Journal of Diabetes Mellitus
Vol.1 No.4(2011), Article ID:8422,5 pages DOI:10.4236/jdm.2011.14017
Iowa medicaid 2: lapse of glycemic control on abrupt transition from insulin glargine to insulin detemir in type 2 diabetes mellitus
![]()
Department of Medicine, University of Iowa, Iowa City, USA. udaya.kabadi@va.gov
Received 29 July 2011; revised 31 August 2011; accepted 11 September 2011.
Keywords: Type 2 Diabetes; Insulin Glargine; Insulin Detemir; Glycemic Control
ABSTRACT
Background: Iowa Care (Iowa Medicaid in State of Iowa, USA), switched insulin glargine to detemir in subjects with Diabetes Mellitus (DM) without the knowledge or approval of healthcare providers beginning 8/2006. Impact of this transition in subjects with Type 1 DM is recently reported. Objective: To examine the impact of this transition on various parameters of diabetes management in Type 2 DM. Subjects and Methods: A retrospective review of the records of subjects with Type 2 DM was conducted until 8/ 2007 in whom the transition had occurred. Only those subjects with adequate glycemic control while receiving insulin glargine [GI] and completing at least 3 months of therapy with insulin detemir [DI] are included in this report. Ten subjects with Type 2 DM, duration 7 ± 2 years with age, 55 ± 3 years who were switched from GI to DI (Group 1) fulfilled the criteria for inclusion. Subjects were switched from GI in Q AM to DI Q HS in the same daily dose. Glycemic control (HbA1c), body weight , daily insulin dose (Units) and severe hypoglycemic events during the last 2 weeks of the period, pre switch and again at the end of 3 months post switch were assessed. Records of 8 subjects matched for age, duration of DM, glycemic control while receiving GI for additional 3 months (Group 2) during the same period were examined for comparison. All subjects were followed in the outpatient clinic at intervals of 3 months. Results: Glycemic control remained stable on continuing GI AM; HbA1c; 7.1% ± 0.3% to 7.1% ± 0.3%, while it worsened on switching to DI Q HS; HbA1c, 7.1% ± 0.3% to 8.1% ± 0.5% [P < 0.01]. A mild weight loss was noted in subjects on transition. No severe hypoglycemic events were reported in any subject in either group. Conclusion: Abrupt transition from insulin glargine to insulin detemir in subjects with Type 2 DM is likely to result in lapse of glycemic control which may cause decreased quality of life. Furthermore, use of insulin detemir may result in increased costs due to need of the higher daily dose as well as additional equipment required for probable twice daily administration to achieve adequate glycemic control. Therefore, insulin glargine and detemir appear to be far from being bioequivalent.
1. INTRODUCTION
In August 2006, Iowa Care (Medicaid in State of Iowa, USA) switched insulin glargine to detemir in subjects with Diabetes Mellitus (DM) without the knowledge or approval of healthcare providers. Several formularies embrace similar policies in an attempt to reduce costs because of the ability to periodically negotiate pricing with manufacturers of drugs including insulins, medical devices as well as other equipment required in clinical practice. The impact of such a policy, especially the trend of interchanging basal insulin analogs is important and has not been well established. Therefore, a retrospective review was conducted to assess the conesquence of this switch from insulin glargine to insulin detemir on several outcomes in subjects with both Type 1 and Type 2 Diabetes Mellitus. The data in subjects with Type 1 DM is already published and showed a lapse of glycemic control despite a significantly higher daily dose of insulin detemir being administered twice daily [1]. In this report, the impact of the abrupt transition from insulin glargine to insulin detemir in subjects with type 2 DM is presented.
2. SUBJECTS AND METHODS
The study protocol was approved by the local IRB at the medical center.
