Journal of Behavioral and Brain Science
Vol. 2 No. 3 (2012) , Article ID: 22092 , 4 pages DOI:10.4236/jbbs.2012.23038
Spatial Memory Deficits and Their Correlations with Clusters of Shrunken Neuronal Soma in the Cortices and Limbic System Following a “Mild’’ Mechanical Impact to the Dorsal Skull in Female Rats
1Centre for Advanced Research in Environmental Genomics, Department of Biology, University of Ottawa, Ottawa, Canada
2Behavioural Neuroscience Laboratory, Department of Biology and Biomolecular Sciences Program, Laurentian University, Sudbury, Canada
Email: *mpersinger@laurentian.ca, wlado038@uottawa.ca
Received June 5, 2012; revised June 27, 2012; accepted July 12, 2012
Keywords: Keyword: TBI
ABSTRACT
Background: Previous results showed that quantitative changes in behavioural accuracies by rats that sustained a “mild” closed head injury were moderately correlated with the total areas (numbers) of anomalous neuronal soma within regions below the impact. Method: Water maze behavioural measures within one day or two months after a single impact of mechanical force over the right dorsal skull, with or without stunning and with or without subsequent pregnancy, were measured and compared to proportions of anomalous neurons under the impact site. Results: The consequences of the impact accommodated about 20% of the variance in the rats’ scores for less proficient spatial learning and memory. There were significantly more anomalous cells within right hemisphere below the impact site that were correlated with poorer initial maze learning. Maternal experience reduced the numbers of anomalous cells in the right limbic area only. Conclusion: These results suggest weak mechanical impacts produce changes in histomorphology within some neurons that are still evident two months later and that the presence of these anomalous clusters, corresponding to less than 1% of the cross-sectional area and below the resolution of contemporary MRI in human cases, are strongly correlated with specific behavioural impairments.
1. Introduction
The recent interest in “concussive” injuries from explosive devices or body impacts during sporting events reflects the intrinsic epidemic of these types of injuries. Disruptions in normal brain functions are common consequences after a closed head injury [1,2] even without loss of consciousness. In addition to behavioural and emotional alterations [3], cognitive domains that include learning and memory are often compromised. The magnitude of these cognitive deficits has been considered a function of the severity and location of the cerebral injury. These cognitive alterations strongly influence the individual’s probabilities to return to the premorbid status. Although many of these cognitive deficits appear to “resolve” within a year after the trauma, some of these deficits may persist as the postconcussive syndrome in humans for decades [4-7].
In many closed head injuries (CHI) or traumatic brain injuries (TBI), prognosis for subsequent adaptability to the same vocational demands is ambiguous. Individuals can appear otherwise normal with no morphological brain defects as inferred by MRI and CT scans. However, these people continue to exhibit various degrees of intermittent amnesia, difficulty in concentration, impaired executive functions, depression, apathy and anxiety [8] that often reflect a continuum of neuroelectrical instability. They are able to perform routine daily tasks well but when presented with novel tasks fail to perform optimally. This has led some observers to suspect the individuals are malingering [7,9].
Biomechanical forces applied to the brain by impact of mechanical pressure waves can cause shearing and sometimes tearing of axons in addition to oedema and transient vascular disruptions [8,10,11]. Occasionally, diffuse global injury may occur. These biomechanical forces cause neurons to undergo cellular death either through necrosis (acute) or apoptosis (secondary). Necrosis occurs immediately as a consequence of the impact while apoptosis is a delayed programmed cellular death due to metabolic perturbation [12-16]. Neuronal death can initiate or at least encourage plasticity leading to alterations in neuronal connectivity [17-20].
We have found that a single impact of mechanical energy from a 200 gm weight dropped 0.9 m (equivalent to an impact of 15 km/hr) upon the right side of the skull in rats produces disruptions in spatial learning and memory (but not in routine or “procedural” behaviours such as ambulation) even though there was no evidence of “stunning” or loss of consciousness. The calculated force of impact was about 2 N. The amplitude of the pressure wave would have varied between 3 kPa and 20 kPa with a transit time through the brain of about 2 msec to 3 msec.
