Green and Sustainable Chemistry
Vol.1 No.3(2011), Article ID:7073,7 pages DOI:10.4236/gsc.2011.13014
Comparison of Cellulose Decomposition by Microwave Plasma and Radio Frequency Plasma
1Hitachi Automotive Systems, Ltd, Development Div. Drive Control Systems Div. 6-3, 1-Chome Fujimi, Kanagawa, Japan
2Department of Life and Environmental Sciences, Faculty of Engineering, Chiba Institute of Technology, Chiba, Japan
3National Institure of Advanced Industrial Science and Technology (AIST), AIST Tsukuba Central 5, Ibaraki, Japan
E-mail: *tatsuaki.yamaguchi@it-chiba.ac.jp
Received June 28, 2011; revised July 29, 2011; accepted August 5, 2011
Keywords: Microwave Plasma, Radio Frequency, Plasma, Cellulose
Abstract
Biomass conversion by plasma has the advantage of mainly producing gaseous products, H2, CO and CO2. Though the thermal plasma has been used for this conversion, the plasma temperature is too high to be unfit for the conversion biomass. The temperature of cold plasma, however, is lower under 3000 K. It expects to be adequate for biomass conversion. Cold plasma can be obtained with irradiation microwave (2.45 GHz) or radio frequency (13.5 MHz) under reduce gas pressure. Therefore, in present study, the effective decomposition of cellulose by microwave plasma (MWP) and radio frequency plasma (RFP) is examined. The conversion of cellulose by MWP (XMWP) is higher than that by RFP (XRFP), irrespective of the reaction time. XMWP and XRFP reach 92.8 wt% at 10 min and 68.1 wt% at 30 min. The maximum yield of gaseous products (Ygas) by MWP is 85.1 wt% at 10 min, higher by 23.2 wt% than Ygas by RFP at 30 min. The amount of H2 and CO obtained by MWP is 18.0 mmol/g and 23.5 mmol/g, it is larger than that obtained by RFP. Comparing the relationship between conversion and yield, Ygas of MWP is slightly higher than that of RFP under X of 60 wt%, and both Ygas is almost same over 60 wt%. The amount of H2 and CO obtained by MWP is larger by 9.3 mmol/g and 9.6 mmol/g than that obtained by RFP. C, H and O element in cellulose is mainly distributed to H2 and CO by MWP. RFP mainly distributes H and O element to the other gases without H2 and CO. In addition, a large amount of C element is remains in the residue. Those results is found that MWP was more suitable for cellulose gasification than RFP, since MWP can highly convert C, H and O element to H2 and CO by higher energy of microwave frequency in comparison with radio frequency.
1. Introduction
Recently, renewable energy is noticed again following reasons: 1) soaring the price of petroleum; 2) the uncertain supply of petroleum; 3) a crisis at the Fukushima No. 1 nuclear power plant. Biomass is a renewable resource by the photosynthesis of H2O and CO2, and biomass widely exists distribution on the earth. The abundance of unused biomass is exists in the field of agriculture, forestry and livestock industry. If those resources convert to the energy resource, for example hydrogen for fuel cell, it will become an expecting technique.
Plasma is considered to be the 4th state of matter, it is gas including ion, electron and neutral particle. Plasma is roughly classified in thermal plasma and cold plasma. Because thermal plasma is equilibrium state of the temperature of electron, ion and neutral particle, the temperature of thermal plasma becomes as high as 3000 K ~ 10000 K. Therefore, plasma method has the advantage of the possible abundance production of H2 and CO from biomass, and gaseous products obtained by plasma contain low tar [1]. Katou et al. reported that the tar yield was low and H2, CO and CO2 were obtained when biomass was converted by DC arc plasma, which is thermal plasma [2]. But the temperature of thermal plasma is too high to be unfit for the energy efficient conversion of biomass.
Cold plasma is non-equilibrium state of the temperature of electron, ion and neutral particle. The electron temperature is only higher than ion and neutral particle temperature. Therefore, plasma temperature is under 3000 K, which might be adequate for the conversion of biomass to gaseous products. Tang et al. studied that the gasification of rice straw by RF plasma (RFP), which was obtained under 3 - 8 kPa at 1600 - 2000 W of power by the irradiation of radio frequency (13.56 MHz). In their results, the yield of gaseous products reached at 66% and H2, CO, CH4 and CO2 was mainly obtained [3].
