Journal of Behavioral and Brain Science
Vol.3 No.2(2013), Article ID:31825,7 pages DOI:10.4236/jbbs.2013.32022
Effect of Atomoxetine on Behavior of Outbred Mice in the Enrichment Discrimination Test
1Laboratory of Radioisotope Researches, Zakusov Institute of Pharmacology, Russian Academy of Medical Sciences, Moscow, Russia
Email: rmsalimov@yandex.ru
Copyright © 2013 Ramiz M. Salimov, Georgy I. Kovalev. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 1, 2013; revised April 10, 2013; accepted May 11, 2013
Keywords: Attention Deficit; Mouse; Atomoxetine
ABSTRACT
Treatment of attention deficit hyperactivity disorder with medications is helpful in less than 60% of cases suggesting the necessity of development of novel drugs. The most accepted animal model of the disease is outbred spontaneously hypertensive rat strain. It was recently found in a novel enrichment discrimination test that the rat strain includes attentionally-low and -high phenotypes and clinically efficient drug for the treatment of the disorder atomoxetine is capable of ameliorating the enrichment discrimination by the attentionally-low rats. The present study aimed to test the generality of these findings in outbred CD-1 mice assessed in the same experimental design. The frequency distribution of the enrichment discrimination ratio differed from the curve expected under the normality hypothesis and had a bimodal shape suggesting the existence of attentionally-low and -high mouse phenotypes. Atomoxetine (3 mg/kg, orally, once daily for 4 days) selectively enhanced enrichment discrimination in mice of attentionally-low phenotype only. The present results generalize and extend findings previously reported in spontaneously hypertensive rats and suggest that the present model could be useful in studies of the neurobiological mechanisms of attention deficiency in rodents and for screening of novel drug candidates for treatment of attention deficit disorder.
1. Introduction
Attention deficit disorder with or without hyperactivity (ADD/ADHD) is described in approximately 8% - 10% of children with greater prevalence in boys than in girls [1]. It is usually first diagnosed in childhood and, if untreated, often lasts into adulthood [2]. Although the patients have problems in academic and job performance their difficulty in selective attention is unrelated to an individual’s overall intelligence and motor skills [1,3,4]. Causal mechanisms of ADD/ADHD are still not known. The disease has considerable heritable components revealed by family and twin studies [4,5], albeit environmental factors may also contribute to the disease [6]. The symptoms of ADD/ADHD could be alleviated by stimulant medications or some antidepressants, particularly, by novel non-stimulating drug atomoxetine [1,7,8]. Nonetheless, the medications are helpful in less than 60% of cases suggesting the necessity of the development of novel drugs for treatment of ADHD [8,9].
Animal models of ADD/ADHD employ rodents of different genetic backgrounds [10-13]. Although the most accepted model is outbred spontaneously hypertensive rat strain capable of demonstrating both inattentiveness and hyperactivity, there is a criticism related to dissimilarity of results obtained across different tests [14] and to the fact that, in instrumental paradigms employed, variability in general activity also contribute to ADD/ ADHD-like behavioral pattern [15] suggesting the necessity of independent evaluation of attention, cognitive performance and general activity. It has been reported that the spontaneously hypertensive rat strain include subpopulations of, so-called, impulsive and non-impulsive individuals [16,17]. Recently, the rat strain was also found to be nonhomogeneous with regard to attention in paradigm of enrichment discrimination that does not involve rule learning and provides separate measures of attention towards enriching objects (ED-ratio), general locomotor activity and spatial orientation [18]. The latter is considered as belonging to cognitive domain that parallel Cattell’s general fluid intelligence [19,20] and has been used for assessment of cognition enhancing drugs [21-23]. The attentionally-low and -high subpopulations did not differ from each other in measures of locomotor activity and blood pressure [18]. Also, the attentionally-high phenotype, as compared with the attentionally-low one, did not show superiority in ability for spatial orientation. The anti-ADD/ADHD drug atomoxetine was capable of improving attention to the environmental cues in the attentionally-low phenotype. Although the attention-enhancing effect of atomoxetine coincided with a decrease in locomotor activity, it was not accompanied by alteration of spatial orientation.
