Journal of Behavioral and Brain Science
Vol.2 No.4(2012), Article ID:25220,6 pages DOI:10.4236/jbbs.2012.24056
Enrichment Discrimination Behavior in Spontaneously Hypertensive Rats
Laboratory of Radioisotope Researches, Zakusov Institute of Pharmacology, Russian Academy of Medical Sciences, Moscow, Russia
Email: rmsalimov@yandex.ru
Received October 11, 2012; revised October 25, 2012; accepted October 31, 2012
Keywords: Attention Deficit; Enrichment Discrimination; Spontaneously Hypertensive Rat; Atomoxetine
ABSTRACT
Objectives: 1) To reveal, among spontaneously hypertensive rats, subpopulations that diverge in attention to objects enriching an empty cross-maze; 2) To evaluate effect of clinically efficient drug for treatment of attention deficiency atomoxetine on the attention to environmental cues in attentionally-low rats. Method: A novel paradigm that provides measure of attention towards enriching objects independent of general locomotor activity and spatial orientation is employed. The apparatus consists of 4-arm radial maze, two arms of which contain objects (enriched compartments). Animals exploring the objects typically stay longer in enriched parts of maze than in empty arms and have a higher score of enrichment discrimination ratio. Results: Frequency distribution of the enrichment discrimination ratio had clear bimodal shape that differed significantly from normal distribution suggesting the existence of subpopulations of attentionally-low and -high individuals. The attentionally-low phenotype did not show inferiority in spatial orientation as compared with attentionally-high phenotype. The phenotypes did not differ from each other in measures of locomotor activity and blood pressure. Atomoxetine (3 mg/kg, orally, once daily for 4 days) enhanced enrichment discrimination in animals of attentionally-low phenotype. Single administration of the drug was ineffective. Conclusion: Population of spontaneously hypertensive rat includes two phenotypes of attentionally-low and -high individuals. Subchronic atomoxetine ameliorates attention to environmental cues in attentionally-low rats. The enrichment discrimination test could be useful in studies of neurobiology of attention deficit condition and for screening of novel drug candidates.
1. Introduction
Attention deficit hyperactivity disorder (ADHD) basically includes inattentiveness that could coincide with hyperactivity and/or impulsiveness and typically is not accompanied by deviation in overall intelligence [1,2] or deficiency in motor skills [3]. Patients with ADHD have difficulties in sustaining attention that lead to problems in academic and job performance [1,2]. If untreated, up to 70% of the children continue to experience symptoms of the disease as adults [4]. The symptoms could be alleviated by psychostimulants or some antidepressants, particularly, by novel non-stimulating drug atomoxetine [1, 5,6]. However, the medications are helpful in less than 60% of cases [6] requiring additional cognitive therapy and suggesting the necessity of development of novel drugs for treatment of ADHD [7].
Animal models of ADHD employ rodents with different genetic backgrounds. Among them, outbred spontaneously hypertensive (SH) rat strain is considered as suitable genetic model of ADHD [8-10]. The SH rats display lower sustained attention to environmental cues [11-13]
greater variability in rewarded operant responses and slower elimination of inefficient responses [14] as compared with normotensive rats. The ADHD-like pattern in SH rats, however, is not related to blood pressure level [15]. In SH rat, learning may be either enhanced or constrained across different tests [16-18] because the SH individuals are more impulsive [8,19,20] and less anxious [21,22] than normotensive animals. Some criticism comes from the fact that, in instrumental paradigms, variability in general activity also contribute to ADHDlike pattern typical for SH rats [23] suggesting the necessity of development of procedures that independently measure attention, cognitive performance and general activity in experimental animal.
Recently it has been found that the SH rat strain is not homogeneous with regard to delayed instrumental responding [14] and include subpopulations of, so-called, impulsive and non-impulsive individuals [19] albeit the result is not illustrated by analysis of frequency distribution and its clinical relevance is not fully supported by effects of drugs [20]. Non-homogeneity of SH strain in measures of attentional behavior per se has never been studied before.