It has been studied in our laboratory that the effective conversion of coal (Yallourn coal and Taiheiyo coal) [4-6] and biomass (cellulose, lignin, saw dust, sugar cane bagasse) [7-9] by microwave plasma (MWP) are performed under reduce pressure by microwave (2.45 GHz), generated by a for lower electric power (300W) than the above mentioned works. When cellulose was used as a raw material, the yield of gaseous products (Ygas) reached at 79 wt% and H2 and CO was mainly obtained [7]. Com-paring those results with that obtained at same temperature of MWP by conventional pyrolysis, Ygas obtained by MWP is higher by 67.7 wt% than that obtained by conventional pyrolysis. Mainly CO2 was obtained at 620 K, CO and CO2 were obtained in the range from 1000 K - 1050 K, and H2, CO and CO2 were obtained over 1100K by conventional pyrolysis [7].
Both cold plasma methods can give the high yield of gaseous products and produce mainly H2 and CO. The difference between microwave plasma and RF plasma is the frequency of electromagnetic wave for obtained plasma. As the difference of energy is depend on the frequency of electromagnetic, the plasma density, the electron state and the activated species might be different between MWP and RFP. It is considered that this affects the decomposition of biomass.
In present study, the decomposition of cellulose by MWP and RFP was compared under Ar at irradiation power of 300 W.
2. Method of Gasification by Plasma
2.1 Raw Material
Cellulose (Merck Co., 200 mesh under), which is main composition of biomass, was used as raw material. The proximate and elemental analysis of cellulose was summarized in Table 1. Cellulose was dried under argon at 110˚C for 3 hours.
2.2. Plasma Apparatus
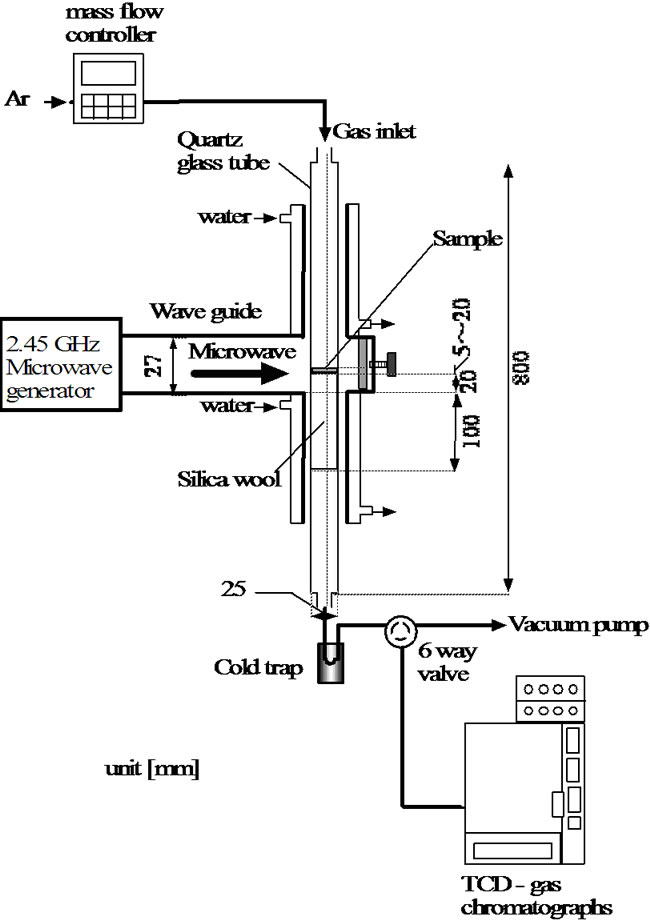
2.2.1. Plasma Reactor with Radio Frequency
Figure 1(a) shows the radio frequency plasma appa
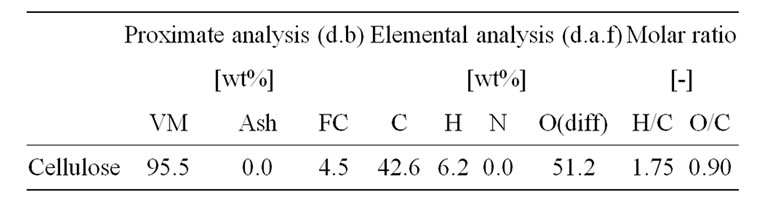
Table 1. Proximate and elemental analysis of cellulose.
ratus. A low-pressure flow reactor was used with a quartz glass reaction tube (12 mm of internal diameter and 600 mm of length). Cellulose was packed in the middle of the vertical reaction tube. The radio frequency (RF) was introduced at 13.56 GHz from two ring copper electrode to produce a plasma in the reaction tube. The space of between two electrodes was 17 mm.