The purpose of this study is to test the generality of the existence of attentionally-low and -high phenotypes among outbred mice, because the behavior of the most accepted models of ADD/ADHD in the species (DAT knockout and Coloboma mutant mice) is primarily characterized by hyperactivity rather than inattentiveness [13]. The specific aims of the present study are to evaluate: 1) If frequency distribution of the ED-ratio in non-selected population of outbred mice diverges from the Gaussian distribution; 2) In case of non-homogeneity of the population, if the EDlow and -high mouse phenotypes differ from each other in general locomotor activity and cognitive ability for spatial orientation and if atomoxetine is capable of improving the enrichment discrimination in mice of ED-low phenotype.
2. Materials and Methods
2.1. Animals
Eighty male mice of CD-1 strain (body weight 20 - 25 g) were purchased by Pushchino animal breeding farm (Moscow region, Russian Federation). The strain was obtained from Charles River Laboratories, USA, in 2001. The animals were kept in standard vivarium conditions with free access to pellets of standard dry chow and sterile drinking water at 12:12 hour light-dark cycle. The care and use of animals and procedures reported in this study were in accordance with the Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes.
2.2. Drugs
Atomoxetine (Strattera, Eli Lilly, USA) was dissolved in sterile water containing 0.5% Tween-80 (P1754, SigmaAldrich, USA). The drug was administered orally using stainless steel feeding needle in a dose of 3 mg/kg once daily. The vehicle for control animals contained the 0.5% Tween-80 in sterile water. The volume of administration was 2.5 ml/kg.
2.3. Apparatus
The cross-maze was purchased by OpenScience Ltd., Russian Federation (catalog # TS0605-2). The apparatus was made of black plastic and consisted of 4 closed arms (12 × 12 × 12 cm) connected to the same central compartment via rectangular doorways (7 × 7 cm). Two cylindrical glass bottles (4.5 cm in diameter, 4 cm high) served as enriching objects. The bottles were placed in opposite arms. Each of them was mounted vertically near the wall that was distant from the doorway. The maze was covered by transparent plastic lid supplied with small ventilation holes and partition numbers. The arms were numbered in clockwise direction 1, 2, 3 and 4; the central compartment was assigned to number 5.
2.4. General Procedure
On day 1, behavior of all 80 animals was evaluated in the first ED-test. Frequency distribution of the ED-ratio (see sections 2.5 and 2.6) was compared with normal distribution. Because there was difference from the normal curve showing the existence of ED-low and -high phenotypes, mice of both phenotypes were randomly divided into subgroups assigned to administration of either vehicle or atomoxetine (15 animals per group). The mice from vehicle and atomoxetine groups received the corresponding treatment on days 4 - 7. On day 7, the animals were subjected to the same second ED-test conducted an hour after last vehicle or atomoxetine administration.
2.5. Enrichment Discrimination Test
The mouse was placed into the central compartment and allowed to explore the maze until 12 visits into arms have occurred with cutoff time of 10 minutes. Each visit was scored after entry into a compartment with all four paws inside. The sequence and timing of arms visited were recorded directly into a personal computer by the use of Behavset 3.0 software. The floor and the objects in arms were cleaned thoroughly with paper towel damped in 70% ethanol and were air-dried after each trial [18]. The position of the objects in a pair of opposite arm (#1 and #3, or #2 and #4) was alternated in a quasi-random order.
Subsequent analysis was performed with the help of Endisc software detecting the following measures:
1) Total time spent in empty or enriched arms. Using the measure, the ED-ratio that was calculated according to the formula:
ED-ratio = 100 × Tenriched/Tempty
where, Tenriched is the total time spent in arms containing objects, Tempty is the total time spent in empty arms. In case of no difference between time spent in enriched and empty arms, the ratio is equal to 100. Animals exploring the objects typically stay longer in enriched part of maze than in empty arms and have the ED-ratio scores higher than 100. Attendance at the objects area and time spent in the area by an animal exploring novel environment is generally considered as measures of an attention directed to the objects [20,24,25].