The present study is aimed to examine homogeneity of SH rat population with regard to attention to objects enriching an empty cross-maze (enrichment discrimination, ED) and, in case of non-homogeneity, to evaluate effects of clinically efficient drug atomoxetine in ED-low SH rats. The study employs a modified paradigm of exploratory behavior that does not involve rule learning and provides separate measures of attention towards enriching objects, general locomotor activity and spatial orienttation which has been previously used for assessment of cognition enhancing drugs [24,25]. The present study is aimed to test: 1) If frequency distribution of the ED-ratio for time spent by the animals in compartments that diverge in complexity differs from normal distribution; 2) In case of non-homogeneity of SH rats, if subpopulations of the ED-low and -high rats differ from each other in blood pressure, general locomotor activity and cognitive ability for spatial orientation; 3) if clinically efficient drug for treatment of attention deficiency atomoxetine is capable of improving the enrichment discrimination in SH rats of ED-low phenotype.
2. Materials and Methods
2.1. Animals
One hundred and nine male rats of SH strain (body weight 205 - 275 g) were purchased by Pushchino animal breeding farm (Moscow region, Russian Federation) and kept in standard vivarium conditions at 12:12 hour lightdark cycle with free access to pellets of standard dry chow and sterile drinking water. The care and use of animals and procedures reported in this study were in accordance with the Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes.
2.2. Drugs
Atomoxetine (Strattera, Eli Lilly, USA.) was dissolved in sterile water containing 0.5% Tween-80 (P1754, SigmaAldrich, USA) and administered orally via stainless steel feeding needle in a dose of 3 mg/kg once daily. The volume of administration was 2.5 ml/kg. The vehicle contained the 0.5% Tween-80 in sterile water.
2.3. Apparatus
The maze apparatus (TS0605-1, OpenScience Ltd., Russian Federation) was made of black plastic and consisted of 4 closed arms (numbered in clockwise direction 1, 2, 3 and 4) connected to the same central compartment via rectangular doorways. The compartment dimensions were 20 × 20 × 20 cm with 10 × 10 cm doorways in each arm. Two of four arms contained enriching objects. Each of the two opposite arms included closed cylindrical glass bottle (7.5 cm in diameter, 13 cm high) mounted vertically in the arm near the wall that was distant from the doorway. The maze was covered by transparent plastic lid supplied with small ventilation holes.
2.4. General Procedure
On day 1 and 2, 80 rats were randomly assigned to blood pressure evaluation. The rats were handled and accustomed to restraining with tail-cuff on the tail for two consecutive days. On Day 3 blood pressure was evaluated using a non-invasive tail-cuff plethysmometry (see section 2.6).
On day 17, behavior of all 109 animals was evaluated in the first ED-test. Frequency distribution of ED-ratio (see sections 2.5 and 2.7) was analyzed in order to estimate its difference from normal distribution. Because of non-homogeneity of the measure revealing existence of two subpopulations of ED-low and -high individuals and due to insufficient number of rats with ED-high phenoltype, the experiment with atomoxetine employed only rats of ED-low phenotype (the ED-ratio was less than 200). Sixty four ED-low rats were randomly divided into four subgroups assigned to either single or multiple administration of vehicle or atomoxetine. Animals from multiple vehicle and atomoxetine groups received the corresponding treatment on days 20 - 23. Animals from single vehicle and atomoxetine groups were treated with either vehicle or atomoxetine, respectively, on day 23. On day 23, behavior of the ED-low rats was evaluated in the same second ED-test conducted an hour after last vehicle or atomoxetine administration.
2.5. Enrichment Discrimination Test
The animal was placed into the central compartment and allowed to explore the maze. The sequence and timing of arms visited were recorded by the use of Behavset 3.0 software directly into a personal computer. Each trial terminated when 12 visits into arms occurred. Animals that failed to make 12 visits during 15 minutes were excluded from the experiment. The criterion for a visit was entry into a compartment with all four paws inside. The floor and the objects in arms were cleaned thoroughly with paper towel damped in 70% ethanol and were airdried after each trial [26,27]. Position of the objects in a pair of opposite arm (#1 and #3, or #2 and #4) was alternated in a quasi-random order. Subsequent analysis was performed with help of Endisc software detecting the following measures:
1) Total time spent in empty or enriched arms. The measure was used for estimation of the ED-ratio that was calculated according to the formula:
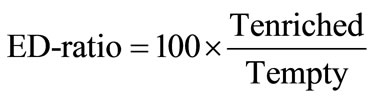
where, Tenriched is total time spent in arms containing objects, Tempty is total time spent in empty arms. In case of no difference in time spent in enriched and empty arms, the ratio is equal to 100. Animals exploring the objects stay longer in enriched part of maze than in empty arms and have higher scores of enrichment discrimination.