2.2.2. Plasma Reactor with Microwave
Figure 1(b) shows the microwave plasma apparatus. A low-pressure flow reactor was used with a quartz glass reaction tube (25 mm of internal diameter and 800 mm of length). Cellulose was packed in the middle of the vertical reaction tube. The microwaves (MW) were introduced at 2.45 GHz from the side of the tube via a wave guide to produce a plasma in the reaction tube.
2.3. Experimental Conditions
Experimental conditions of MWP and RFP are summarized in Table 2.
2.4. Calculation of Conversion and Product Yield
The conversion (X) and the yield (Y) were calculated, based on the weight decrease of the post-reaction residues dried at 380 K for 3 h. The yield of the liquid products was also calculated, based on the weight difference, before and after extraction with benzene. Equations are showed as follows.
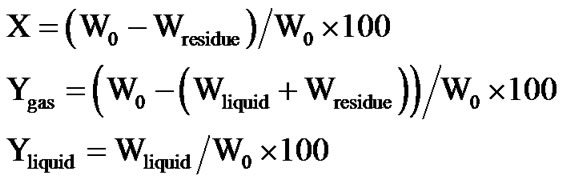
X: conversion (XMWP: conversion by microwave plasma, XRFP: conversion by RFP)
Ygas: yield of gaseous products Yliquid: yield of liquid products W0: weight of cellulose Wresidue: weight of residue Wliquid: weight of liquid products
2.5. Analysis of Products
On-line gas chromatography (SHIMADZU Co. Ltd, GC-
 (a)
(a)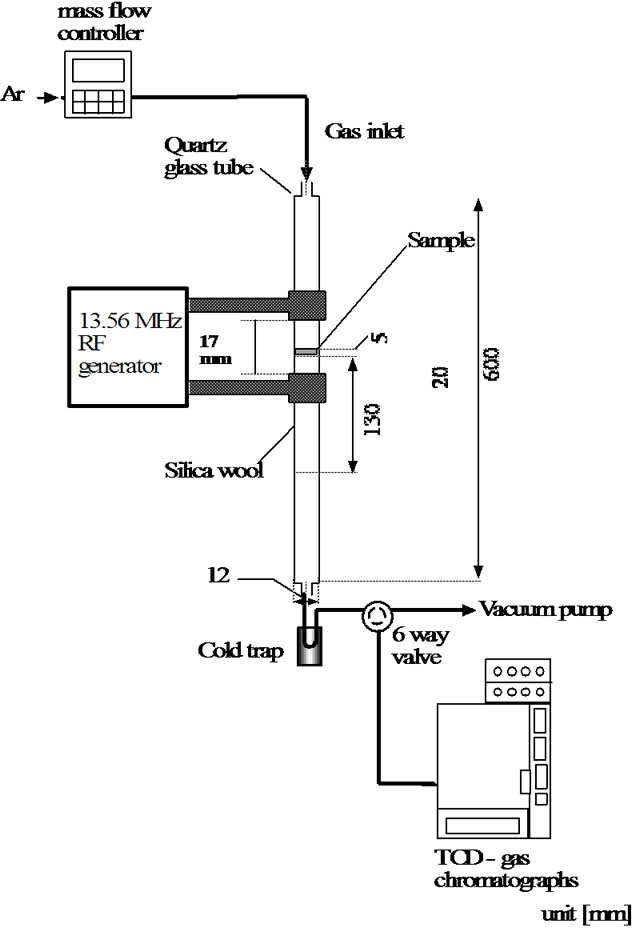 (b)
(b)
Figure 1. Plasma reactor. (a) Radio frequency plasma reactor; (b) Microwave plasma reactor.