2) Total time in maze until an animal completes 12 visits to arms, i.e. when it returns to central partition from the arm entered on 12th visit. The variable is considered as measure of locomotor activity because it highly negatively correlates with ambulation in the open-field test [26].
3) Size of first patrolling episode scored as number of entries by an animal into arms until each of four arms has been visited at least ones. For instance, if the sequence of arm entered is 124141334132, then the size of first patrolling episode is 7, because the episode is completed with entry into arm #3 on 7th visit. The more visits a patrolling episode takes, the less efficient is maze exploration. The shortest patrolling episode includes 4 visits. In that case, it is analogous to cognitive behavioral alternation in the Y-maze exploratory test (i.e. visiting of all 3 arms in a row without repetitions) that has been considered as the measure of short-term memory dependent spatial orientation [27,28].
4) The total number of patrolling episodes made by an animal during the test. In the example above, the measure equals two, because the second patrolling episode is completed on the 12th entry into arm #2. The more patrolling episodes are made during the test, the more efficient is exploration. It was possible for an animal to make maximum 3 patrolling episodes during the test. Both the measures of patrolling behavior are considered to represent the ability for spatial orientation [21,26,29]. The cognitive behavior is sensitive to cognition enhancing drugs [21-23], ageing [29] and L-glutamate applied in neurotoxic concentration to frontal cortex [30].
2.6. Statistical Analysis
The Chi-Square test was employed for comparison of Gaussian distribution with frequency distribution of the ED-ratio. The T-tests for independent and dependent samples were used for comparison of measures from ED-low and ED-high phenotypes. A two-way ANOVA with phenotype (ED-low or -high) and drug (vehicle or atomoxetine) as independent variables was performed for estimation of atomoxetine effects on behavior of mice in the second ED-test. Also, difference between pairs of means was evaluated by the use of ANOVA’s univariate test of significance for planned comparison. The analysis was made using Statictica 6.0 package.
3. Results
The ED-ratio of time spent in enriched and empty arms in the first ED-test had frequency distribution with apparent bimodal shape (Figure 1). The distribution differed significantly from the curve expected under the normality hypothesis (Chi-Square = 12.93, df = 4, p < 0.012) revealing the existence of two phenotypes that diverge in attention to enriched partitions. Because local minimum between the modes was near score 100, the mice with ED-ratio below 100 were accepted as ED-low (mean ± SEM of the ED-ratio = 76.3 ± 2.2), while the rest of animals were considered as ED-high (138.4 ± 3.4, respectively). The ED-low phenotype was present in 45% individuals of unselected population.
The ED-low mice spend less time in the enriched than in the empty arms of the maze (t(35) = 6.73, p < 0.001; Table 1). On the contrary, the ED-high mice had preference for enriched partitions (t(43) = 12.01, p < 0.001). The phenotypes did not differ from each other in measures of patrolling behavior and in time spent in the maze until 12 visits into arms have occurred.
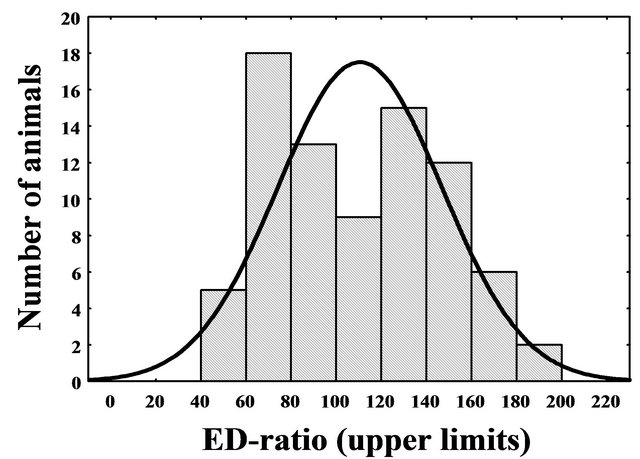
Figure 1. Frequency distribution of ED-ratio in CD-1 mice evaluated during first ED-test (represented by bars) has bimodal shape and differs significantly from theoretical normal curve (represented by line) (ChiSquare = 12.93, df = 4, p = 0.012).