2) Total time in maze before an animal completes 12 visits to arms served as measure of locomotor activity. The variable highly negatively correlates with locomotor activity in the open-field [28].
3) Length of the first episode patrolling behavior, i.e., the number of entries performed by an animal until every maze arm has been visited. For instance, if the arm-entering sequence of the 12 entries is 124141334132, then the length of first patrolling episode is 7, because the episode is completed with entry into arm #3 on 7th visit. The more visits a patrolling episode takes, the less efficient is maze exploration. Obviously, an optimal patrolling episode includes 4 visits.
4) The total number of patrolling episodes made by an animal during the test. In the example above, the measure equals two, because the second patrolling episode is completed on the 12th entry into arm #2. The more patrolling episodes are made during the test, the more efficient is exploration. The patrolling behavior is considered to represent ability for spatial orientation that might parallel so-called fluid intelligence in human [26,28,29].
2.6. Blood Pressure Evaluation
Blood pressure and heart rate were determined non-invasively in conscious rats by tail-cuff plethysmograph (Panlab, Spain). Rats were handled and accustomed to 15-min restraining with tail-cuff unit on the tail for two consecutive days. On the third day the rats were kept in a warm environment (30˚C - 32˚C) for 15 - 20 min, after which the measurements were taken. An average of four pressure readings was taken for each rat.
2.7. Statistical Analysis
The results were analyzed by the use of Statistica 6.0 software. The frequency distribution of the ED-ratio was compared with a normal distribution using Chi-Square test. The Mann-Whitney U-Test and Wilcoxon Matched Pairs Test were used for comparison of behavioral variables from ED-low and ED-high subpopulations. A twoway ANOVA was employed for analysis of atomoxetine effects on behavior of ED-low SH rats with type (vehicle or atomoxetine) and duration (single or multiple administration) of treatment as independent variables. Difference between pairs of means was additionally evaluated by the use of ANOVA’s univariate test of significance for planned comparison.
3. Results
3.1. First ED-Test
In the first ED-test, frequency distribution of the EDratio had a clear bimodal shape (Figure 1, the data are represented by bars) that differed significantly from the curve expected under the normality hypothesis (Chi-Square = 48.65, df = 5, p < 0.001). The result indicates existence of two subpopulations diverging in attention to environmental enrichment. Although there was subpopulation overlap at ED-ratio near 200, ED-low rats were designnated as those if the ED-ratio was below 200; otherwise the animals were considered as belonging to ED-high subpopulation.
The ED-low phenotype represented by left part of the distribution was characterized by the ED-ratio range 0 - 199 and mean 104.8 ± 6.2. The ratio shows that the rats spend almost the same time in the enriched arms of the maze as in the empty arms. The phenotype was present in 81% of the rat population. The right part of distribution represented the phenotype of ED-high rats had the EDratio in the range of 200 - 600 and mean 326.7 ± 12.6. The ratio indicates clear preference of the rats for enriched environment. The individuals with ED-low phenotype demonstrated more episodes of patrolling behavior than did animals of ED-high phenotype. The ED-low and -high subpopulations differed neither in time spent in the maze until 12 visits into arms (Table 1) nor in blood pressure measures (Table 2).
3.2. Second ED-Test
In the second ED-test performed with ED-low rats hav-
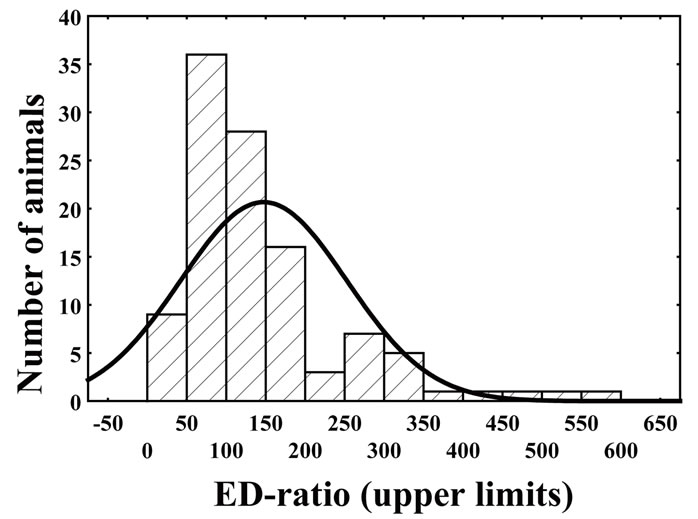
Figure 1. Frequency distribution of ED-ratio in SH rats evaluated during first ED-test (represented by bars) has bimodal shape and differs significantly from theoretical normal curve (represented by line) (Chi-Square = 48.65, df = 5, p < 0.001).