The impact results in very obvious morphological changes in neurons (even evident to the naïve observer at 40×) within the cerebral cortices immediately below the impact and in the “countercoup” areas within the piriform lobes, amygdala and entorhinal cortices. These neurons, present for more than 60 days after the impact, are darkly-stained with obvious shrunken soma. They are distributed between neurons with normal soma and may comprise 50% of the population within an affected area. Within the limits of conventional histological stains and light microscopy the soma appear to be in a state of dormancy.
To assess the effects of CHI on motivation, visual acuity, learning and memory, mechanical energy was propagated vertically by the weight-drop method through the right hemisphere of female Wistar rats. The rats were tested in a Morris Water Maze (MWM) paradigm. Two protocols of the MWM paradigm were adopted. Firstly, a hidden platform MWM task was used to assess spatial memory [21] 24-hour and a fortnight after the CHI. Secondly, a visible platform MWM task was utilized to test for sensory, motor or motivation problems [22] immediately after the TBI. The hidden platform MWM was different from the visible platform MWM in that the former had extramaze cues around the pool and required a platform be maintained in the same fixed position for the duration of the experiment. The latter had no extramaze cues and involved shifting the platform randomly in all four quadrants in all four trials per session.
In line with our previous findings for the radial maze [23] we hypothesized that CHI relative to sham animals would exhibit spatial memory deficits. Furthermore, we hypothesized CHI animals would loose more body weight than sham animals. Neurons in the hippocampus and related structures are susceptible to mechanically induced injuries [8,24]. Because the hidden platform MWM is a hippocampal-related task [2,25] disruptions in the hippocampus or its inputs from the entorhinal cortices should impair spatial memory.
After a CHI or TBI, neurotropic factors and cytokines are released to promote neuronal survival and to stimulate neurogenesis and plasticity in the nervous system [26-28]. In our (M. A. Persinger, L. S. St-Pierre, S. G. Tiller) 20 years of clinical experience we have encountered several cases where young women who became pregnant after a brain injury showed conspicuously fewer neuropsychological deficits than expected for the energies involved with the impact and the emergency room assessment. We reasoned pregnancy might be neuroprotective because of neuronal proliferations and plasticity seen in discreet parts of pregnant rodents’ brains [29]. We hypothesized that rats that had sustained CHIs and then became mothers would show fewer deficits than those that were never mothers.
2. Methods
2.1. Animals
The subjects were 4 - 12 months old female albino Wistar rats (N = 74). Each rat weighed between 250 - 450 g and was housed singly or in pairs in standard wire cages. The rats had been obtained at 60 days of age from Charles River Breeders (Quebec). The animals had access to food and tap water ad libitum except when their weights were measured every two days and when they were removed for testing in another room containing a custom-built Morris Water Maze. The ambient temperature in the colony was maintained at 20˚C ± 1˚C, and the light: dark cycle was kept at 12:12 with a photophase onset at 07:30 h. All experiments were preformed in the midphotophase period. Animals were maintained and treated according to the Canadian Council for Animal Care (CCAC) guidelines in the ethical treatment of animals and with the approval of the Laurentian University Animal Care Committee. Animals were monitored quailtatively by experienced Animal Care Technicians.
2.2. General Procedures
For any given experiment, rats were randomly divided into two conditions—sham and closed head injury (CHI). The procedure of CHI induction has been described elsewhere [23]. Briefly, rats were brought to an experimental room not associated with the experiments. CHI rats were held with forelimbs crossed briefly by one experimenter so the head was resting on a Formica-surface table. A 200 gm weight from an analytical balance was dropped from a height of 0.9 m through a cardboard tube onto their right dorsal skull. The sham animals were just held briefly against the table and had the cardboard tube placed on their right dorsal skull, but no weight was dropped. The entire procedure required about 10 - 15 sec. Animals that were stunned or rendered unconscious or immobile for 2 - 3 min were ranked as 2. Those stunned for 30-sec or less were ranked as 1. Sham animals as well as the rats that received the blow and showed no stunning received a score of 0. After recovery, as assessed through their righting reflex and normal gait, the animals were returned to their home cages.