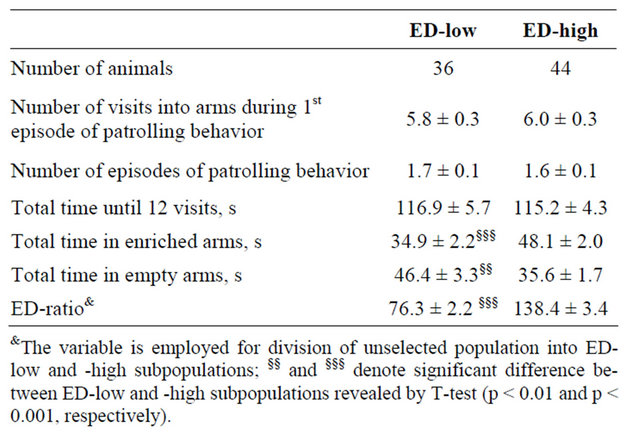
Table 1. Behavioral measures from the first enrichment discrimination test in the ED-low and -high subpopulations of CD-1 mice (mean ± S.E.M.).
In the second ED-test, mice of ED-low and -high phenotypes having been treated with vehicle retained their main characteristics (Table 2). The phenotypes diverged from each other both in time spent in enriched (t(28) = 2.59, p = 0.015) and empty arms (t(28) = 2.54, p = 0.05). The ED-low mice spent less time in enriched arms as compared with empty ones (t(14) = 2.25, p = 0.041), while the ED-high mice displayed preference for enriched partitions (t(14) = 2.22, p = 0.043). Correspondingly, the EDratio for mice of ED-low phenotype was again lower than in ED-high mice (t(28) = 3.43, p = 0.002).
Two-way ANOVA of time spent in enriched arms by the ED-low and -high mice having been treated with either atomoxetine or vehicle revealed the only significant effect of drug (F (1,56) = 7.16, p = 0.01; there was a 47% and 22% increase in the measure, correspondingly).
On time spent in empty arms, the ANOVA yielded significant effect of drug (F(1,56) = 4.23, p = 0.044) and interaction of phenotype with drug (F(1,56) = 5.74, p = 0.02). Paired comparison revealed that atomoxetine produced a 46% reduction in the measure in mice of ED-low phenotype (F(1,56) = 9.91, p = 0.003). The effect of atomoxetine on time spent in empty arms by the ED-high mice did not reach statistical significance. After atomoxetine, time spent in enriched partitions was greater than that in empty ones in mice of both ED-low (t(28) = 3.99, p = 0.001) and -high (t(28) = 2.86, p = 0.013) phenotype.
On the ED-ratio, the ANOVA revealed significant effect of drug (F(1,56) = 7.72, p = 0.007) and its interaction with phenotype (F(1,56) = 10.26, p = 0.002). In mice of ED-low phenotype, atomoxetine produced a 130% increase in the measure as compared with corresponding vehicle group (F(1,56) = 17.89, p < 0.001). In the EDhigh mice, the effect of atomoxetine on ED-ratio was insignificant.