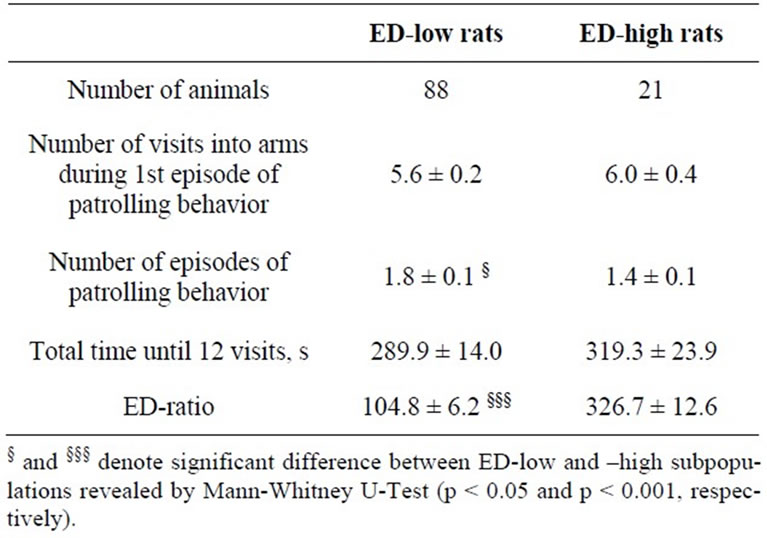
Table 1. Behavioral measures in the first enrichment discrimination test in the ED-low and -high subpopulations of SH rats (mean ± SEM).
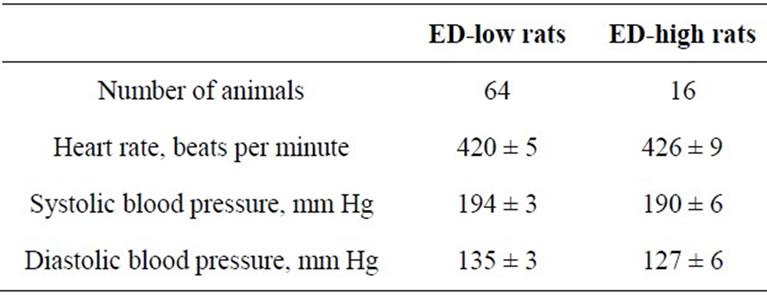
Table 2. Blood pressure in the ED-low and -high subpopulations of SH rats (mean ± SEM).
ing received single or multiple vehicle administration, the ED-ratio did not differ significantly from the measure obtained for the same animals in the first test: 98 ± 9.4 in the second test versus 96.4 ± 7.4 in the first test (Wilcoxon T = 243, N = 31, Z = 0.1, p = 0.922).
Two-way ANOVA of the data from ED-low rats having received single or multiple administration of either atomoxetine or vehicle revealed significant interaction between treatment type and duration (F(1,60) = 4.56, p = 0.036). Paired comparison yielded that the ED-ratio was higher after multiple atomoxetine as compared with multiple vehicle (F(1,60) = 8.29, p = 0.006) or single atomoxetine groups (F(1,60) = 6.57, p = 0.013) (Table 3). The ED-ratio after multiple atomoxetine was by 79% higher than after multiple vehicle (F(1,60) = 8.29, p < 0.006). The effect of the drug was not observed in single atomoxetine group which did not differ significantly from single vehicle group (F(1,60) = 0.05, p = 0.825).
Two-way ANOVA did not reveal any significant effects of the treatment on measures of patrolling behavior. On time spent in maze until 12 visits into arms, the ANOVA yielded only significant effect of treatment duration (F(1,60) = 26.22, p < 0.001). Effect of interaction between drug type and treatment duration only approached significance level (F(1,60) = 3.51, p < 0.066). Despite to difference between singleand multiple treatment conditions, ANOVA’s univariate test for difference between pairs of means revealed that atomoxetine increased the measure after 4-day administration as compared with multiple vehicle group (F(1,60) = 6.83, p < 0.011). The drug, however, had no significant effect after single administration compared with single vehicle group (F(1,60) = 0.01, p < 0.911).