A retrospective review of records of subjects with Type 2 DM manifesting good glycemic control [HbA1c 6.6% - 7.5%] who were switched from insulin glargine to insulin detemir from 08/06 until 03/07 was conducted. Only subjects with adequate glycemic control while receiving insulin glargine were included in the study because the primary aim of the study was to assess comparative efficacy of the same daily dose of individual insulin on glycemic control. The other reason was to avoid variability of glycemic control prior to transition from insulin glargine to insulin detemir as well as the subjects in whom insulin glargine was continued. The duration of therapy with detemir of at least 3 months was deemed to be appropriate for the review because it is the usual duration over which assessment of HbA1c is accepted in clinical practice. The duration of therapy with insulin glargine was also at least 3 months prior to switch. Concomitant therapy with same oral agents, e.g. Metformin and Sulfonlyurea in the same dosage was continued following and throughout the period of transition. Subjects requiring hospitalization during the study period were excluded. Medications for other disorders used prior to switch were continued in the same daily dosage for the entire period of observation. Records of 10 subjects, 6 men and 4 women fulfilling the criteria until 03/07 mentioned above were reviewed [Group 1]. Records of additional 8 subjects [5 men and 3 women] matched for age, duration of DM, body weights, glycemic control [HbA1c] while receiving treatment with same antihyperglycemic drugs in identical daily dose as subjects in Group 1 and continuing insulin glargine covered by their insurance policies during the same period were used as controls [Group 2]. The diagnosis of Type 2 DM was previously established by criteria recommended by American Diabetes association [2] as well as by history of adequate glycemic control maintained for several years by diet and oral agents and at the time of transition by combination therapy with oral agents and insulin glargine.
The pertinent demographic characteristics of subjects are summarized in Table 1. In all subjects, the daily dose of insulin detemir at the time of switch was kept exactly the same as insulin glargine and was administered at bedtime as recommended by the pharmacy initiating the transition or at the time of the routine clinic visit based on product information provided by the manufacturer. Prior to transition, insulin glargine was administered in AM prior to breakfast since the AM administration in combination with oral agents has resulted in better glycemic control without weight gain in some previous studies [3-6]. Moreover, once daily administration of insulin glargine may be appropriate at any dose since the 24 hour profile remains stable without peaks and troughs even at a dose as high as 2 units/KG body weight in subjects with type 2 Diabetes [7,8]. Outcomes for assessment were predetermined and included glycemic control (HbA1c), body weight and daily insulin dose. Severe hypoglycemic events during 2 weeks prior to switch as well as 2 weeks prior to the end of 3 months on therapy with insulin detemir were also reviewed. Only the severe hypoglycemic events were included for review because of the definitive documentation by history obtained from subjects, their caregiver or their next of kin as well as by the recordings in blood glucose diaries. Severe hypoglycemic event is defined as the one requiring a visit to emergency room or assistance by a paramedic or a third party at home for resuscitation (2). Mild hypoglycemic episodes not requiring secondary attention were not included because of lack of adequate documentation by participating subjects.
All these outcomes were determined prior to switch and at 3 months following therapy with insulin detemir in Group 1.The same endpoints were also examined on two occasions at interval of 3 months in 8 control subjects [Group 2]. All subjects were always being followed in outpatient diabetes clinic by the same physician at intervals of 8 - 12 weeks for assessment and management of diabetes and other disorders.
3. RESULTS
Serum creatinine, liver enzymes and other chemistries in all subjects in both groups were within normal limits on both occasions, at the initiation and at the end of period of observation. A significant rise [p < 0.01] in HbA1c was noted in Group 1 at the end of the period of observation in comparison to the time of switch as well as at both times of assessment in Group 2 while continuing the same the daily insulin dose (Table 2). Body
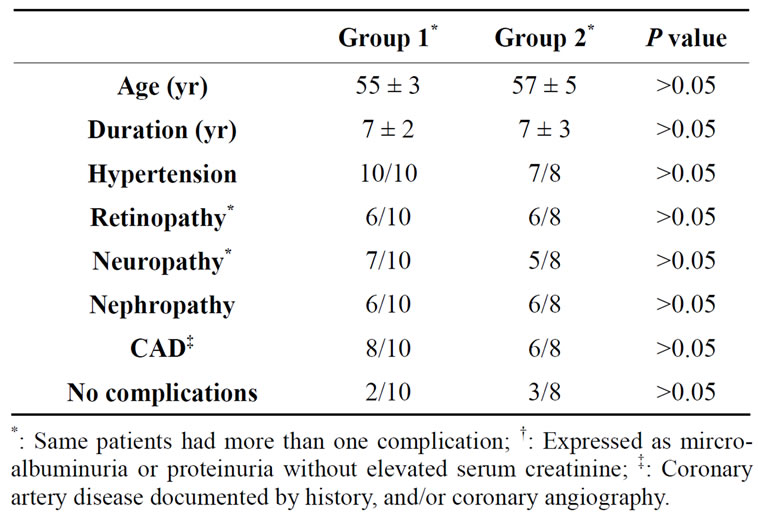
Table 1. Demographic characteristics of 10 subjects who were switched from insulin glargine to detemir [Group 1] and 8 subjects [Group 2], who remained on insulin glargine.