2.3. Apparatus
All animals were transported in carrying cages to the testing room and tested singly in a hidden platform Morris Water Maze (MWM) paradigm for seven sessions. For the open-platform MWM six sessions were involved. The MWM was a child’s swimming pool, measuring 1.5 m in diameter by 0.45 m in height. It was divided into four quadrants by transparent fishing lines. A hidden platform that was a transparent Tupperware (15 cm × 15 cm) filled with opaque black non-toxic water-soluble dye was placed about 0.38 m from the centre of the pool (in the centre of one quadrant—the target quadrant).
The pool was filled with darkly-dyed water until the platform was submerged about 2.5 cm below the surface. Water temperature was maintained at 19˚C. Visual cues were placed on the walls around the pool to aid rats in locating the platform. Each session consisted of four trials with an inter-trial interval of 30-sec. The rats’ escape latencies (time to find a hidden platform) were measured for the four trials (each trial lasted for 120-sec) to the nearest second. Rats were allowed to remain on the platform for 30-sec after they found it before they were removed or 120-sec had elapsed. The release schedules, for each session and each trial, were randomized.
All experiments were essentially the same except for the open-platform MWM task and probe tests. The openplatform MWM was used to test the rats’ motivation and visual acuity [22]. It utilized the same pool as above. However, the platform was elevated 2.5 cm out of the water. Furthermore, the platform had visible cues (a small paper flag and a Christmas-tree like object placed on opposite sides). After each trial, the rats were removed from the MWM and the platform was moved to a new location in a randomized manner until rats were tested in all four quadrants.
The probe test, completed during the seventh session after the hidden platform MWM task, measured the strength of the rats’ spatial memory [30]. The probe test consisted of assessing the rats’ spatial memories in the MWM devoid of the platform but with the visible cues left intact. The strength of the rats’ spatial memory was inferred from the time spent in the target quadrant (where the hidden platform was maintained for the hidden platform MWM). After each session, the rats were placed under a heating lamp until they dried before being returned to their home cages. In addition, to the MWM performances, body weights for all rats were taken every two days both before and after the CHI (before the start of the sessions) on the weighing days to monitor their health status.
In Experiment 1, 11 rats (CHI = 6, sham = 5) were tested beginning one day after the treatments in the hidden platform MWM for one week. In Experiment 2, 16 rats (CHI = 9, sham = 7) were tested in the hidden platform setting for seven daily sessions two weeks after the treatment. To test visual acuity and motivation [22] in Experiment 3, 12 rats (CHI = 6, sham = 6) were tested 24 hrs after the treatment in the MWM where the platform was visible. In Experiment 4, because of the possible beneficial role of pregnancy in neuronal plasticity [29], a total of 34 rats (CHI = 17, sham = 17) were selected; 9 of the CHI rats and 9 of the sham rats were immediately placed with males. The other rats were placed with a novel female.
The 34 rats were transferred and housed singly in plastic cages with wood shaving bedding after 19 days. The latency to deliver after first exposure to the male and the numbers of pups delivered were recorded. After parturition, pups were weaned (21 days of age), and the mothers were transferred from plastic cages to standard wire cages. The female rats’ spatial memory was assessed in the hidden platform MWM for seven sessions in 4 blocks approximately 50 days after the trauma.
2.4. Neurohistology
About 60 days after the impact the brains of eight mothers (four bred, four not bred; two from each being either stunned or not stunned) were removed and fixed in ethanol-formalin-acetic acid for at least 48 hr. After processing and embedding in paraffin, ten more or less equally spaced 10 micrometer sections were obtained between the caudal cerebrum and the level of the anterior commissure. We reasoned that if the neuropathological effects of the impact were consistent and strong the pattern of distribution of neuronal anomalies or any effect from maternal experience should be conspicuous even with a relatively small sample size.
