4: C, 45.25; H, 3.80; N, 8.60. Found: C, 45.16; H, 3.79; N, 8.78%.
4.5. Synthesis of 6,7-Dichloro-3-(D-erythro-glycerol-1-Yl)-1- phenyl-1H-pyrazolo[3,4-b]quinoxaline (8)
4.5.1. By Condensation of 7 and NNBPHH
A suspension of 7 (0.1 g, 0.3 mmol) in water (10 mL) was heated with NNBPHH (0.36 g, 1.5 mmol), and AcOH (1 mL) in a sealed flask for 8 h in a boiling water bath. The flask was cooled, opened, and the precipitate obtained was filtered off, washed successively with water, 50% EtOH, and Et2O, then dried; yield 0.06 g (46.2%). It was recrystallized from 1-propanol, to give yellow needles of 8, m.p. 222˚C - 223˚C; TLC (solvent D) Rf 0.57; 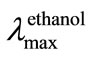 271, 343, and 412 nm (log ε 4.4, 3.8, and 3.7);
271, 343, and 412 nm (log ε 4.4, 3.8, and 3.7); ![]() 3340 (OH), 1605, and 1560 cm–1 (C=N); 1H NMR: (400 MHz; Me2SO-d6): δ 3.69-3.71 (m, 1 H, H-3”), 3.85 - 3.89 (m, 1 H, H-3’), 4.43-4.46 (m, 1 H, H-2’), 4.56 (t, 1 H, 3’-OH, J3’,OH 5.4 Hz), 4.66 (d 1 H 2’-OH J2’,OH 5.4 Hz), 5.10 (dd, 1 H, H-1’, J1’,2’ 8.8, J1’,OH 4.9 Hz), 5.90 (d 1 H 1’-OH J1’,OH 4.9 Hz), 7.38 (1 H, H-p,), 7.63 (2 H, H-m,), 8.36 (2 H, H-o,), 8.54 (s, 1 H, H-5), and 8.63 (s, 1 H, H-8); after addition of CD3CO2D, the three hydroxyl protons disappeared: 3.71 (q, 1 H, H-3”, J2’,3’ 5.9, J3’,3” 11.7 Hz), 3.88 (dd, 1 H, H-3’, J2’,3’ 2.9 Hz), 4.43 - 4.47 (m, 1 H, H-2’), and 5.11 (d, 1 H, H-1’, J1’,2’ 8.8 Hz); 13C NMR: (100.4 MHz; Me2SO-d6): 63.07 (C-3’), 68.03 (C-1’), 72.99 (C-2’), 119.49 (C-o), 125.98 (C-p), 129.31 (C-8), 129.42 (C-m), 130.52 (C-5), 131.16, 134.27, 137.93, 138.53, 138.88, 139.41 (six quaternary carbons; *C-6, *C-7, *C-13, *C-12, *C-11, C-10), 142.21 (C-a), and 149.05 (C-3).Anal. Calc. for C18H14Cl2N4O3: C, 53.35, H, 3.48; N, 13.83- Found: C 53.61; H, 3.20; N, 13.70%.
3340 (OH), 1605, and 1560 cm–1 (C=N); 1H NMR: (400 MHz; Me2SO-d6): δ 3.69-3.71 (m, 1 H, H-3”), 3.85 - 3.89 (m, 1 H, H-3’), 4.43-4.46 (m, 1 H, H-2’), 4.56 (t, 1 H, 3’-OH, J3’,OH 5.4 Hz), 4.66 (d 1 H 2’-OH J2’,OH 5.4 Hz), 5.10 (dd, 1 H, H-1’, J1’,2’ 8.8, J1’,OH 4.9 Hz), 5.90 (d 1 H 1’-OH J1’,OH 4.9 Hz), 7.38 (1 H, H-p,), 7.63 (2 H, H-m,), 8.36 (2 H, H-o,), 8.54 (s, 1 H, H-5), and 8.63 (s, 1 H, H-8); after addition of CD3CO2D, the three hydroxyl protons disappeared: 3.71 (q, 1 H, H-3”, J2’,3’ 5.9, J3’,3” 11.7 Hz), 3.88 (dd, 1 H, H-3’, J2’,3’ 2.9 Hz), 4.43 - 4.47 (m, 1 H, H-2’), and 5.11 (d, 1 H, H-1’, J1’,2’ 8.8 Hz); 13C NMR: (100.4 MHz; Me2SO-d6): 63.07 (C-3’), 68.03 (C-1’), 72.99 (C-2’), 119.49 (C-o), 125.98 (C-p), 129.31 (C-8), 129.42 (C-m), 130.52 (C-5), 131.16, 134.27, 137.93, 138.53, 138.88, 139.41 (six quaternary carbons; *C-6, *C-7, *C-13, *C-12, *C-11, C-10), 142.21 (C-a), and 149.05 (C-3).Anal. Calc. for C18H14Cl2N4O3: C, 53.35, H, 3.48; N, 13.83- Found: C 53.61; H, 3.20; N, 13.70%.
4.5.2. From D-Glucose, 6 and Phenylhydrazine Hydrochloride in a One Pot Reaction
A solution of D-glucose (1 g, 5 mmol) in water (50 mL) was heated with 6 (0.9 g, 5 mmol), phenylhydrazine hydrochloride (3.6 g, 30 mmol), and AcOH (1.3 mL) in a sealed flask for 6 h in a boiling water-bath. The flask was cooled, opened, and the precipitate obtained was filtered off, washed successively with water, 50% EtOH, and Et2O, then dried; yield 0.9 g (40%). It was recrystallized from 1-propanol to give yellow needles of 8, m.p. and mixed m.p with 8 (from method 4.5.1), 222˚C - 223˚C; TLC (solvent D) showed the same Rf and the same NMR spectral pattern.