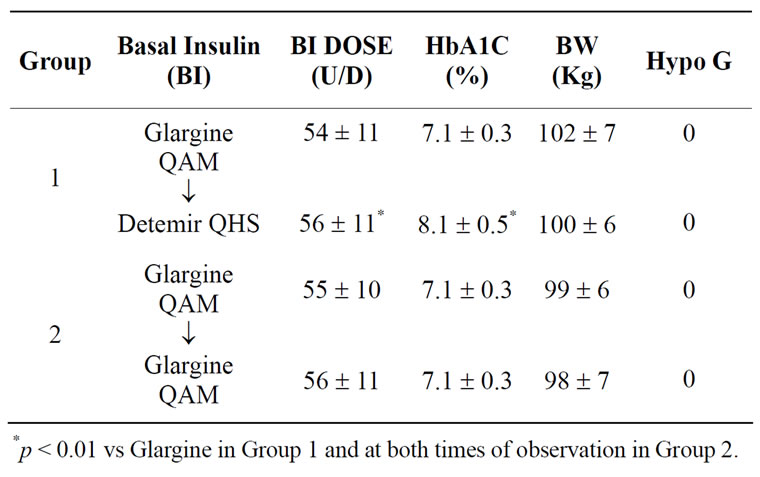
Table 2. Glycemic Control [HbA1c], Body Weight [BW], and Severe Hypoglycemic Events [HYPO G] in subjects switching from Glargine to Detemir [Group 1] and continuing Glargine [Group 2] at the time of transition and at the end of 3 months.
weights were slightly lower in group1 at the end of observation period in comparison to the time of transition (Table 2). However, the changes were not statistically significant. No severe hypoglycemic events were reported by any subject in either group.
4. DISCUSSION
This study in subjects with type 2 DM confirms our previous observation reported in subjects with Type 1 DM, in whom, an abrupt transition from stable insulin glargine regimen to insulin detemir induced a lapse of glycemic control (1). This finding is consistent with observations in another study in which a substantial reduction in daily dose of insulin glargine was noted with a transition from insulin detemir in subjects with type 2 Diabetes [9]. Several other studies have also documented a requirement of a higher daily dose of insulin detemir in comparison to insulin glargine to attain comparable glycemic control [10-19]. Thus, a higher daily dose and twice daily administration of insulin detemir may have been required to achieve desirable glycemic goal in this study as documented in previous studies in most subjects with both Type 1 and Type 2 diabetes [1,10-19]. The requirement for a higher daily dose for insulin detemir in comparison to insulin glargine in order to achieve identical glycemic control in type 2 Diabetes is further confirmed in a recent study examining pharmacokinetics and pharmacodynamics of these insulins [20].
A significantly greater weight gain was documented in subjects with type 2 Diabetes on attaining similar glycemic control with insulin glargine administered once daily in comparison with insulin detemir administered once or twice daily despite a significantly smaller daily dose [12-15,17]. In contrast, no significant weight gain was noted in subjects continuing insulin glargine in this study. The differences in changes in body weights observed in earlier studies [12-15,17] in comparison to our present data may be attributed to the different times during the day at which insulin glargine was administered. Lack of significant weight gain in our subjects in Group 2 is likely to be secondary to the administration of insulin glargine in AM as reported in other previous studies [3,5-8] whereas the significant weight gain noted in other studies [12-15,17] may be attributed to bedtime administration of insulin glargine. Weight gain in subjects receiving insulin glargine at bedtime may be due to a consumption of a snack following insulin administration because of the concern of nocturnal hypoglycemia on part of both patients and providers alike especially because of a fairly large dose required by most obese subjects with type 2 Diabetes. Alternatively, a bedtime snack is not advised and frequently not consumed by subjects on AM administration of insulin glargine because of a distinctly less concern of nocturnal hypoglycemia on part of both patients and providers since nocturnal hypoglycemia is significantly less frequently documented in studies using insulin glargine in AM [3,4-8]. Thus, the difference in total daily caloric consumption may contribute to these different outcomes regarding body weights in subjects receiving insulin glargine. Finally, a slight clinical, though not significant, weight loss was noted in our subjects in Group 1 and may be attributed to lapse of glycemic control and not because of a specific effect of insulins detemir on body weight.