The sections were stained with toluidine blue O. Each section was examined thoroughly at 40×, 100×, and 400× by light microscopy. Neurons that displayed shrunken soma appeared in discrete clusters (about 3 to 6 distributed amongst a similar number of neurons with normal soma per 0.026 mm2) within specific areas (Figure 1). The numbers of squares (0.026 mm2 at 100×) containing the anomalous cells within a grid of 36 squares were counted. The areas that displayed these criteria included the dorsal (parietal, occipital, frontal and retrosplenial)
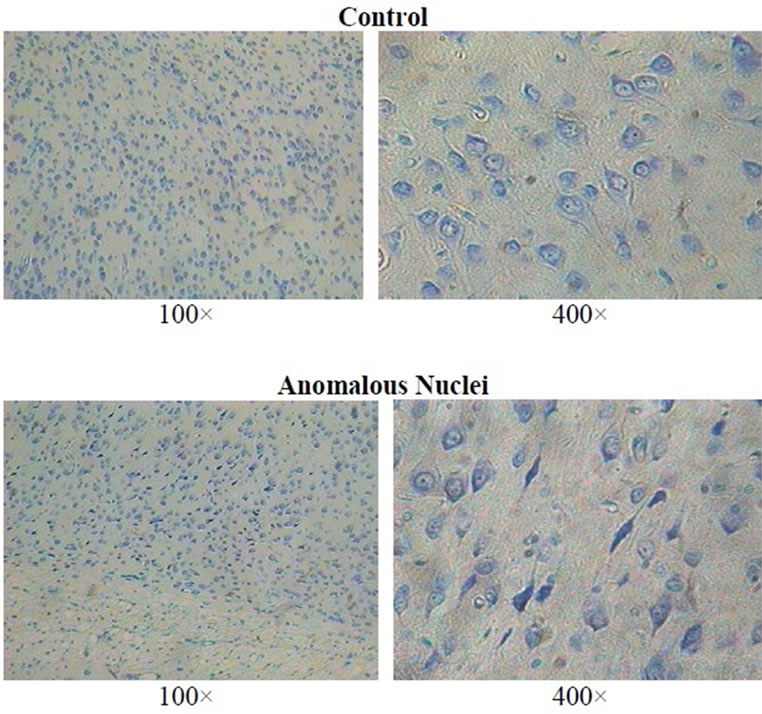
Figure 1. Examples of normal cortical neurons in the left hemisphere (non-injured side) and anomalous soma (dark shrunken soma) in the right hemisphere within the dorsal cortices below the impact site of a female rat (100× and 400×; toluidine blue O) approximately 60 days after the mechanical impact.
cortices, and the amygdala, entorhinal cortices, piriform cortices, endopiriform area, and the temporal cortices.
The sum of the areas containing aberrant neuronal morphology was calculated by computer for each of these areas for the left and right side of the brain for each section separately. For the purposes of statistical analyses, the mean of the sum of squares containing clusters of aberrant neurons in the dorsal cortices (near the impact sight) and within the other (lower) regions were calculated as well for all 10 sections.
2.5. Statistical Analyses
All analyses involved SPSS software on a Vax 4000 computer. Post hoc tests were Tukey’s for main effects and paired t tests for repeated measures. The body weights were transformed to percentage relative differences  to accommodate individual differences. The effect size (h2) was included. It is defined as the amount of variance in the dependent variables explained by the independent variables.
to accommodate individual differences. The effect size (h2) was included. It is defined as the amount of variance in the dependent variables explained by the independent variables.
3. Results
3.1. Body Weight
Weight losses by CHI rats compared to controls were apparent for the days after the treatments but were no longer apparent two weeks after the treatment or after weaning of offspring for the maternal study. For Experiment 1, an analysis of variance (ANOVA) with one within subject level (body weight) and one between subject level (conditions) revealed a main effect for conditions [F (1,8) = 5.57, p < 0.05, h2 = 41%] but not for days (p > 0.05). The means and SEMs for percentage (to baseline) weight changes were –2.9% (0.4%) for the CHI rats and +0.4% (1.1%) for sham rats. For Experiment 3, the means and SEMs for weight changes for the CHI rats were –6.0% (1.0%) and –1.7% (0.5%) for the sham rats [F(1,10) = 28.28, p < 0.01; h2 = 0.74].