4. Discussion
The present study shows that frequency distribution of ED-ratio of time spent in enriched and empty partitions by mice from non-selected population differs from the curve expected under normality hypothesis and has a bimodal shape. The result reveals the existence, among CD-1 mice, of two subpopulations that diverge in attention to enriched partitions in the maze. Mice from the ED-high phenotype prefer enriched partitions while those of the ED-low one do not have that property. The ED-low mice spent even less time in enriched than in empty arms, probably, because in the former case the floor was partially occupied by enriching objects and there was less space for exploratory ambulation. The divergence between ED-low and -high mice seems to be specific toward attention to environmental cues, because the phenotypes differ neither in spatial orientation (patrolling behavior) nor in locomotor activity. The outcomes of the second ED-test demonstrate the relative stability of the behavioral patterns displayed by the ED-low and -high phenoltypes. Atomoxetine selectively enhances the attentional behavior in individuals of ED-low phenotype only: the drug increases the ED-ratio, however, has no significant effect on both locomotor activity and spatial orientation.
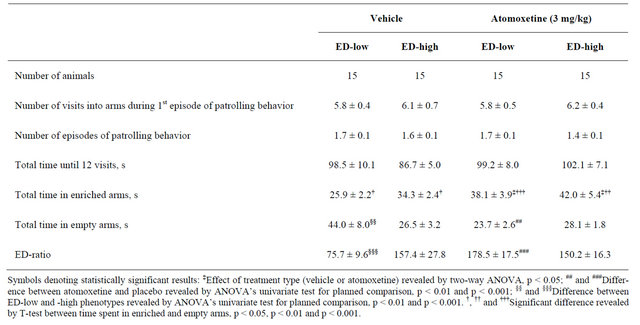
Table 2. Behavioral measures (mean ± S.E.M.) from the second ED-test in ED-low CD-1 mice after vehicle or atomoxetine administration (orally, once daily, for 4 days).
The results are in general agreement with those reported in hyperactive DAT knockout and Coloboma mice: there was no significant difference from corresponding genetic controls in measures of spatial orientation in the Y-maze [31,32] and atomoxetine did not reduce ambulatory activity by Coloboma mice in the square arena [33].
The findings of the present study in mice generalize and extend results previously reported in spontaneously hypertensive rats [18]. Both the species include individuals of attentionally-low and -high phenotypes. The rodent species only diverge in local minimum between two modes of the ED-ratio distributions (100 in mice vs. 200 in rats). As compared with spontaneously hypertensive rats, the mouse population consequently includes fewer ED-low animals (45% vs. 81% in the rats) and the EDlow mice have slightly lower ED-ratio scores (76.3 ± 2.2 vs. 104.8 ± 6.2, correspondingly). Both absolute and relative distance between the ED-low and -high phenotypes seems to be larger among the rats (ED-low ratio = 104.8 ± 6.2, ED-high ratio = 326.7 ± 12.6) than in CD-1 mice (ED-low ratio = 76.3 ± 2.2, ED-high ratio = 138.4 ± 3.4). In both rodent species, atomoxetine is capable of ameliorating the enrichment discrimination in the ED-low animals. At the same time, atomoxetine does not produce improvement of patrolling behavior that is considered as marker for cognition enhancing drug activity [23,29]. On this basis the ED-enhancing effect of the drug cannot be attributed to its potential cognition-enhancing property. It might be interesting in the future to estimate the effects of different medications in the ED-test including those that have been used for treatment of the ADD/ADHD. Because mouse genome is well characterized, already existing [34-36] and novel specific gene knockout and other transgenic strains could be used to evaluate mechanisms underlying the attention deficiency revealed in the ED-test.
To the best of our knowledge, the enrichment discrimination in rats and mice is the first simple paradigm that could serve as the model of attention deficiency suitable for drug screening and recognition of novel antiADD/ADHD drug candidates as well as for variety of neuroscience experiments and translational studies of molecular mechanisms of attention deficiency in rodents.
5. Acknowledgements
The present study was supported by research funds from Russian Academy of Medical Sciences.