Therefore, it is apparent that in order to attain glycemic control similar to that achieved in subjects in Group 2 continuing insulin glargine, a much larger daily dose of insulin detemir would be required and that too, to be administered twice daily in majority of subjects . Thus, use of detemir may adversely affect quality of life in the short term, since several studies have documented that higher the daily insulin dose, more frequent the daily injections and more the peaks of insulin during the day, greater is the occurrence of hypoglycemic events and greater is the weight gain [21-26]. Moreover, in the long term, there are always several untoward consequences of weight gain, e.g. cardiovascular outcomes which could not be addressed in these short term studies [21-26]. Lack of severe hypoglycemic events noted in this study among both groups is not an unusual finding in subjects with type 2 Diabetes (2). However, hypoglycemic events are likely to rise with a greater daily dose of insulin detemir used once daily and may be further exacerbated in subjects with twice daily administration because of induction of multiple daily insulin peaks in the circulation as documented in previous studies using other insulin’s achieving peak concentrations [21-26].
The cost efficacy is likely to decline following transition from insulin glargine to insulin detemir because of a rise in expenditure caused by additional equipment [syringes, alcohol pads, etc.], needed for twice daily administration in most subjects due to increased number of injections; higher daily insulin dose as well at least twice daily monitoring of blood glucose as documented in several recent studies [16,27,28]. Switching from insulin glargine to insulin detemir is also likely to increase costs because of consequences induced by a lapse of glycemic control [29]. Lapse of glycemic control may also lead to: decline in quality of life due to return of some symptoms as well as increase in morbidity and even mortality [29- 35].
Thus, this study demonstrates that an abrupt transition from insulin glargine to insulin detemir especially in subjects in whom a desirable glycemic control has been attained and maintained is inappropriate. Certainly, there are limitations of the study, e.g. a relatively small number of subjects and a relatively short duration. However, the findings of the study have been documented in other previous reports and therefore are very relevant to clinical practice in management of subjects with type 2 diabetes.
In conclusion, insulin glargine and detemir are apparently far from being bioequivalent in terms of peak and duration of action as well as efficacy. Moreover, nonchalant transition from one to another may be detrimental and hazardous to quality of life and not cost effective. Therefore, such a transition may be uncalled for and should not become a routine clinical practice.
REFERENCES
- Kabadi, U.M. (2008) Deleterious outcomes after abrupt transition from insulin glargine to insulin detemir in patients with type 1 diabetes mellitus. Clinical Drug Investigation, 28, 697-701. doi:10.2165/00044011-200828110-00003
- American Diabetes Association (2011) Standards of medical care in diabetes-2011. Diabetes Care, 34, S11-S61.
- Fritsche, A., Schweitzer, M.A. and Häring, H.U. (2003) Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Annals of Internal Medicine, 138, 952-959.
- Schreiber, S.A., Ferlinz, K. and Haak, T. (2008) The long-term efficacy of insulin glargine plus oral antidiabetic agents in a 32-month observational study of everyday clinical practice. Diabetes Technology & Therapeutics, 10, 121-127. doi:10.1089/dia.2007.0265
- Hammer, H. and Klinge, A. (2007) Patients with type 2 diabetes inadequately controlled on premixed insulin: Effect of initiating insulin glargine plus oral antidiabetic agents on glycemic control in daily practice. International Journal of Clinical Practice, 61, 2009-2018. doi:10.1111/j.1742-1241.2007.01598.x
- Standl, E., Maxeiner, S., Raptis, S., Karimi-Anderesi, Z. and Schweitzer, M.A. (2005) Good glycemic control with flexibility in timing of basal insulin supply: A 24-week comparison of insulin glargine given once daily in the morning or at bedtime in combination with morning glimepiride. Diabetes Care, 28, 419-420. doi:10.2337/diacare.28.2.419
- Wang, Z.H., Hedrington, M.S., Joy, N.G., Briscoe, V.J., Richardson, M.A., Younk, L., Nicholson, W., Tate, D.B. and Davis, S.N. (2010) Dose-response effects of insulin glargine in type 2 diabetes. Diabetes Care, 33, 1555-1560. doi:10.2337/dc09-2011
- Swinnen, S.G. and De Vries, J.H. (2009) Higher dose requirements with insulin detemir in type 2 diabetes— Three cases and a review of the literature. Diabetes Research and Clinical Practice, 84, 24-26. doi:10.1016/j.diabres.2009.02.009
- Bott, S., Tusek, C., Jacobsen, L.V., et al. (2006) Insulin detemir under steady-state conditions: No accumulation and constant metabolic effect over time with twice daily administration in subjects with type 1 diabetes. Diabetic Medicine, 23, 522-528.