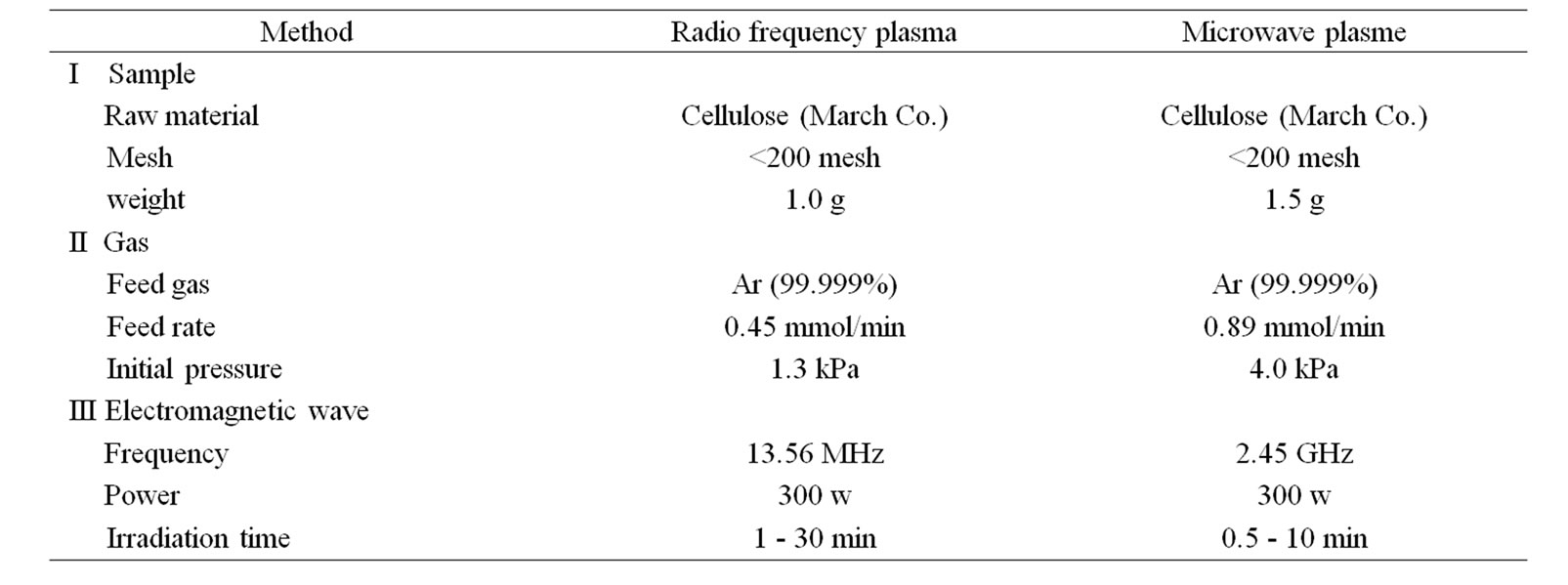
Table 2. Experimental conditions.
14B) was used for the analysis of the gaseous products. The element analysis of residue was measured with CHN coder (Yanaco Co., MT-6)
3. Results and Discussion
3.1. Comparison of the Effect of Time Change on Reaction
3.1.1. Comparison of Conversion and Products Yield
Figure 2 shows the relationship between reaction time (t) and X. XMWP immediately increases until 5 min and is constant at 92.8 wt% after 5 min. XRFP gently increases until 15 min and is constant at 65 wt% after 15 min. Comparing XMWP with XRFP, XMWP is higher than XRFP irrespective of t. This shows that the conversion rate of MWP is faster than that of RFP. From this result, it is found that MWP can convert cellulose in the short time and indicates high conversion.
Figure 3 shows the relationship between t and Ygas, Yliquid. Ygas by MWP reaches 86 wt% at 10 min. Yliquid by MWP indicates the maximum value of 13 wt% at 5 min. Ygas by RFP increases until 15 min and is constant at 65 wt% after 15 min. Yliquid by RFP indicates the maximum
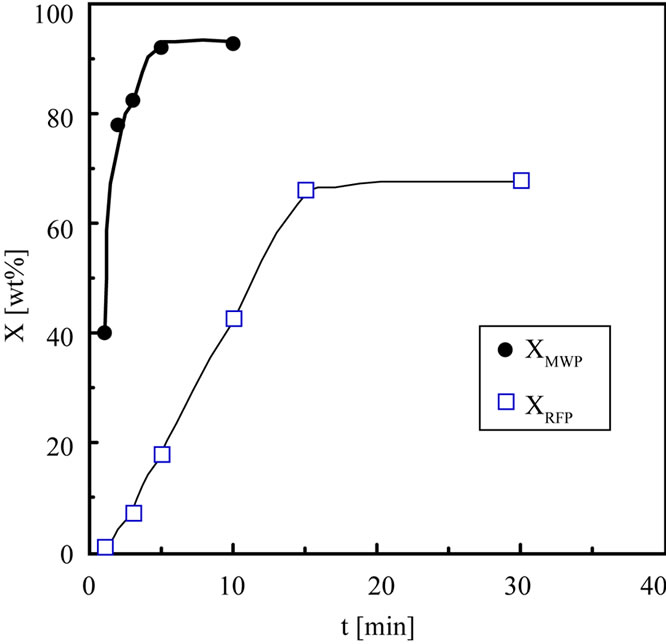
Figure 2. Comparison of the conversion with the time change.