REFERENCES
- American Academy of Pediatrics, “Clinical Practice Guideline: Diagnosis and Evaluation of the Child with Attention-Deficit/Hyperactivity Disorder,” Pediatrics, Vol. 105, No. 5, 2000, pp. 1158-1170. doi:10.1542/peds.105.5.1158
- S. Mannuzza, R. G. Klein, A. Bessler, P. Malloy and M. LaPadula, “Adult Outcome of Hyperactive Boys. Educational Achievement, Occupational Rank and Psychiatric Status,” Archives of General Psychiatry, Vol. 50, No. 7, 1993, pp. 565-576. doi:10.1001/archpsyc.1993.01820190067007
- K. Al-Menabbawy, A. El-Gerzawy, A. Ezzat and H. Mottawie, “Developmental, Behavioral and Genetic Factors in Correlation with Attention Deficit Hyperactivity Disorder in Egyptian Children,” Journal of Medical Sciences, Vol. 6, No. 4, 2006, pp. 569-576. doi:10.3923/jms.2006.569.576
- Y. Paloyelis, F. Rijsdijk, A. C. Wood, P. Asherson and J. Kuntsi, “The Genetic Association between ADHD Symptoms and Reading Difficulties: The Role of Inattentiveness and IQ,” Journal of Abnormal Child Psychology, Vol. 38, No. 8, 2010, pp. 1083-1095. doi:10.1007/s10802-010-9429-7
- C. M. Freitag, L. A. Rohde, T. Lempp and M. Romanos, “Phenotypic and Measurement Influences on Heritability Estimates in Childhood ADHD,” European Child and Adolescent Psychiatry, Vol. 19, No. 3, 2010, pp. 311-323. doi:10.1007/s00787-010-0097-5
- A. Brassett-Harknett and N. Butler, “Attention-Deficit/ Hyperactivity Disorder: An Overview of the Etiology and a Review of the Literature Relating to the Correlates and Lifecourse Outcomes for Men and Women,” Clinical Psychology Review, Vol. 27, No. 2, 2007, pp. 188-210. doi:10.1016/j.cpr.2005.06.001
- P. Asherson, W. Chen, B. Craddock and E. Taylor, “Adult Attention-Deficit Hyperactivity Disorder: Recognition and Treatment in General Adult Psychiatry,” The British Journal of Psychiatry, Vol. 190, No. 1, 2007, pp. 4-5. doi:10.1192/bjp.bp.106.026484
- N. Kates, “Attention Deficit Disorder in Adults. Management in Primary Care,” Canadian Family Physician, Vol. 51, No. 1, 2005, pp. 53-59.
- F. Naderi, A. Heidarie, L. Bouron and P. Asgari, “The Efficacy of Play Therapy on ADHD, Anxiety and Social Maturity in 8 to 12 Years Aged Clientele Children of Ahwaz Metropolitan Counseling Clinics,” Journal of Applied Sciences, Vol. 10, No. 3, 2010, pp. 189-195. doi:10.3923/jas.2010.189.195
- E. Davids, K. Zhang, F. I. Tarazi and R. J. Baldessarini, “Animal Models of Attention-Deficit Hyperactivity Disorder,” Brain Research. Brain Research Reviews, Vol. 42, No. 1, 2003, pp. 1-21. doi:10.1016/S0165-0173(02)00274-6
- T. Sagvolden, V. A. Russell, H. Aase, E. B. Johansen and M. Farshbaf, “Rodent Models of Attention-Deficit/Hyperactivity Disorder,” Biological Psychiatry, Vol. 57, No. 11, 2005, pp. 1239-1247. doi:10.1016/j.biopsych.2005.02.002