- Pieber, T.R., Treichel, H.C., Hompesch, B., et al. (2007) Comparison of insulin detemir and insulin glargine in subjects with type 1 diabetes using intensive insulin therapy. Diabetic Medicine, 24, 635-642. doi:10.1111/j.1464-5491.2007.02113.x
- Dornhorst, A., Luddeke, H.J., Sreenan, S., et al. (2007) Safety and efficacy of insulin detemir in clinical practice: 14-week follow-up data from type 1 and type 2 diabetes patients in the predictive European cohort. International Journal of Clinical Practice, 61, 523-528. doi:10.1111/j.1742-1241.2007.01316.x
- Rosenstock, J., Davies, M., Home, P.D., Larsen, J., Koenen, C. and Schernthaner, G. (2008) A randomized, 52- week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucoseowering drugs in insulin-naive people with type 2 diabetes. Diabetologia, 51, 408-416. doi:10.1007/s00125-007-0911-x
- Hollander, P., Cooper, J., Bregnhøj, J. and Pedersen, C.B. (2008) A 52-week, multinational, open-label, parallelgroup, noninferiority, treat-to-target trial comparing insulin detemir with insulin glargine in a basal-bolus regimen with mealtime insulin aspart in patients with type 2 diabetes. Clinical Therapeutics, 30, 1976-1987.
- Raskin, P., Gylvin, T., Weng, W. and Chaykin, L. (2009) Comparison of insulin detemir and insulin glargine using a basal-bolus regimen in a randomized, controlled clinical study in patients with type 2 diabetes. Diabetes/Metabolism Research and Reviews, 25, 542-548. doi:10.1002/dmrr.989
- Johnson, C.K. and Shimshi, M. (2009) When a unit of insulin is not a unit: Detemir dosing and insulin cost in type 2 diabetes mellitus. Insulin, 4, 87-93. doi:10.1016/S1557-0843(09)80017-1
- Swinnen, S.G., Dain, M.P., Aronson, R., Davies, M., Gerstein, H.C., Pfeiffer, A.F., Snoek, F.J., Devries, J.H., Hoekstra, J.B. and Holleman, F.A. (2010) 24-week, randomized, treat-to-target trial comparing initiation of insulin glargine once-daily with insulin detemir twice-daily in patients with type 2 diabetes inadequately controlled on oral glucoselowering drugs. Diabetes Care, 33, 1176-1178. doi:10.2337/dc09-2294
- Dailey, G., Admane, K., Mercier, F. and Owens, D. (2010) Relationship of insulin dose, A1c lowering, and weight in type 2 diabetes: Comparing insulin glargine and insulin detemir. Diabetes Technology & Therapeutics, 12, 1019- 1027. doi:10.1089/dia.2010.0063
- Kato, T., Tokubuchi, I., Muraishi, K., Sato, S., Kato, T., Hara, K., Tanaka, K., Kaku, H., Tajiri, Y. and Yamada, K. (2010) Distinct pharmacodynamics of insulin glargine and insulin detemir: Crossover comparison in type 1 and type 2 diabetic patients on basal-bolus regimen. Diabetes Research and Clinical Practice, 90, 64-66. doi:10.1016/j.diabres.2010.08.011
- Lucidi, P., Porcellati, F., Rossetti, P., Candeloro, P., Cioli, P., Marzotti, S., Andreoli, A.M., Fede, R., Bolli, G.B. and Fanelli, C.G. (2011) Pharmacokinetics and pharmacodynamics of therapeutic doses of basal insulins NPH, glargine, and detemir after 1 week of daily administration at bedtime in type 2 diabetic subjects: A randomized cross-over study. Diabetes Care, 34, 1312-1314. doi:10.2337/dc10-1911
- Malone, J.K., Kerr, L.F., Campaigne, B.N., Sachson, R.A. and Holcombe, J.H. (2004) Combined therapy with insulin lispro Mix 75/25 plus metformin or insulin glargineplus metformin: A 16-week, randomized, open-label, crossover study in patients with type 2 diabetes beginning insulin therapy. Clinical Therapeutics, 26, 2034-2044. doi:10.1016/j.clinthera.2004.12.015