4. Discussion
The first main outcome of the present study is that distribution of ED-ratio for rats from non-selected population has bimodal shape that differs from the expected Gaussian curve. The result reveals the existence among SH rats of two phenotypes that diverge in attention to stimuli enriching the environment. The difference between ED-low and -high subpopulation seems to be related neither to spatial orientation ability (because EDhigh-individuals do not display superiority in patrolling behavior) nor to general locomotor activity (because the phenotypes do not differ in total time of maze exploration). Therefore, the data suggest relative selectivity of attentional deficit displayed by ED-low rats that has been suggested as desirable for future models of ADHD [23]. The ED-low or -high group membership seems to be relatively stable group characteristic because the EDratio did not change significantly in the second test after vehicle administration.
The fact that rats of both phenotypes have similar blood pressure levels is consonant with the data observed in SH rats [15]. Notwithstanding the fact that most of SH rats belong to attentively-low phenotype, the population includes 19% of attentively-high rats. The heterogeneity could at least partially account for high variability of the ADHD-like behavior having been reported in SH rats [14, 19,23] and the ED-test might be useful for obtaining of more robust results in the tests for exploratory behavior and attention.
The second main outcome of the present study is that multiple administration of clinically efficient anti-ADHD drug atomoxetine administered for 4 days improves enrichment discrimination in ED-low individuals suggesting improvement of attention to the enriching objects. Interestingly, there was no improvement after single atomoxetine. The efficacy of subchronic atomoxetine could be hardly attributed to drug cumulation, because its halflife time in rats is near 3 hours [30], suggesting involvement of neuronal plasticity in the result. Since diverse medications have been used to treat the ADHD, it would be interesting in the future studies to estimate effects of different drugs and their combinations in the ED-test.
Although the ED-enhancing effect of atomoxetine coincided with some decrease in locomotor activity, it was not accompanied by change in patrolling behavior. Because improvement in patrolling behavior is typical after
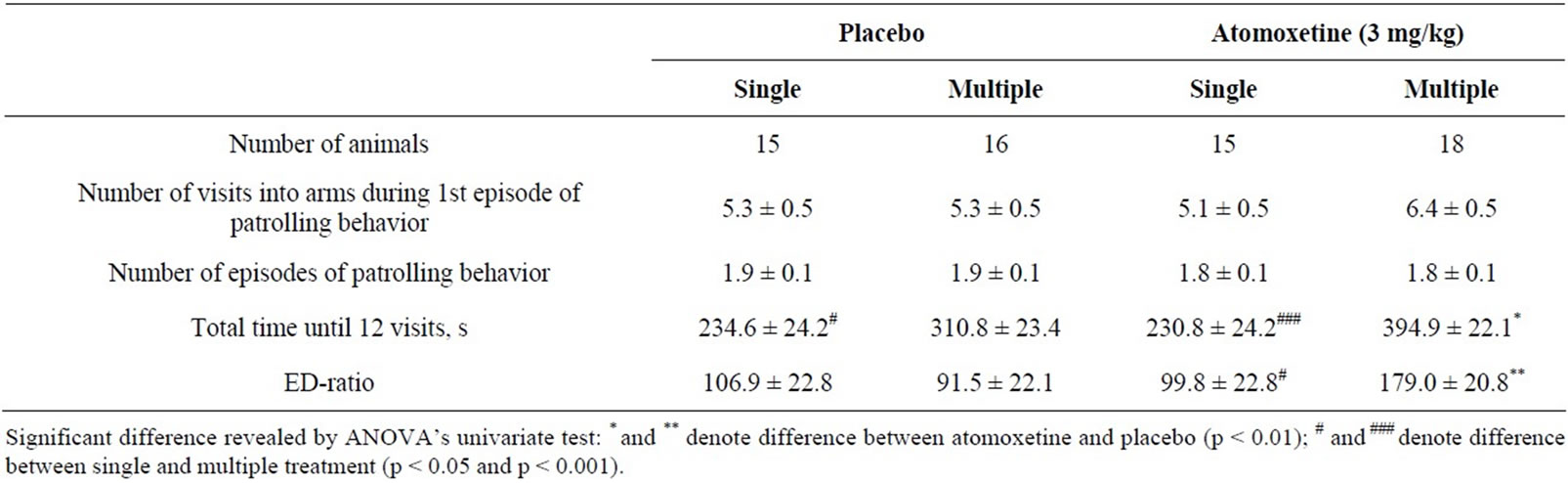
Table 3. Behavioral measures from the second ED-test by ED-low SH rats (mean ± S.E.M.) after single or multiple (once daily, for 4 days) atomoxetine administration.
cognition enhancing drugs [25,26], the result demonstrates that atomoxetine does not exert a cognition-enhancing property in the test.