- T. Sagvolden, T. Dasbanerjee, Y. Zhang-James, F. Middleton and S. Faraone, “Behavioral and Genetic Evidence for a Novel Animal Model of Attention-Deficit/Hyperactivity Disorder Predominantly Inattentive Subtype,” Behavioral and Brain Functions, Vol. 4, No. 1, 2008, p. 56. doi:10.1186/1744-9081-4-56
- Y. Arime, Y. Kubo and I. Sora, “Animal Models of Attention-Deficit/Hyperactivity Disorder,” Biological and Pharmaceutical Bulletin, Vol. 34, No. 9, 2011, pp. 1373-1376. doi:10.1248/bpb.34.1373
- F. S. Van den Bergh, E. Bloemarts, J. S. Chan, L. Groenink, B. Olivier and R. S. Oosting, “Spontaneously Hypertensive Mice Do Not Predict Symptoms of AttentionDeficit Hyperactivity Disorder,” Pharmacology Biochemistry and Behavior, Vol. 83, No. 3, 2006, pp. 380-390. doi:10.1016/j.pbb.2006.02.018
- B. Alsop, “Problems with Spontaneously Hypertensive Rats (SHR) as a Model of Attention-Deficit/Hyperactivity Disorder (AD/HD),” Journal of Neuroscience Methods, Vol. 162, No. 1-2, 2007, pp. 42-48. doi:10.1016/j.jneumeth.2006.12.002
- W. Adriani, A. Caprioli, O. Granstrem, M. Carli and G. Laviola, “The Spontaneously Hypertensive-Rat as an Animal Model of ADHD: Evidence for Impulsive and NonImpulsive Subpopulations,” Neuroscience & Biobehavioral Reviews, Vol. 27. No. 7, 2003, pp. 639-651. doi:10.1016/j.neubiorev.2003.08.007
- B. Langen and R. Dost, “Comparison of SHR, WKY and Wistar Rats in Different Behavioural Animal Models: Effect of Dopamine D1 and Alpha2 Agonists,” Attention Deficit and Hyperactivity Disorders, Vol. 3, No. 1, 2011, pp. 1-12. doi:10.1007/s12402-010-0034-y
- R. M. Salimov and G. I. Kovalev, “Enrichment Discrimination Behavior in Spontaneously Hypertensive Rats,” Journal of Behavioral and Brain Science, Vol. 2, No. 4, 2012, pp. 479-484. doi:10.4236/jbbs.2012.24056
- L. J. Horn and R. B. Cattell, “Age Differences in Fluid and Crystallized Intelligence,” Acta Psychologica (Amst), Vol. 26, No. 2, 1967, pp. 107-129. doi:10.1016/0001-6918(67)90011-X
- L. D. Matzel and S. Kolata, “Selective Attention, Working Memory, and Animal Intelligence,” Neuroscience and Biobehavioral Review, Vol. 34, No. 1, 2010, pp. 23-30. doi:10.1016/j.neubiorev.2009.07.002
- R. M. Salimov, “Measurement of the Ranking of the Choice of Route in the Exploratory Behavior of Mice,” Zhurnal Vyssheï Nervnoï Deiatenlnosti Imeni I P Pavlova, Vol. 38, No. 3, 1988, pp. 569-570.
- R. M. Salimov, I. I. Poletaeva, G. I. Kovalev, N. B. Salimova and R. R. Gainetdinov, “Interstrain Differences in Extrapolation Capacity and Exploration of a Cruciform Maze Correlate with Various Indices of Monoamine Metabolism in the Brain,” Zhurnal Vyssheï Nervnoï Deiatenlnosti Imeni I P Pavlova, Vol. 45, No. 5, 1995, pp. 914- 924.
- G. I. Kovalev, I. Firstova and R. M. Salimov, “Effects of Piracetam and Meclofenoxate on the Brain NMDA and Nicotinic Receptors in Mice with Different Exploratory Efficacy in the Cross Maze Test,” Eksperimental’naia i Klinicheskaia Farmakologiia, Vol. 71, No. 1, 2008, pp. 12-17.