- Kabadi, M.U. and Kabadi, U.M. (2003) Efficacy of sulfonylureas with insulin in type 2 diabetes mellitus. The Annals of Pharmacotherapy, 37, 1572-1576, doi:10.1345/aph.1C492
- Janka, H.U., Plewe, G., Riddle, M.C., Kliebe-Frisch, C., Schweitzer, M.A. and Yki-Järvinen, H. (2005) Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetes. Diabetes Care, 28, 254-259. doi:10.2337/diacare.28.2.254
- Raskin, P., Allen, E., Hollander, P., Lewin, A., Gabbay, R.A., Hu, P., Bode, B. and Garber, A. (2005) Initiating insulin therapy in type 2 diabetes: A comparison of biphasic and basal insulin analogs. Diabetes Care, 28, 260-265.
- Kabadi, U.M. (2006) Comparative efficacy of glimepiride and/or metformin with insulin in type 2 diabetes. Diabetes Research & Clinical Practice, 72, 265-270. doi:10.1016/j.diabres.2005.10.024
- Ligthelm, R.J., Gylvin, T., DeLuzio, T. and Raskin, P.A. (2011) Comparison of twice-daily biphasic insulin aspart 70/30 and once-daily insulin glargine in persons with type 2 diabetes mellitus inadequately controlled on basal insulin and oral therapy: A randomized, open-label study. Endocrine Practice, 17, 41-50. doi:10.4158/EP10079.OR
- Pscherer, S., Dietrich, E.S., Dippel, F.W. and Neilson, A.R. (2010) Comparison of one-year costs of type 2 diabetes treatment with insulin glargine or insulin detemir in a basal supported oral therapy (BOT) in Germany. International Journal of Clinical Pharmacology and Therapeutics, 48, 129-137.
- Bierwirth, R.A., Kohlmann, T., Moock, J., Holle, R. and Landgraf, W. (2010) Costs of diabetes care and treatment satisfaction in type 2 diabetes patients treated with a basal-bolus (ICT) insulin regimen in outpatient care: Results of the LIVE-COM study. Medizinische Klinik (Munich), 105, 792-801. doi:10.1007/s00063-010-1136-1
- Gilmer, T.P., O’Conner, P.J., Manning, W.G., et al. (1997) The cost to health plans of poor glycemic control. Diabetes Care, 20, 1847-1853. doi:10.2337/diacare.20.12.1847
- Gray, A., Raikoi, M., McGuire, A., et al. (2000) Cost effectiveness of an intensive blood glucose control policy in patients with type 2 diabetes; economic analysis alongside randomized controlled trial (UKPDS 41). British Medical Journal, 320, 1373-1378. doi:10.1136/bmj.320.7246.1373
- Testa, M.A. and Simonson, D.C. (1998) Health economic benefits and quality of life during improved glycemic control in patients with type 2 diabetes mellitus: A randomized, controlled, double-blind trial. The Journal of the American Medical Association, 208, 1490-1496. doi:10.1001/jama.280.17.1490
- Adler, A.I., Stratton, I.M., Neil, H.A.W., et al. (2000) Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): Prospective observational study. British Medical Journal, 321, 412-419. doi:10.1136/bmj.321.7258.412
- Skyler, J.S. (1996) Diabetic complications. The importance of glucose control. Endocrinology Metabolism Clinics of North America, 252, 243-254.
- DCCT Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The New England Journal of Medicine, 329, 977-986. doi:10.1056/NEJM199309303291401
- Ohkubo, Y., Kishikawa, H., Araki, E., et al. (1995) Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: A randomized prospective 6-year study. Diabetes Research and Clinical Practice, 28, 103-117. doi:10.1016/0168-8227(95)01064-K
- United Kingdom Prospective Diabetes Study (UKPDS) (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). The Lancet, 353, 837-853.