The observed effects of atomoxetine are in general agreement with results obtained in Wistar rats during choice behavior in T-maze [8] and in Lister hooded rats in the five-choice serial reaction time task [31]. The results also parallel improvement in visuo-spatial performance in children with ADHD after subchronic atmoxetine [32].
In general, data of the present study contribute to face and predictive validity of the ED-test that could be useful in comparative studies having been suggested in rodents of different genetic background [9], for targeted selective breeding of experimental animals as well as in studies of neurobiology of the ADHD and, especially, for screening of novel anti-ADHD drug candidates.
5. Acknowledgements
The present study was supported by research funds from Russian Academy of Medical Sciences.
REFERENCES
- American Academy of Pediatrics, “Clinical Practice Guideline: Diagnosis and Evaluation of the Child with Attention-Deficit/Hyperactivity Disorder,” Pediatrics, Vol. 105, No. 5, 2000, pp. 1158-1170. doi:10.1542/peds.105.5.1158
- Y. Paloyelis, F. Rijsdijk, A. C. Wood, P. Asherson and J. Kuntsi, “The Genetic Association between ADHD Symptoms and Reading Difficulties: The Role of Inattentiveness and IQ,” Journal of Abnormal Child Psychology, Vol. 38, No. 8, 2010, pp. 1083-1095. doi:10.1007/s10802-010-9429-7
- K. Al-Menabbawy, A. El-Gerzawy, A. Ezzat and H. Mottawie, “Developmental, Behavioral and Genetic Factors in Correlation with Attention Deficit Hyperactivity Disorder in Egyptian Children,” Journal of Medical Sciences, Vol. 6, No. 4, 2006, pp. 569-576. doi:10.3923/jms.2006.569.576
- S. Mannuzza, R.G. Klein, A. Bessler, P. Malloy and M. LaPadula, “Adult Outcome of Hyperactive Boys. Educational Achievement, Occupational Rank and Psychiatric Status,” Archives of General Psychiatry, Vol. 50, No. 7, 1993, pp. 565-576. doi:10.1001/archpsyc.1993.01820190067007
- P. Asherson, W. Chen, B. Craddock and E. Taylor, “Adult Attention-Deficit Hyperactivity Disorder: Recognition and treatment in General Adult Psychiatry,” The British Journal of Psychiatry, Vol. 190, No. 1, 2007, pp. 4-5. doi:10.1192/bjp.bp.106.026484
- N. Kates, “Attention Deficit Disorder in Adults. Management in Primary Care,” Canadian Family Physician, Vol. 51, No. 1, 2005, pp. 53-59.