- A. Ennaceur, “One-Trial Object Recognition in Rats and Mice: Methodological and Theoretical Issues,” Behavioural Brain Research, Vol. 215, No. 2, 2010, pp. 244-254. doi:10.1016/j.bbr.2009.12.036
- E. D. Levin, P. J. Bushnell and A. H. Rezvani, “Attention-Modulating Effects of Cognitive Enhancers,” Pharmacology, Biochemistry and Behavior, Vol. 99, No. 2, 2011, pp. 146-154. doi:10.1016/j.pbb.2011.02.008
- N. V. Markina, R. M. Salimov and I. I. Poletaeva, “Behavioral Screening of Two Mouse Lines Selected for Different Brain Weight,” Progress in Neuro-Psychopharmacology & Biological Psychiatry, Vol 25, No. 5, 2001, pp. 1083-1109. doi:10.1016/S0278-5846(01)00169-5
- L. Kokkinidis and H. Anisman, “Perseveration and Rotational Behavior Elicited by d-Amphetamine in a Y-Maze Exploratory Task: Differential Effects of Intraperitoneal and Unilateral Intraventricular Administration,” Psychopharmacology (Berl), Vol. 52, No. 2, 1977, pp. 123-128. doi:10.1007/BF00439098
- M. Sarter and T. Steckler, “Spontaneous Exploration of a 6-Arm Radial Tunnel Maze by Basal Forebrain Lesioned Rats: Effects of the Benzodiazepine Receptor Antagonist Beta-Carboline ZK 93426,” Psychopharmacology (Berl), Vol. 98, No. 2, 1989, pp. 193-202. doi:10.1007/BF00444691
- R. Salimov, N. Salimova, L. Shvets and N. Shvets, “Effect of Chronic Piracetam on Age-Related Changes of CrossMaze Exploration in Mice,” Pharmacology Biochemistry and Behavior, Vol. 52, No. 3, 1995, pp. 637-640. doi:10.1016/0091-3057(95)00179-Z
- R. M. Salimov and N. B. Salimova, “L-Glutamate Abolishes Differential Responses to Alcohol Deprivation in Mice,” Alcohol, Vol. 10, No. 4, 1993, pp. 251-257. doi:10.1016/0741-8329(93)90001-5
- R. K. Gunn, M. E. Keenan and R. E. Brown, “Analysis of Sensory, Motor and Cognitive Functions of the Coloboma (C3Sn.Cg-Cm/J) Mutant Mouse,” Genes, Brain and Behavior, Vol. 10, No. 5, 2011, pp. 579-588. doi:10.1111/j.1601-183X.2011.00697.x
- B. Li, Y. Arime, F. S. Hall, G. R. Uhl and I. Sora, “Impaired Spatial Working Memory and Decreased Frontal Cortex BDNF Protein Level in Dopamine Transporter Knockout Mice,” European Journal of Pharmacology, Vol. 628, No. 1-3, 2010, pp. 104-107. doi:10.1016/j.ejphar.2009.11.036
- K. J. Bruno and E. J. Hess, “The Alpha(2C)-Adrenergic Receptor Mediates Hyperactivity of Coloboma Mice, a Model of Attention Deficit Hyperactivity Disorder,” Neurobiology of Disease, Vol. 23, No. 3, 2006, pp. 679-688. doi:10.1016/j.nbd.2006.05.007
- E. J. Hess, H. A. Jinnah, C. A. Kozak and M. C. Wilson, “Spontaneous Locomotor Hyperactivity in a Mouse Mutant with a Deletion Including the Snap Gene on Chromosome 2,” The Journal of Neuroscience, Vol. 12, No. 7, 1992, pp. 2865-2874.
- R. R. Gainetdinov, W. C. Wetsel, S. R. Jones, E. D. Levin, M. Jaber and M. G. Caron, “Role of Serotonin in the Paradoxical Calming Effect of Psychostimulants jn Hyperactivity,” Science, Vol. 283, No. 5400, 1999, pp. 397-401. doi:10.1126/science.283.5400.397
- T. Furuse, Y. Wada, K. Hattori, I. Yamada, T. Kushida, Y. Shibukawa, et al., “Phenotypic Characterization of a New Grin1 Mutant Mouse Generated by ENU Mutagenesis,” European Journal of Neuroscience, Vol. 31, No. 7, 2010, pp. 1281-1291. doi:10.1111/j.1460-9568.2010.07164.x