3.2. Maze Performance
In addition to the expected trial and days differences, the only relevant interaction [F(15,120) = 2.53, p < 0.05, h2 = 24%] was between conditions, days, and trials. Post hoc analysis indicated that the CHI animals performed poorly relative to sham animals two days following the trauma but on the third and subsequent days their performance was indistinguishable from the sham animals (Figure 2).
In addition to the expected day and trial effects for Experiment 2, there was a statistically significant three-
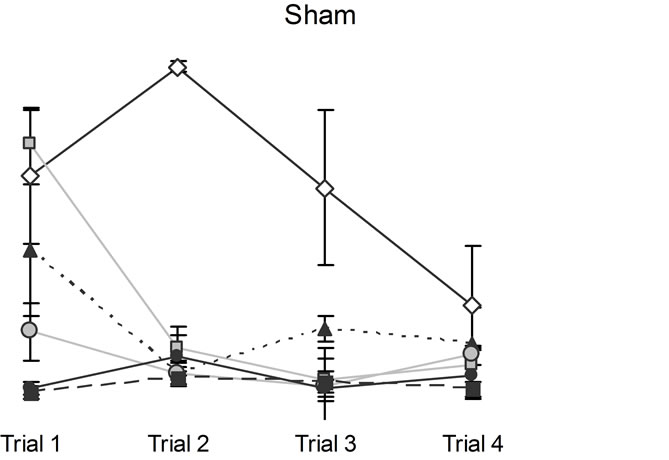
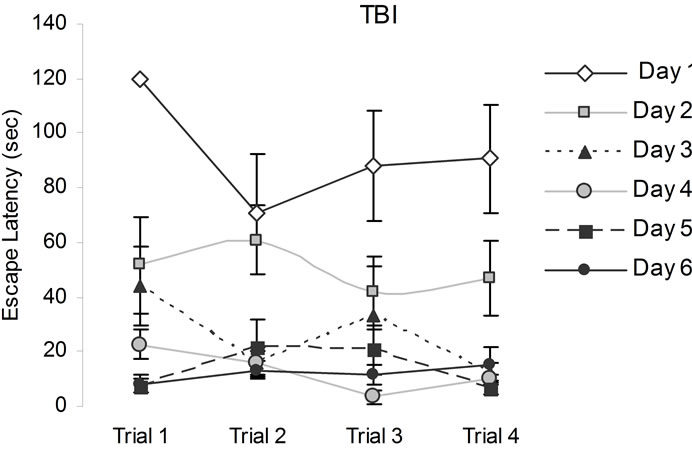
Figure 2. Animals’ performance in the hidden platform Morris Water Maze measured over six days immediately after the CHI. Note that CHI animals performed poorly relative to sham animals on days 1 and 2, but all animals were indistinguishable from each other on subsequent days. Vertical bars represent the standard errors of the mean (SEM).
way interaction [F(15,210) = 1.78, p < 0.05, h2 = 11%] between conditions and days and trials. Post hoc analysis indicated that the CHI animals performed poorly relative to sham animals a fortnight following the trauma. Three days after the initial introduction to the MWM during subsequent days their performance became indistinguishable from the sham animals (Figure 3).
For Experiment 3, there were statistical significant interactions between conditions and days [F(6,48) = 3.34, p < 0.05, h2 = 30%], and conditions and trials [F(3,24) = 3.85, p < 0.05, h2 = 33%]. The interactions between conditions and days and trials approached statistical significance (p = 0.07). Post hoc analyses indicated that overall the CHI animals (M = 36.02 s, SEM = 5.61 s) required longer to find the visible platform positions relative to sham animals (M = 14.74 s, SEM = 1.85 s). Over days, the TBI animals took longer to find the new platform positions (Figure 4).