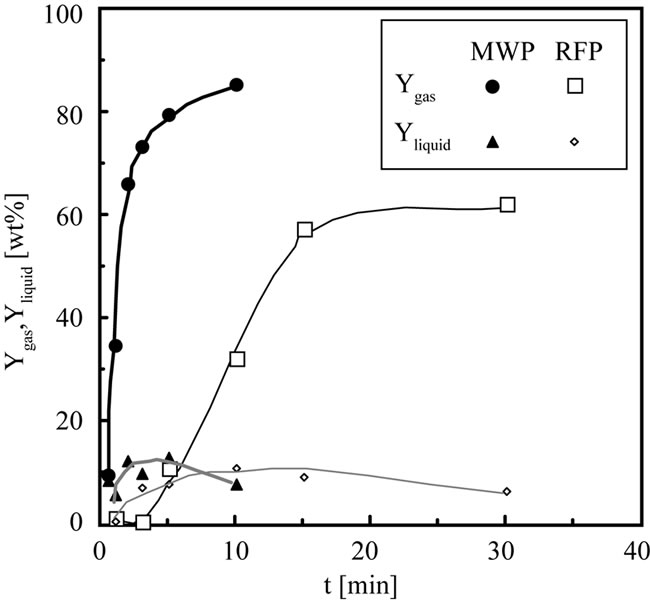
Figure 3. Comparison of the product yield with the time change.
value of 11 wt% at 10 min. Comparing Ygas and Yliquid by MWP with that by RFP, main products is gas irrespective of t, and Ygas by MWP is higher than that of RFP irrespective of t. This result shows the gasification rate of MWP is faster than that of RFP and the maximum Ygas by MWP is higher than that by RFP. From above those results, it is found that the maximum value, conversion rate and Ygas rate are different by frequency. This is probably due to the higher temperature of MWP in comparison of the temperature of RFP. It may be cause of the difference energy by frequency and the plasma state obtained by different of frequency. The decomposition of biomass by pyrolysis generally indicates higher conversion and Ygas with an increase in temperature. The energy of MW is larger than that of RF, because the frequency of MW (2.45 GHz) is larger than that of RF (13.56 MHz). In addition, the different frequency caused the change of a plasma density. As the frequency of MW is higher than that of RF, the plasma density of MW should be higher than that of RF. In high plasma density, electron, ion and neutral particle easily collide to each other. Therefore, gas temperature of MWP is assumed to be high. We measured the temperature of graphite heated by Ar plasma obtained under 1 torr at 300W by MW. The temperature reached 1074 K until 3min and was constant over 5 min. On the other hand, the temperature of N2 plasma obtained under 1 torr at 308 W by RF was 783 K [10]. It is suggested that the difference of conversion and Ygas between MWP and RFP results from the different plasma temperature by a frequency.
3.1.2. Comparison of the Amount of Gaseous Products
Figure 4 shows the relationship between t and the amount of gaseous products. The component of gaseous products obtained by MWP is mainly H2 and CO, and the others are a few CH4, CO2, C2H2 and C2H4. The amount of H2 and CO increases with an increase in t and indicates a maximum value at 10 min. The amount of H2 and CO is 18.0 mmol/g and 23.5 mmol/g at 10 min. The component of gaseous products obtained by RFP is mainly H2 and CO, and the others are a few CH4, CO2, C2H2, C2H4 and C2H6. The amount of H2 and CO increases with an increase in t and indicates maximum value at 30 min. The amount of H2 and CO is 8.7 mmol/g and 13.9 mmol/g at 30 min. The amount of H2 and CO obtained by MWP is larger than that obtained by RFP irrespective of t. It is found that MWP can produce the large amount of H2 and CO in the short time of 10min. This results from the higher temperature of MWP than that of RFP. Producing syngas (H2 and CO) from biomass by a general pyloysis, Dufour et al. reported the amount of hydrogen obtained from wood increased from 0.06 Nm3/kg to 0.24 Nm3/kg with an increase in temperature from 700˚C to 1000˚C [11]. It is suggested that the obtained large amount of H2 and CO by MWP is probably due to the high temperature.
3.2. Comparison of the Product Yield with the Conversion Change
Figure 5 shows the relationship between X and Ygas, Yliquid. Ygas increase with an increase in X irrespective of method. Under 60 wt% of X, Ygas by MWP is higher than as that of RFP. Over 60 wt% of X, Ygas by MWP is almost same as that by RFP. Yliquid is constant irrespective of X. Yliquid by MWP is lower than that of RFP under 60 wt% of X. From this result, it is found that Ygas is almost same over X of 60wt% irrespective of method.
