Open Journal of Urology
Vol. 2 No. 2 (2012) , Article ID: 19128 , 3 pages DOI:10.4236/oju.2012.22013
Giant Angiomyolipoma of the Kidney Presenting as Anaemia—A Rare Presentation*
Department of Surgery, Maulana Azad Medical College and Lok Nayak Hospital, New Delhi, India
Email: sudhirkumar11@gmail.com
Received December 23, 2011; revised January 30, 2012; accepted February 16, 2012
Keywords: Kidney; Angiomyolipoma; Anaemia
ABSTRACT
Angiomyolipoma presenting with severe anaemia is a rare entity. Urgent operative intervention is warranted in such patients especially if the tumour is “Giant” i.e. greater than 10 cm and involves the whole kidney. We chronicle the case of a 35-year-old lady who presented with a rapidly enlarging renal lump in the left loin associated with severe anaemia. MRI was highly suggestive of angiomyolipoma. She underwent nephrectomy in view of continuous intratumoral haemorrhage. The tumour measured 18 × 13 × 8 cm and weighed approximately 1.6 kilograms after removing blood and blood clots. The histopathology was suggestive of angiomyolipoma. Angioembolization may be considered for tumours involving a portion of the kidney, as a part of the nephron sparing approach.
1. Introduction
An angiomyolipoma measuring more than 10 cm in size is considered as “Giant” and is unusual. We report one such case which appears to be one of the largest reported so far as per a literature search, weighing approximately 1.6 kilograms and measuring 18 × 13 × 8 cm, and presented as a painful abdominal lump with severe anemia.
2. Case Report
A 35 year old lady presented to the outpatient department with the complaint of a rapidly enlarging, painful lump in the left flank for the last 5 days along with a recent onset feeling of weakness and lethargy. There were no urinary symptoms and no history of fever or weight loss. On examination, the patient had marked pallor with tachycardia (pulse rate of 100 beats per minute) with normal jugular venous pressure and no evidence of pedal edema. Examination of the abdomen revealed a 15 × 12 cm tender, bimanually palpable, globular lump in the left lumbar region which was moving with respiration. One could insinuate the digits of the examining hand between the costal margin and the lump. The renal angle was found to be tender and full. No other lump was palpable and there was no free fluid in the abdomen. She was found to be anaemic, with haemoglobin of 6 g/dL at presentation. After transfusion of 4 units of blood, the haemoglobin rose to 9 g/dL but again fell to 7 g/dL while she underwent further evaluation for the renal lump. Her renal function tests were within the normal range and urine routine microscopy did not show microscopic haematuria.
Ultrasonography revealed a well circumscribed, hyperechoic lesion in the left kidney with a few hypoechoic areas suggestive of haemorrhage or necrosis, replacing the upper and mid pole and displacing the hilum, suggestive of a mitotic etiology likely angiomyolipoma.
Magnetic Resonance Imaging (MRI) revealed a large altered signal intensity mass lesion (Figure 1) of size 18 × 13 × 8 cm with well defined margins replacing almost the whole of the left kidney. It appeared heterogeneously hyperintense on T1 and heterogeneously isointense on T2, which combined with the displacement of the hilum (signifying a mass effect), was highly suggestive of an angiomyolipoma.
The patient underwent a nephrectomy through a transperitoneal route. Per-operatively, a large 18 × 13 × 8 cm renal mass was found to be displacing the abdominopelvic structures. The renal hilum was displaced inferiorly. The specimen consisted of a soft, yellow tumour with areas of haemorrhage. The tumour weighed approximately 1.6 kilograms after removing blood and blood clots. The histopathology report of the above specimen showed a tumour of 18 × 11 × 7 cm occupying the upper, middle and part of the lower pole, with histological features of angiomyolipoma (Figure 2). The tumour was seen compressing the capsule and was separated from it by a hematoma. The renal pelvis, ureter and vessels identified were normal.