3.3. Effects of Pregnancy and Mothering
A four-way ANOVA for escape latency with two within levels (days and trials) and two between levels (rank of post impact behaviour and motherhood) did not reveal statistically significant main effects for rank or motherhood. There were significant differences between trials Sham
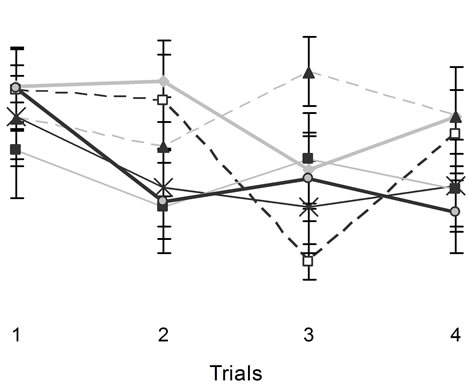
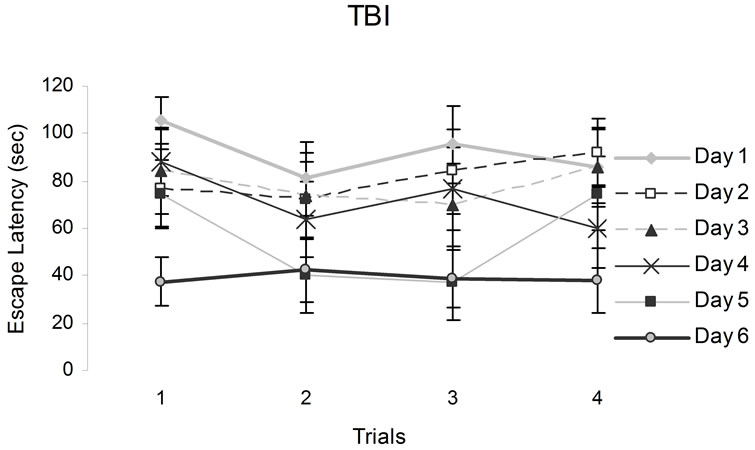
Figure 3. The figures above show animals performances in a hidden platform Morris Water Maze. Vertical bars denote SEM. Note the poor performances of CHI animals relative to the sham on the first two days. The numbers from 1 to 6 in the legend refer to training sessions.
[F(3,84) = 11.44, p < 05, h2 = 29%] and days [F(5,140) = 47.39, p < 0.05, h2 = 63%]. There were statistical significant interactions between the ranks for stunning, motherhood, and trials [F(6,84) = 2.25, p < 0.05, h2 = 14%] and between days and trials [F(15,420) = 17.17, p < 0.05, h2 = 38%]. No other statistically significant interactions were observed. Post hoc analysis indicated that motherhood following mild stunning from a CHI interacted deleteriously for the first performances during the first trial of the successive days of testing (Figure 5).
An ANOVA for escape latency (after the data were transformed into percentages to accommodate individual differences by dividing each trial by 120 and multiplying by 100) with two levels repeated (trials by sessions) and two levels not repeated (breeding and level of stun) did not show main effects (p > 0.05) for rank, motherhood, or trials. There were main effects for days [F(5,140) = 69.66, p < 0.05, h2 = 71%] and trials [F(3,84) = 32.76, p < 0.05, h2 = 54%]. There were two-way interactions between rank and days [F(10,140) = 2.02, p < 0.05, h2 = 13%] and days and trials [F(15,420) = 1.72, p < 0.05, h2 = 6%]. Post hoc analysis indicated that rats that had a CHI but were mildly or not stunned performed worse than the sham group on day 4 (Figure 6). The results of the probe tests did not reveal any statistically significant difference between rats that had sustained the mechanical impact or the controls for any of the four experiments.
