World Journal of Vaccines
Vol.2 No.3(2012), Article ID:21521,3 pages DOI:10.4236/wjv.2012.23021
The Prevalence of Mycoplasma hyopneumoniae in Commercial Suckling Pigs in Thailand
![]()
1Faculty of Veterinary Medicine, Kasetsart University, Kamphaeng Saen Campus, Nakonpathom, Thailand; 2Pfizer Animal Health (Thailand) Limited, Bangkok, Thailand.
Email: *fvetakb@ku.ac.th
Received February 15th, 2012; revised March 18th, 2012; accepted April 20th, 2012
Keywords: Natural Infection; M. hyopneumoniae; Suckling Pig; Nested PCR
ABSTRACT
The aim of this study was to determine the prevalence of M. hyopneumoniae infection in suckling pigs. Nasal swabs were collected from 300 suckling pigs originating from five farrow-to-finishing farms. One farm had a confirmed PRDC problem (farm A) and four other farms previously had a PRDC problem (farms B, C, D and E). Thirty (30) lactating sows in parity 1, 2 and 3 were selected from each farm (Ten sows per parity). Two piglets from each sow were randomly sampled for nasal swab at 3 weeks of age. The samples were analyzed by the nested PCR technique. Forty five per cent (27/60) of nasal swabs from farm A were found positive. On the other hand, a total of 2.08 per cent were found positive (5/240) from farm B, C, D and E. The tendency of piglet infection per sow by parity showed that first parity had more prevalence than the second and the third parity (60%, 55%, 20%), respectively. We have found a correction between M. hyopneumoniae early infection in suckling pigs and a confirmed PRDC problem (farm A) as oppose to farms that did not have a PRDC problem. The strategies to prevent M. hyopneumoniae early infection are to maintain good lactation, antibiotic prevention program and early M. hyopneumoniae vaccination.
1. Introduction
M. hyopneumoniae is the primary agent of porcine enzootic pneumonia and associated with respiratory diseases. It also plays a crucial role in the porcine respiratory disease complex (PRDC). It causes major economic losses in the swine industry worldwide mainly due to reduced performance and increased susceptibility to other infections. M. hyopneumoniae is normally transmitted to susceptible pigs by direct contact or sharing of the same air space with infected pigs. Piglets may become infected in the farrowing unit through shedding sows. The chance of transmission from a sow to its offspring is higher in gilts and low parity sows [1]. A recent study showed that pigs can remain infectious for at least 200 days post infection [2]. Nested PCR is a sensitive method to detect M. hyopneumoniae infection in young pig [3]. The purpose of this study was to determine the extent of natural infection caused by M. hyopneumoniae in suckling pigs in commercial pig farms in Thailand.
2. Materials and Methods
Farm; five commercial farrow-to-finish pig farms in Rachaburi and Chonburi provinces in Thailand were chosen for this study. As normal practice, Mycoplasmal vaccine was administered to piglets at 3 weeks of age. PRDC status of these farms were categorized based on their clinical records in nursery pigs. If clinical records showed over 5 percent of losses in nursery pigs due to PRDC, farms were classified as PRDC positive. Farms were also categorized based on the average daily litter weight gain (ADLWG) of piglets, classified as “A” when the ADLWG is more than 2300 g/d and as “B” when the ADLWG is less than 2300 g/d.
Sample and sampling; in total 300 samples of nasal swabs were collected from suckling pigs from the five farms. The farms were divided into two groups depending on their individual situation; one farm had confirmed PRDC problem (farm A) and other four farms previously had a PRDC problem (farms B, C, D and E). Lactating sows in parity 1, 2 and 3 from each farm were selected (10 sows per group). Nasal swab samples were randomly obtained from two suckling pigs at 3 weeks old from each sow. Samples were kept in a PBS solution stored at 4˚C and sent to the Diagnostic and Laboratory Unit, Faculty of Veterinary Medicine, Kasetsart University, Kamphaeng Saen campus, Nakhon Pathom province for PCR analysis. The specific primers for M. hyopneumoniae used in nested PCR method was designed as described by Calsamiglia et al., 1999 [4]. Finally, the PCR products were analyzed by electrophoresis in a 1% agarose gel with 0.5 µg/ ml of ethidium bromide and then observed under UV lamp.
Statistics; the results were explained and analysed through a descriptive statistical technique, as prevalent of the M. hyopneumoniae infection in piglets. A Chi square test or Fischer’s exact test was used to analyse correlation between PCR result and parity, lactation status and PRDC status. A P value of <0.05 was considered statistically significant.
3. Results
The extent of natural infection caused by M. hyopneumoniae in piglets on farms A, B, C, D and E were 45%, 8.33%, 0%, 0% and 0%, respectively (Table 1). Piglets from parity 1 sows in farm A showed the highest prevalence of M. hyopneumoniae whereas piglets from parity 3 sows showed the lowest level (Table 1).