- F. Naderi, A. Heidarie, L. Bouron and P. Asgari, “The Efficacy of Play Therapy on ADHD, Anxiety and Social Maturity in 8 to 12 Years Aged Clientele Children of Ahwaz Metropolitan Counseling Clinics,” Journal of Applied Sciences, Vol. 10, No. 3, 2010, pp.189-195. doi:10.3923/jas.2010.189.195
- J. C. Bizot, S. David and F. Trovero, “Effects of Atomoxetine, Desipramine, d-Amphetamine and Methylphenidate on Impulsivity in Juvenile Rats, Measured in a TMaze Procedure,” Neuroscience Letters, Vol. 489, No. 1, 2011, pp. 20-24. doi:10.1016/j.neulet.2010.11.058
- E. Davids, K. Zhang, F. I. Tarazi and R. J. Baldessarini, “Animal Models of Attention-Deficit Hyperactivity Disorder,” Brain Research. Brain Research Reviews, Vol. 42, No. 1, 2003, pp. 1-21. doi:10.1016/S0165-0173(02)00274-6
- T. Sagvolden, V.A. Russell, H. Aase, E. B. Johansen and M. Farshbaf, “Rodent Models of Attention-Deficit/Hyperactivity Disorder,” Biological Psychiatry, Vol. 57, No. 11, 2005, pp. 1239-1247. doi:10.1016/j.biopsych.2005.02.002
- A. C. Meyer, S. Rahman, R. J. Charnigo, L. P. Dwoskin, J. C. Crabbe and M. T. Bardo, “Genetics of Novelty Seeking, Amphetamine Self-Administration and Reinstatement Using Inbred Rats,” Genes, Brain and Behavior, Vol. 9, No. 7, 2010, pp. 790-798. doi:10.1111/j.1601-183X.2010.00616.x
- M. B. Calzavara, R. Levin, W. A. Medrano, V. Almeida and A. P. Sampaio, L. C. Barone, R. Frussa-Filho and V. C. Abílio, “Effects of Antipsychotics and Amphetamine on Social Behaviors in Spontaneously Hypertensive Rats,” Behavioural Brain Research, Vol. 225, No. 1, 2011, pp. 15-22. doi:10.1016/j.bbr.2011.06.026
- B. Langen and R. Dost, “Comparison of SHR, WKY and Wistar Rats in Different Behavioural Animal Models: Effect of Dopamine D1 and Alpha2 Agonists,” Attention Deficit and Hyperactivity Disorders, Vol. 3, No. 1, 2011, pp. 1-12. doi:10.1007/s12402-010-0034-y
- E. B. Johansen, P. R. Killeen and T. Sagvolden, “Behavioral Variability, Elimination of Responses and Delay-ofReinforcement Gradients in SHR and WKY Rats,” Behavioral and Brain Functions, Vol. 3, 2007, pp. 60. doi:10.1186/1744-9081-3-60
- J. R. Wickens, J. Macfarlane, C. Booker and N. McNaughton, “Dissociation of Hypertension and Fixed Interval Responding in Two Separate Strains of Genetically Hypertensive Rat,” Behavioural Brain Research, Vol. 152, No. 2, 2004, pp. 393-401. doi:10.1016/j.bbr.2003.10.023
- W. Danysz, A. Plaznik, O. Pucilowski, M. Plewako, M. Obersztyn and W. Kostowski, “Behavioral Studies in Spontaneously Hypertensive Rats,” Behavioral and Neural Biology, Vol. 39, No. 1, 1983, pp. 22-29. doi:10.1016/S0163-1047(83)90569-1
- I. Lukaszewska and G. Niewiadomska, “The Differences in Learning Abilities between Spontaneously Hypertensive (SHR) and Wistar Normotensive Rats Are Cue Dependent,” Neurobiology of Learning and Memory, Vol. 63, No. 1, 1995, pp. 43-53. doi:10.1006/nlme.1995.1004
- A. Tamburella, V. Micale, C. Mazzola, S. Salomone and F. Drago, “The Selective Norepinephrine Reuptake Inhibitor Atomoxetine Counteracts Behavioral Impairments in Trimethyltin-Intoxicated Rats,” European Journal of Pharmacology, Vol. 683, No. 1-3, 2012, pp. 148-154. doi:10.1016/j.ejphar.2012.02.045
- W. Adriani, A. Caprioli, O. Granstrem, M. Carli and G. Laviola, “The Spontaneously Hypertensive-Rat as an Animal Model of ADHD: Evidence for Impulsive and Non-Impulsive Subpopulations,” Neuroscience & Biobehavioral Reviews, Vol. 27. No. 7, 2003, pp. 639-651. doi:10.1016/j.neubiorev.2003.08.007
- T. E. Wooters and M. T. Bardo, “Methylphenidate and Fluphenazine, but Not Amphetamine, Differentially Affect Impulsive Choice in Spontaneously Hypertensive, Wistar-Kyoto and Sprague-Dawley Rats,” Brain Research, Vol. 1396, 2011, pp. 45-53. doi:10.1016/j.brainres.2011.04.040
- M. B. Calzavara, G. B. Lopez, V. C. Abilio, R. H. Silva and R. Frussa-Filho, “Role of Anxiety Levels in Memory Performance of Spontaneously Hypertensive Rats,” Behavioural Pharmacology, Vol. 15, No. 8, 2004, pp. 545- 553. doi:10.1097/00008877-200412000-00003
- S. A. Ferguson and E. P. Gray, “Aging Effects on Elevated Plus Maze Behavior in Spontaneously Hypertensive, Wistar-Kyoto and Sprague-Dawley Male and Female Rats,” Physiology & Behavior, Vol. 85, No. 5, 2005, pp. 621-628. doi:10.1016/j.physbeh.2005.06.009
- B. Alsop, “Problems with Spontaneously Hypertensive Rats (SHR) as a Model of Attention-Deficit/Hyperactivity Disorder (AD/HD),” Journal of Neuroscience Methods, Vol. 162, No. 1-2, 2007, pp. 42-48. doi:10.1016/j.jneumeth.2006.12.002
- R. M. Salimov, I. I. Poletaeva, G. I. Kovalev, N. B. Salimova and R. R. Gainetdinov, “Interstrain Differences in Extrapolation Capacity and Exploration of a Cruciform Maze Correlate with Various Indices of Monoamine Metabolism in the Brain,” Zhurnal Vyssheï Nervnoï Deiatenlnosti Imeni I P Pavlova, Vol. 45, No. 5, 1995, pp. 914-924.