The females that had sustained the mechanical impact
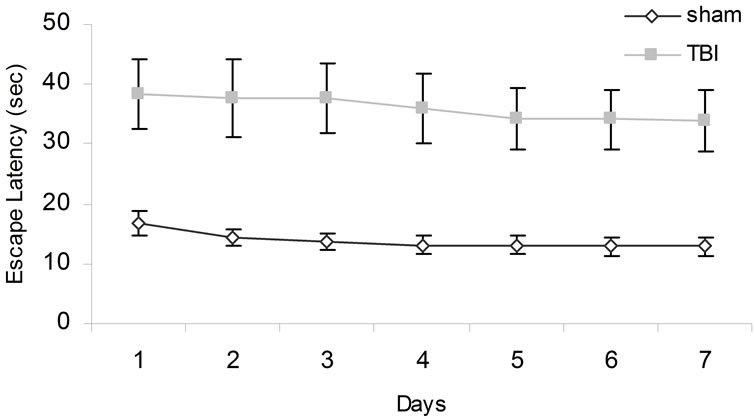
Figure 4. Animals’ performances tested over days when the escape platform was visible. Error bars denote SEM.
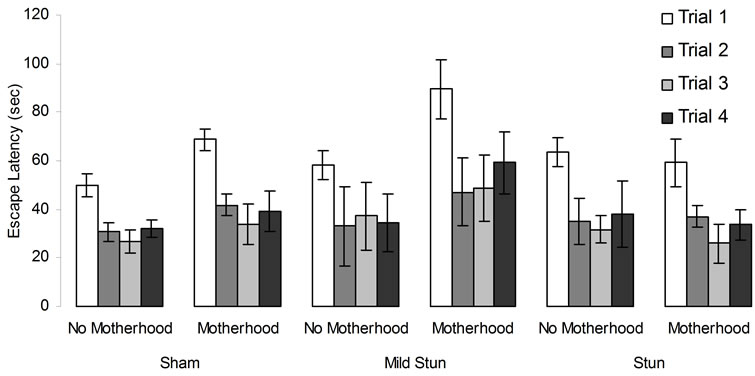
Figure 5. Sham and CHI animals’ performances in the hidden platform MWM. Vertical bar denotes SEM.
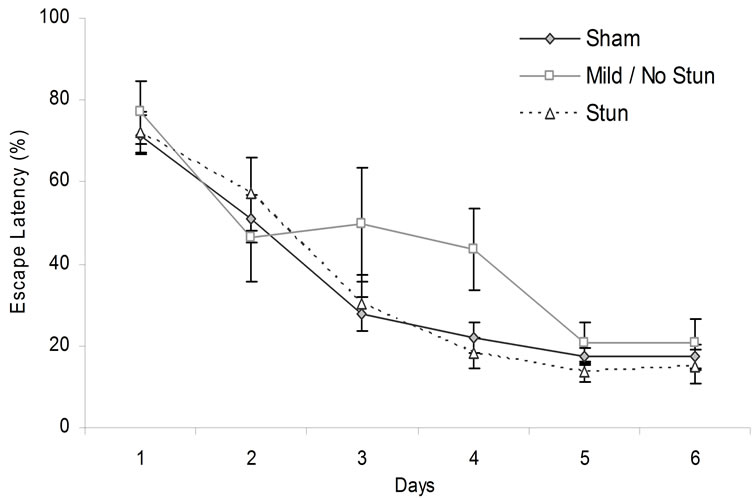
Figure 6. Animals escape latency over days in the hidden platform MWM. Vertical bars stand for SEM.
did not differ significantly (all dfs = 1,15, Fs < 0.5) from control females with respect to the latencies of birth (M = 25.7 days, SD = 4.6 days), total numbers of pups per litter (M = 13.7, SD = 3.5), the numbers of males (M = 7.3, SD = 2.6), or the numbers of females (M = 6.4, SD = 2.9) per litter. However litter sizes were significantly correlated with escape latencies for the second trial on day 6 were (due to numbers of females, rho = 0.65) while this correlation on the third trial on day three was due to the numbers of males (0.43).
3.4. Histological Analyses
Even though not all rats showed obvious post-concussive behaviors (such as stunning), the brain of every rat that received the mechanical impact to the dorsal right skull showed the same type of scattered neurons with shrunken soma within the (primarily) right cerebral cortices and the limbic structures. However the total area of these interspersed shrunken somas varied. Only two of 12 rats showed one small area of conspicuous, circumscribed neuronal dropout and gliosis typical of ischemic damage. Only one rat showed a cystic lesion in the temporal lobe (much like the “burst lobe” phenomena) and massive, dense accumulation of Nissl and cell debris within the right medial geniculate. This debris was identical to that found in the thalamus several weeks after seizure-induced necrosis within greater than 50% of the neuronal population had occurred [31]. In other words only 16% of the population that sustained impact from the pressure wave displayed the type of histopathology that in human contexts would be revealed by MRI or CT methods.