5. Acknowledegments
We are grateful to The Research Council of Norway for a fellowship to M. A. S.
REFERENCES
- M. A. Mostafa, “Aspects on Pyrazolo[3,4-b]quinoxaline Formation,” Bulletin of the Faculty of Science, Alexandria University, Vol. 29, No. 2-3, 1989, pp. 35-44.
- A. S. Shawali, M. M. Zayed and T. A. Farghaly, “Synthesis and Biological Activity of New 1H-Pyrazolo[3,4-b] quinoxalines (Flavazoles),” Journal of Heterocyclic Chemistry, Vol. 42, No. 2, 2005, pp. 185-189. doi:10.1002/jhet.5570420202
- N. P. Buu-Hoi, J. N. Vallat, G. Saint-Ruf and G. Lambelin, “Chimie, Physico-Chimie et Pharmacologie due Flavazole et de Quelques-Uns de Ses Derives Substitutes,” Chimie Therapeutique, Vol. 6, No. 4, 1971, pp. 245-250.
- Y. Kurasawa, M. Muramatsu, K. Yamazaki, S. Tajima, Y. Okamoto and A. Takada, “A Convenient Synthesis and Antifungal Activity of 1-Aryl-1Hand 1-Aryl-3-heteroaryl- 1H-pyrazolo [3,4-b]quinoxalines,” Journal of Heterocyclic Chemistry, Vol. 23, No. 5, 1986, pp. 1379-1382. doi:10.1002/jhet.5570230524
- Y. Kurasawa, M. Muramatsu, K. Yamazki, S. Tajima, Y. Okamoto and A. Takada, “A facile Synthesis of 1-Aryl-3- heteroaryl-1H-pyrazolo[3,4-b]quinoxalines and Related Compounds with Antifungal Activity,” Journal of Heterocyclic Chemistry, Vol. 23, No. 5, 1986, pp. 1391-1394. doi:10.1002/jhet.5570230527
- M. M. Abbasi, S. M. El-Kousy, Y. E. El-Moghazy and S. El-Kafrawy, “Synthesis of Pyrazoloquinoxaline-C-Acyclic Nucleosides with Expected Antiviral Activity,” International Journal of Chemistry, Vol. 15, No. 2, 2005, pp. 77-84.
- M. A. Ortega, M. E. Montoya, B. Azrranz, A. Jaso, I. Aldana, S. Leclere, L. Meijer and A. Monge, “Pyrazolo [3,4-b]quinoxalines. A New Class of Cyclin-Dependent Kinases Inhibitors,” Bioorganic and Medicinal Chemistry, Vol. 10, No. 7, 2002, pp. 2177-2184. doi:10.1016/S0968-0896(02)00069-X
- S. Young, M. K. Lee, Y. Kurasawa and A. Takada, “Synthesis and Evaluation of 1H-Pyrazolo[3,4-b]quinoxalines and 1-Aryl-3-quinoxalinyl-1,2,4-triazoles as Antibacterial Agents,” Journal of Heterocyclic Chemistry, Vol. 33, No. 6, 1996, pp. 1855-1858. doi:10.1002/jhet.5570330649
- A. Mongae, J. A. Palop, A. Ochoa de Retana, I. Urbasos and A. E. Fernandez, “New Quinoxalines and Pyrazolo [3,4-b]quinoxalines with Antihypertensive Activity,” Anales de Quimica, Serie C: Quimica Organica y Bioquimica, Vol. 84, No. 3, 1988, pp. 364-366.
- H. Ohle and G. A. Melkonian, “1-Phenyl-3-(D-erythrotrioxypropyl)-flavazol. Die Konstitution der Seitenkette,” Berichte der Deutschen Chemischen Gesellschaft, Vol. 74, No. 2, 1941, pp. 279-291. doi:10.1002/cber.19410740220
- M. A. E. Sallam, H. M. El Nahas, S. M. E. Abdel Megid and T. Anthonsen, “1-Aryl-6,7-dichloro-3-β-D-erythrofuranosyl-pyrazolo[3,4-b]quinoxalines C-Nucleoside Analogs,” Carbohydrate Research, Vol. 280, No. 1, 1996, pp. 127-138. doi:10.1016/0008-6215(95)00187-5
- H. Ohle and A. Iltgen, “Flavazole, IV. Mitteil: Die Stammverbindung: Das Flavazol,” Berichte der Deutschen Chemischen Gesellschaft, Vol. 76, No. 1-2, 1943, pp. 1-14. doi:10.1002/cber.19430760102
- H. El Khadem, “Chemistry of Osazones,” Advances in Carbohydrate Chemistry, Vol. 20, 1965, pp. 139-181. doi:10.1016/S0096-5332(08)60298-2
- H. Ohle and M. Hielscher, “Nolez zur Konstitution des 1-Phenyl-d-fructosons,” Berichte der Deutschen Chemischen Gesellschaft, Vol. 74, No. 1, 1941, pp. 18-19. doi:10.1002/cber.19410740105
- B. Nordin, “1-Phenyl Flavazoles,” In: R. L. Whitler, M. L. Wolfrom and J. N. Bemiller, Eds., Methods in Carbohydrate Chemistry, Academic Press, New York, 1963, pp. 136-137.
- R. Lohmar and K. P. Link, “Note on the Reaction of d-Glucosamine with o-Phenylene Diamine,” The Journal of Biological Chemistry, Vol. 150, 1943, pp. 351-352.
NOTES
*For preliminary communication see reference [1].
#Corresponding author.