- G. I. Kovalev, I. Firstova and R. M. Salimov, “Effects of Piracetam and Meclofenoxate on the Brain NMDA and Nicotinic Receptors in Mice with Different Exploratory Efficacy in the Cross Maze Test,” Eksperimental’naia i Klinicheskaia Farmakologiia, Vol. 71, No. 1, 2008, pp. 12-17.
- R. Salimov, N. Salimova, L. Shvets and N. Shvets, “Effect of Chronic Piracetam on Age-Related Changes of CrossMaze Exploration in Mice,” Pharmacology Biochemistry and Behavior, Vol. 52, No. 3, 1995, pp. 637-640. doi:10.1016/0091-3057(95)00179-Z
- R. M. Salimov, “Different Behavioral Patterns Related to Alcohol Use in Rodents: A Factor Analysis,” Alcohol, Vol. 17, No. 2, 1999, pp. 157-162. doi:10.1016/S0741-8329(98)00049-4
- N. V. Markina, R. M. Salimov and I. I. Poletaeva, “Behavioral Screening of Two Mouse Lines Selected for Different Brain Weight,” Progress in Neuro-Psychopharmacology & Biological Psychiatry, Vol 25, No. 5, 2001, pp. 1083-1109. doi:10.1016/S0278-5846(01)00169-5
- I. I. Poletaeva, and R.M. Salimov, “A Factor Analysis of Behavioral Organization in Mice,” Zhurnal Vyssheï Nervnoï Deiatenlnosti Imeni I P Pavlova, Vol. 42, No. 2, 1992, pp. 314-324.
- E. L. Mattiuz, G. D. Ponsler, R. J. Barbuch, P. G. Wood, J. H. Mullen, R. L. Shugert, Q. Li, W. J. Wheeler, F. Kuo, P. C. Conrad and J.-M. Sauer, “Disposition and Metabolic Fate of Atomoxetine Hydrochloride: Pharmacokinetics, Metabolism and Excretion in the Fischer 344 Rat and Beagle Dog,” Drug Metabolism and Disposition, Vol. 31, No. 1, 2003, pp. 88-97. doi:10.1124/dmd.31.1.88
- A. B. Fernando, D. Economidou, D. E. Theobald, M. F. Zou, A. H. Newman, M. Spoelder, D. Caprioli, M. Moreno, L. Hipólito, A. T. Aspinall, T. W. Robbins, J. W. Dalley, “Modulation of High Impulsivity and Attentional Performance in Rats by Selective Direct and Indirect Dopaminergic and Noradrenergic Receptor Agonists,” Psychopharmacology (Berl), Vol. 219, No. 2, 2012, pp. 341- 352. doi:10.1007/s00213-011-2408-z
- C. G. de Jong, S. van de Voorde, H. Roeyers, R. Raymaekers, A. J. Allen, S. Knijff, H. Verhelst, A. H. Temmink, L. M. Smit, R. Rodriques-Pereira, D. Vanden-berghe, W. van I, L. ter Schuren, M. Al Hakim, A. Amin, L. Vlasveld, J. Oosterlaan, and J. A. Sergeant, “Differential Effects of Atomoxetine on Executive Functioning and Lexical Decision in Attention-Deficit/Hyperactivity Disorder and Reading Disorder,” Journal of Child Adolescent Psychopharmacology, Vol. 19, No. 6, 2009, pp. 699-707. doi:10.1089/cap.2009.0029