Park et al. proposed decomposition mechanism of wood pyrolysis and obtained activation energy [12]. Ac-
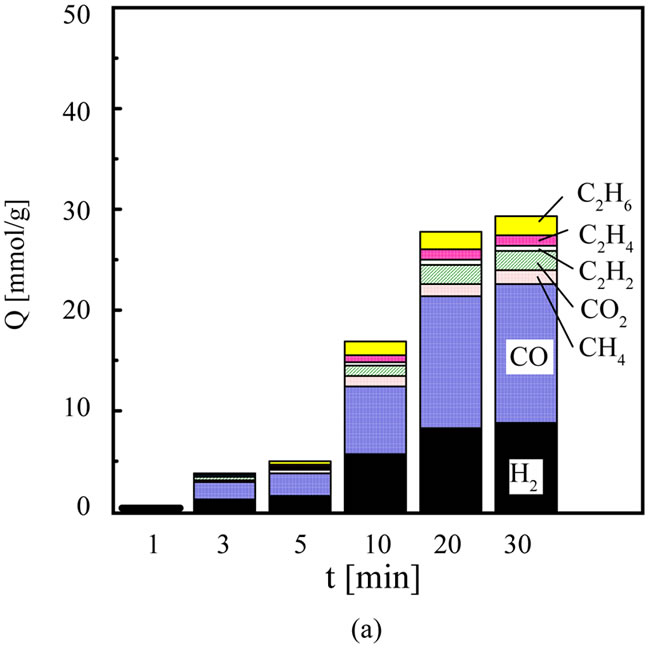
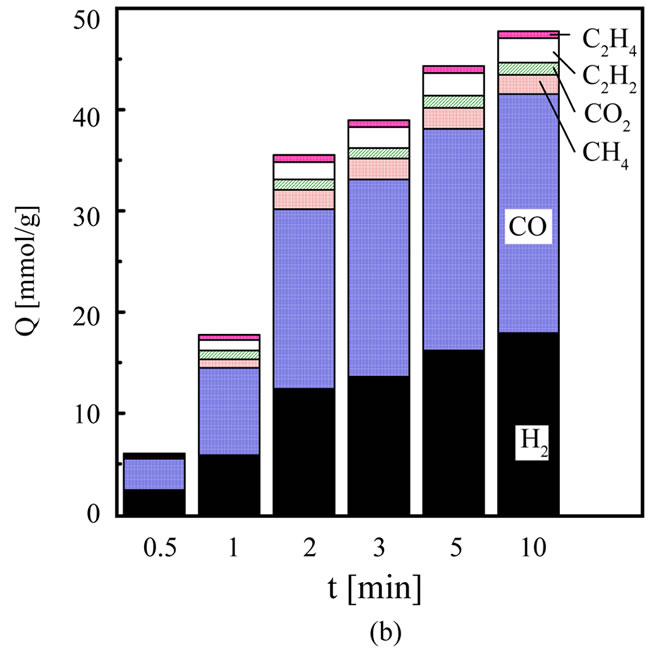
Figure 4. Comparison of the amount of gaseous products. (a) Radio frequency plasma; (b) Microwave plasma.

Figure 5. Relationship between conversion and product yield (Ygas, Yliquide).
cording to their results, gas was produced directly from wood or obtained from the decomposition of tar produced by wood pyrolysis. Moreover, they investigated activation energy (E) of the each decomposition process, E of wood to gas was 152.7 kJ/mol, E of wood to tar was 148 kJ/mol, and E of tar to gas was 108 kJ/mol. Those results are found directly gasification from wood needed large energy and production of tar was easier than that of gas. Moreover, obtained tar was easily converted to gas. It is suggested that the higher Ygas of MWP than that of RFP under X of 60 wt% is due to the higher MW energy in comparison with RF energy. Over X of 60 wt%, Ygas of RFP is as same as that of MWP. This is due to the conversion of obtained liquid products to gaseous products.