Pig farm that had positive PRDC status tend to have positive M. hyopeumoniae from their piglet nasal swabs as compared to negative PRDC status farms (Table 2). The study result demonstrated that farm with a high quality lactating unit (ADLWG ≥ 2300 g/d) had zero PCR positive result (Table 3). Moreover, the study also showed higher PCR positive results from parity 2 sows than parity 1 sows and parity 3 (Table 4).
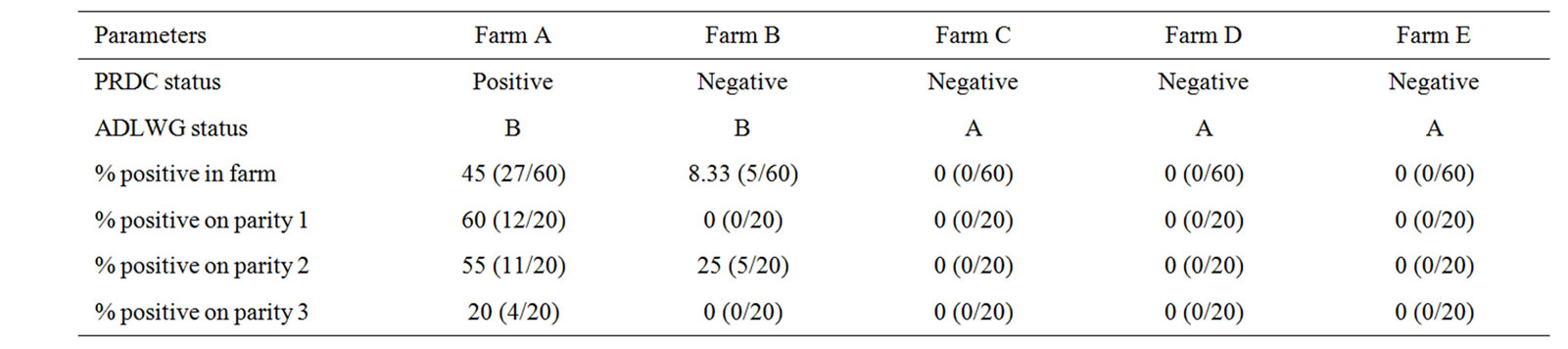
Table 1. Shows farm information and percentage of positive M. hyopneumoniae nested PCR results from suckling pig nasal swabs (positive sample/total sample).
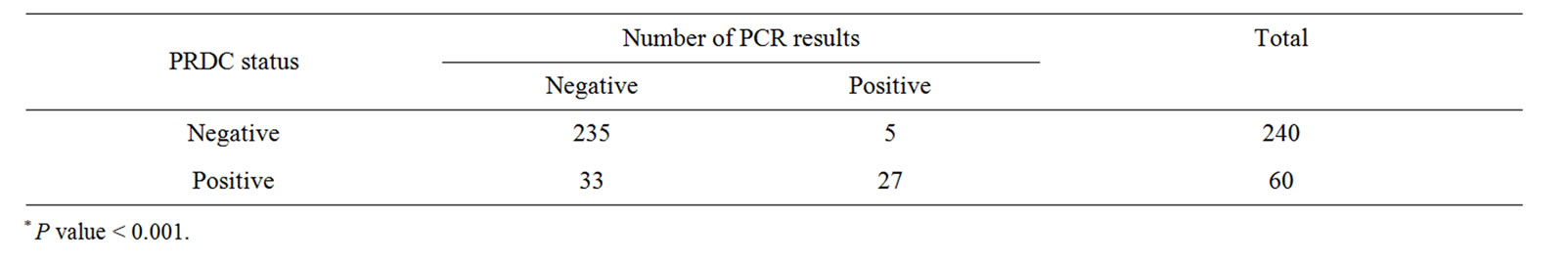
Table 2. Shows correlation between PRDC status and number of nested PCR results*.
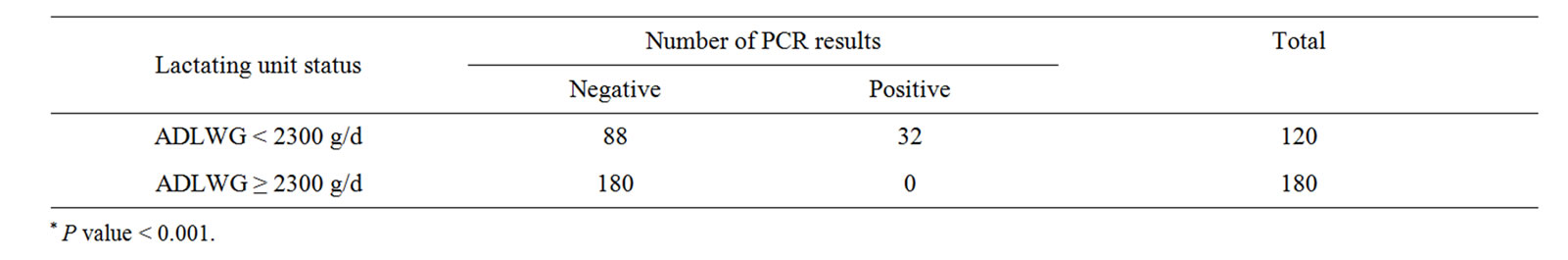
Table 3. Shows relationship between a lactation status and number of PCR results*.