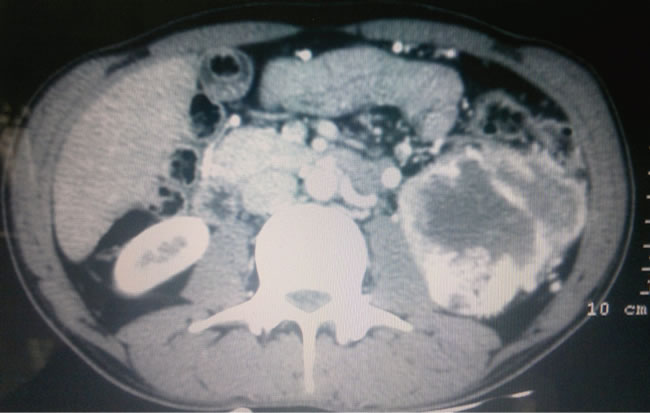
Figure 1. MRI showing a large altered signal intensity mass lesion in the left kidney (areas of haemorrhage indicated by black arrow).
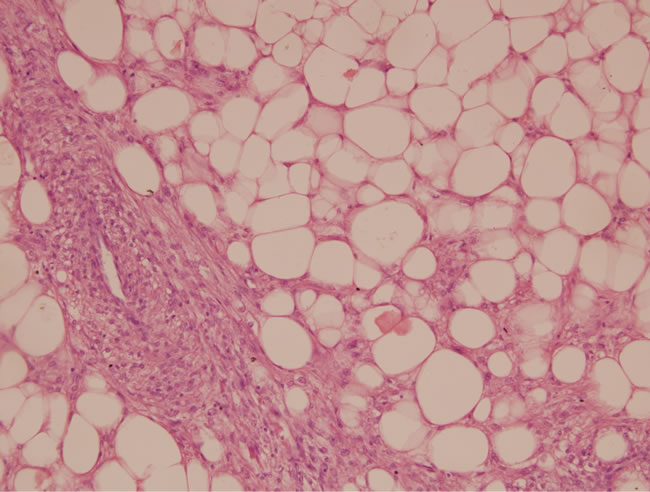
Figure 2. Photomicrograph: A typical abnormally thick walled blood vessel surrounded by spindle shaped smooth muscle cells along with mature adipocytes (Haemotoxylin and eosin stain, 100× magnification).
Postoperative period was uneventful and she was discharged after 7th post-operative day. She remained well during one year of follow up.
3. Discussion
Angiomyolipoma (AML) is a rare benign tumour of the kidney consisting of a mixture of mature adipose tissue, smooth muscle and thick-walled vessels [1]. It is found in 0.3% of all autopsies and 0.13% of the population undergoing an ultrasonographic screening. Approximately 20% - 30% of all angiomyolipomata are detected in patients with tuberous sclerosis syndrome [2]. In these patients, the lesions tend to be multiple, bilateral, affecting younger individuals with a female to male ratio of 2:1 [3].
Patients commonly present with flank pain, abdominal mass and haematuria (Lenk’s triad) and on occasion, hypertension. In case of massive retroperitoneal haemorrhage, a known complication found in up to 10% of cases, they can present with hypovolemic shock (Wunderlich syndrome) [4].
In our case, the patient presented with a unilateral, rapidly enlarging, painful abdominal lump and severe anaemia although she did not have features of hypovolemic shock. Her haemoglobin levels did not improve despite blood transfusion leading us to suspect haemorrhage into the tumour.
The diagnostic modality of choice in a case of AML is Contrast Enhanced Computed Tomography (CECT) or MRI. The hallmark feature of AML on a non-contrast CT is the presence of fat (attenuation of less than or equal to -20 HU) [5,6]. MRI with fat suppressed image is also helpful in characterizing a lesion as AML. Although AML can be diagnosed on CT, clinical dilemma occurs with liposarcoma and a fat containing RCC. However differentiation can still be done as a liposarcoma does not distort the renal parenchyma and a fat containing RCC may have calcifications, whereas in AML these features are almost never seen [6].