3.3. Comparison of the Amount of H2 and CO with the Change of Gaseous Yield
Figure 6 shows the relationship between Ygas and the amount of H2 or CO. The amount of H2 and CO increases with an increase in Ygas irrespective of method. The amount of H2 and CO obtained by MWP is larger than that obtained by RFP irrespective of Ygas. This results from the difference of energy between MW and RF. According to Figure 4, the amount of CH4, CO2, C2H4 and C2H6 obtained by RFP was larger than that obtained by MWP. Cho et al. studied oxidative coupling of methane with microwave and RF plasma catalytic reaction over transitional metals loaded on ZSM-5 [13]. From their results, comparing product selectivity obtained by MWP with that obtained by RFP when CH4/O2 (4:1) was reacted on non-catalyst, CO selectivity of MWP was higher than that of RFP and CO2 selectivity of MWP was lower than that of RFP. This result was found that CO selectivity is higher with larger energy. This result suggested that the difference of composition of gaseous products between MWP and RFP is due to the difference of energy between MW and RF.
3.4. Comparison of the Element Distribution
Figure 7 shows the comparison of the element distribution at the maximum conversion between MWP and RFP. H and O element were mainly distributed to gaseous products irrespective of method. By means of MWP, H element was mainly converted to H2. On the other hand, H element was mainly distributed to other gases as CH4 and C2 hydrocarbon by using RFP. O element was mainly converted to CO by using MWP. O element was mainly distributed to other gas as CO2 by using RFP. C element was gasificated to CO irrespective of method. Although, the large amount of C element remained in the residue obtained by RFP, and C element remained little in the
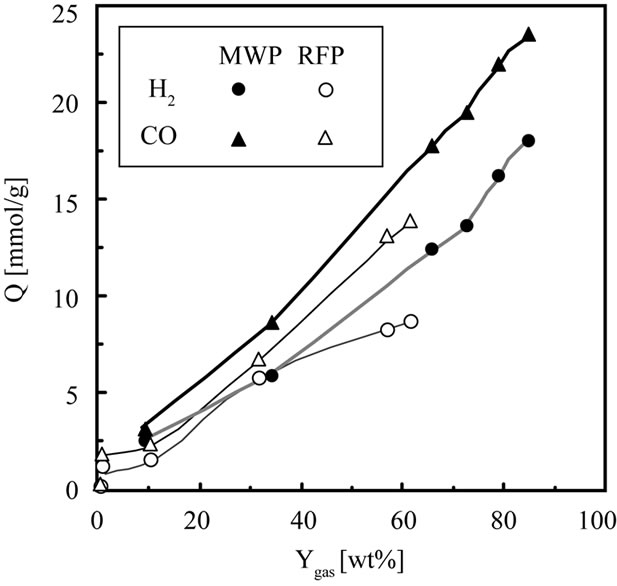
Figure 6. Relationship between conversion and the amount of H2, CO in.
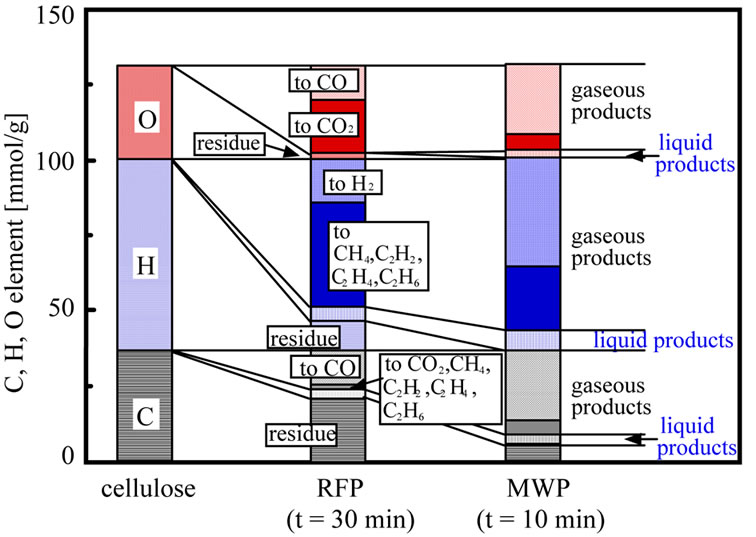
Figure 7. Comparison of element distribution between MWP and RFP.
residue obtained by MWP. Form above result, it is found that MWP can distribute C, H and O element to mainly H2 and CO. This is probably due to the adequate energy of MWP to give active species for H2 and CO.
4. Conclusions
MWP can highly convert cellulose to gaseous products at short time (10 min) in comparison with RFP. Gaseous products mainly comprise H2 and CO. The amount of H2 and CO obtained by MWP is 18.0 mmol/g and 23.5 mmol/g, it was larger than that obtained by RFP. The gaseous yields by MWP were almost same as that by RFP over X of 60 wt%. However, the amounts of H2 and CO by MWP were higher than that by RFP irrespective of gaseous yield. By MWP, C, H and O element in cellulose could be converted to mainly H2 and CO. Those results suggest that MWP is suitable for cellulose gasification in comparison with RFP, since MWP highly convert the element of C, H and O to H2 and CO by the higher energy of microwave frequency than that of radio frequency.