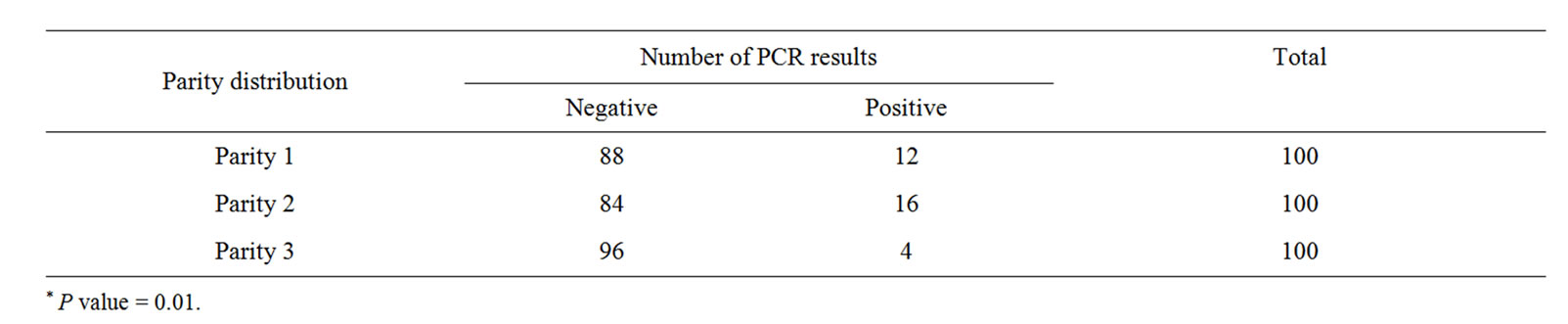
Table 4. Shows correlation between parity distribution and number of nested PCR results*.
4. Discussion and Conclusion
The percentage of M. hyopneumoniae infected pigs at weaning varies between studies. In most reports, approximately 8% - 20% of the piglets are positive at or shortly after weaning using nested PCR on nasal swabs [3,5]. In the present study 45% of 3 weeks old piglets from farm A were positive. The high prevalence of M. hyopneumoniae PCR positive at weaning resulted in high infectious load in the farm and correlated with farm PRDC status. Nasal swab results of piglets from the remaning farms (except farm B) were found negative, correlated with farm PRDC status. Farm B had more population of gilts and parity one sows than others farm in the PRDC status negative group. In addition, this study confirms that gilts can shed M. hyopneumoniae to their piglets more readily than older parities [1]. Moreover, piglets from high ADLWG group showed lower M. hyopneumoniae positive compared with piglets from low ADLWG group. ADLWG indicates piglet’s health during lactation. Furthermore, antibiotic protocols and early M. hyopneumoniae vaccination programs are a forceful technique for prevention.
REFERENCES
- E. Fano, C. Pijoan, S. Dee and M. Torremorell, “Assessment of the Effect of Sow Parity on the Prevalence of Mycoplasma hyopneumoniae in Piglets at Weaning,” Proceedings of 19th International Pig Veterinary Society, Copenhagen, 16-19 July 2006, p. 96.
- M. Pieters, C. Pijoan, E. Fano and S. Dee “An Assessment of the Duration of Mycoplasma hyopneumoniae Infection in an Experimentally Infected Population of Pigs,” Veterinary Microbiology, Vol. 134, No. 3-4, 2009, pp. 261- 266. doi:10.1016/j.vetmic.2008.08.016
- M. Sibila, R. Bernal, D. Torrents, P. Riera, D. Llopart, M. Calsamiglia and J. Segales, “Effect of Sow Vaccination against Mycoplasma hyopneumoniae on Sow and Piglet Colonization and Seroconversion, and Pig Lung Lesions at Slaughter,” Veterinary Microbiology, Vol. 127, No. 1-2, 2008, pp. 165-170.
- M. Calsamiglia, C. Pijoan and A. Trigo, “Application of a Nested Polymerase Chain Reaction Assay to Detect Mycoplasma hyopneumoniae from Nasal Swabs,” Journal of Veterinary Diagnostic Investigation, Vol. 11, No. 3, 1999, pp. 246-251.
- I. Villarreal, K. Vranckx, L. Duchateau, F. Pasmans, F. Haesebrouck, J. Jensen and D. Maes, “Prevalence and Risk Factors for Early Mycoplasma hyopneumoniae Infections in Suckling Piglets in Different EU Countries,” Proceedings of 21st IPVS Congress, Vancouver, 18-21 July 2010, p. 101.
NOTES
*Corresponding author.