The lesions can be classified as small (<4 cm), medium (4 - 8 cm) and large (>8 cm) based on the single largest lesion in each kidney. Smaller lesions are asymptomatic and found incidentally whereas lesions greater than 8 cm are responsible for significant morbidity and usually require treatment [7-9]. Larger tumours are at a greater risk of spontaneous or traumatic rupture resulting in haemorrhagic complications [4,10].
In view of haemorrhage into the tumour and the low haematocrit of the patient, urgent intervention was required in our case. Although angioembolization is one of the available modalities to control haemorrhage, nephrectomy was done because the tumour had involved almost the entire renal parenchyma. The excised specimen weighed 1.6 kilograms which is one of the largest reported in the literature.
4. Conclusion
Although anaemia is a rare presentation of a renal angiomyolipoma, it should be considered as a differential in the patients who present with a tender renal mass associated with severe anemia. Active intervention should be done in these patients as an angiomyolipoma with bleeding can lead to severe complications, increasing the morbidity. The nature of the lesion can be confirmed on MR/ CT imaging and in cases of giant angiomyolipomata with intratumoral haemorrhage, nephrectomy is a good treatment option. However, if the bleeding tumour is localized to one of the poles, angioembolization may be considered as it has the advantage of sparing nephrons.
REFERENCES
- P. Tamboli, J. Y. Ro, M. B. Amin, et al., “Benign Tumours and Tumour Like Lesions of the Adult Kidney, Part 2: Benign Mesenchymal and Mixed Neoplasms and Tumour Like Lesions,” Advances in Anatomic Pathology, Vol. 7, No. 1, 2000, pp. 47-66. doi:10.1097/00125480-200007010-00007
- J. N. Eble, “Angiomyolipoma of Kidney,” Seminars in Diagnostic Pathology, Vol. 15, No. 1, 1998, pp. 21-40.
- T. S. Lendvay and F. F. Marshall, “The Tuberous Sclerosis Complex and Its Highly Variable Manifestations,” The Journal of Urology, Vol. 169, No. 5, 2003, pp. 1635-1642. doi:10.1097/01.ju.0000058253.40352.60
- J. E. Oesterling, E. K. Fishman, S. M. Goldman and F. F. Marshall, “The Management of Renal Angiomyolipoma,” The Journal of Urology, Vol. 135, 1986, pp. 1121-1124.
- M. A. Bosniak, A. J. Megibow, D. J. Hulnick, et al., “CT Diagnosis of Renal Angiomyolipoma: The Importance of Detecting Small Amounts of Fat,” American Journal Roentgenology, Vol. 151, No. 3, 1998, pp. 497-501.
- L. Lemaitre, M. Claudon, F. Dubrulle and F. Mazeman, “Imaging of Angiomyolipoma,” Seminars in Ultrasound, CT, and MR, Vol. 18, No. 2, 1997, pp. 100-114. doi:10.1016/S0887-2171(97)90054-8
- M. Dickinson, H. Ruckle, M. Beaghler, et al., “Renal Sngiomyolipoma: Optimal Treatment, Size and Symptoms,” Clinical Nephrology, Vol. 49, No. 5, 1998, pp. 281-286.
- M. S. Steiner, S. M. Goldman, et al., “The Natural History of Renal Angiomyolipoma,” The Journal of Urology, Vol. 150, No. 6, 1993, pp. 1782-1786.
- M. L. Blute, R. S. Malek and J. W. Sigura, “Angiomyolipoma: Clinical Metamorphosis and Concerns,” Urology, Vol. 139, No. 1, 1988, pp. 20-24.
- C. P. Nelson and M. G. Sanda, “Contemporary Diagnosis and Management of Renal Angiomyolipoma,” The Journal of Urology, Vol. 168, No. 1, 2002, pp. 1315-1325.
NOTES
*It is certified that this manuscript has been read and approved by all the authors. The authors declare that they have no conflict of interest.