5. References
[1] L. hang, C. C. Xu and P. Champagne, “Overview of Recent Advances in Thermo-Chemical Conversion of Biomass,” Energy Conversion and Manegement, Vol. 51, No. 5, 2010, pp. 969-982. doi:10.1016/j.enconman.2009.11.038
[2] K. Katou, T. Asou, Y. Karauchi and R. Sameshima, “Melting Municipal Solid Waste Incineration Residue by Plasma Melting Furnace with a Graphite Electrode,” Thin Solid Films, Vol. 386, No. 2, 2001, pp. 183-188. doi:10.1016/S0040-6090(00)01640-0
[3] L. Tang and H. Huang,” Biomass Gasification Using Capacitively Couples RF Plasma Technology,” Fuel, Vol. 84, No. 16, 2005, pp. 2055-2063. doi:10.1016/j.fuel.2005.04.015
[4] O. Kamei, W. Marushima, M. Kobayashi, K. Onoe, T. Yamaguchi, S. Kawai and Y. Ito, “Product Distribution from Yallourn Coal by Methane Microwave Plasma Conversion,” Journal of the Japan Petroleum Institute, Vol. 42, No. 5, 1999, pp. 335-341. doi:10.1627/jpi1958.42.335
[5] O. Kamei, W. Marushima, M. Kobayashi, K. Onoe, T. Yamaguchi, S. Kawai and Y. Ito, “Conversion of Yallourn Coal by Microwave Plasma—Effect of Plasma Gas Spiesis on Products,” Journal of the Japan Petroleum Institute, Vol. 78, No. 8, 1999, pp. 664-669. doi:10.1016/S0016-2361(98)00055-6
[6] O. Kamei, K. Onoe, W. Marushima and T. Yamaguchi, “Brown Coal Conversion by Microwave Plasma Reactions under Successive Supply of Methane,” Fuel, Vol. 77, No. 13, 1998, pp. 1503-1506.
[7] M. Kobayashi, K. Konno, H. Okamura, T. Yamaguchi and K. Onoe, “Decomposition of Biomass by Microwave Plasma Reactions,” Journal of the Japan Petroleum Institute, Vol. 84, No. 6, 2005, pp. 468-473.
[8] K. Konno, H. Okamura, M. Kobayashi, K. Onoe and T. Yamaguchi, “Hydrogen Production from Wet Biomass (Lignin) Using Microwave Plasma Technique,” Gekkan Kinouzairyou, Vol. 25, No. 1, 2005, pp. 56-61.
[9] K. Konno, M. Kobayashi, K. Onoe and T. Yamaguchi, “Decomposition of Biomass Using Microwave Plasma,” The Proceeding of 16th International Symposium on Plasma Chemistry, 2003, S-11.
[10] W.K Tu, J. L Shie, C. Y Chang, C. F. Chang, C. F. Lin, S. Y. Yang, J. T. Kuo, D. G. Shaw and D. J. Lee, “Pyrolysis of Rice Straw Using Radio-Frequency Plasma,” Energy and Fuel, Vol. 22, No. 1, 2008, pp. 24-30. doi:10.1021/ef7002848
[11] A. Dufour, P. Girods, E. Masson, Y. Rogaume and A. Zoulalian, “Synthesis Gas Production by Biomass Pyrolysis—Effect of Reactor Temperature on Product Distribution,” Hydrogen Energy, Vol. 34, No. 4, 2009, pp. 1726-1734. doi:10.1016/j.ijhydene.2008.11.075
[12] W.C. Park, A. Atreya and H. R. Baum, “Experimental and Theoretical Investigation of Heat and Mass Transfer Processes during Wood Pyrolysis,” Combustion and Flame, Vol. 157, No. 3, 2010, pp. 481-494. doi:10.1016/j.combustflame.2009.10.006
[13] W. Cho, Y. Baek, S. K. Moon and Y. C. Kim, “Oxidative Coupling Methane with Microwave and RF Plasma Catalytic Reaction over Transitional Metals Loaded on ZSM-5,” Catalysis Today, Vol. 74, No. 3-4, 2002, pp. 207-223. doi:10.1016/S0920-5861(02)00030-5