Four way analysis of variance as a function of no stun vs. stun and history of motherhood for the sums of areas containing neurons with shrunken soma and densely stained Nissl substance indicated significantly [F(1,7) = 28.26, p < 0.001; 80% of variance explained] larger areas of anomalous neurons within the right hemisphere compared to the left hemisphere. The means and standard deviations (in parentheses) for the areas containing anomalous neurons within four regions were left dorsal cortices 0.05 (0.05) mm2, left ventral region 0.06 (0.06) mm2, right dorsal cortices 0.13 (0.08) mm2, and right ventral region 0.12 (0.09) mm2. For comparison the cross-sectional area of the typical coronal section is about 120 mm2.
There was a statistically significant interaction [F(1,7) = 6.72, p < 0.01; h2 = 49%] between a history of motherhood and side of the impact. According to post hoc Tukey's (p < 0.05) in conjunction with correlated t-tests, the major source of this interaction was the smaller areas (M = 0.07 mm2, SD = 0.04 mm2) of anomalous neurons within the right ventral region (limbic area) of rats that had been mothers compared to those who had not (M = 0.18 mm2, SD = 0.11 mm2). There was no difference between the two groups for the areas of anomalous neurons immediately beneath the impact site (M = 0.14 mm2 and 0.12 mm2, respectively). There were no other statistically significant differences for the other main effects or the remaining interactions.
3.5. Behavioral and Brain Correlates
Specific components of the learning and memory paradigm were correlated with the measures of neurohistology. The relative times required to complete the first trial on each of the six days (defined as the ratio of the value of each first trial per day divided by the mean of the first trials for all six days) were significantly correlated with the area of anomalous neurons within the right dorsal cortices only (see Table 1). Partial correlation analyses for the first trials for the second and third days of testing showed the association remained significant (partial rs = 0.70 and –0.55) even after the shared variance with the area of anomalous cells in the left dorsal cortices was first removed.
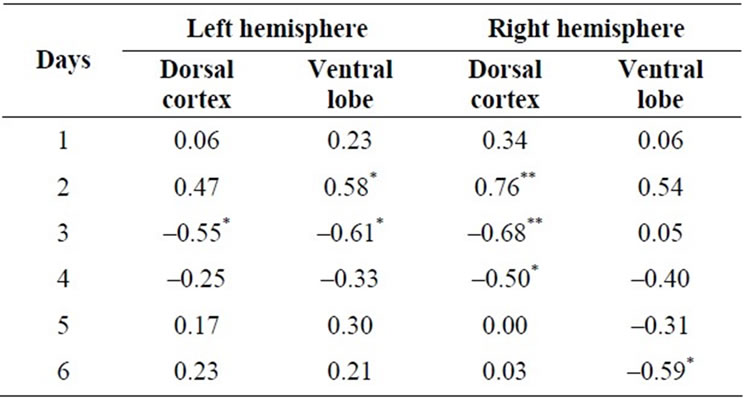
Table 1. Correlations (Spearman rho) between the relative times required to complete the first trial each day (each day divided by the mean of all six days) and the area of anomalous neurons within the dorsal cortices and ventral (limbic) area in the left and right hemisphere 60 days after the single impact (equivalent to 15 km/hr) of mechanical energy to the right dorsal skull. *p < 0.05, **p < 0.01.
When the shared variance between the area of anomalous neurons within the right dorsal cortices and either the left ventral or right ventral areas were first removed the correlations between areas of anomalous neurons in the right dorsal cortices and the behavior remained significant statistically. Removing the shared variance between area of anomalies with each of the other three regions and the dorsal right cortices before the analyses eliminated any statistically significant effects.
To ensure that specific structural effects were not masked by over-inclusion of unrelated structures. Spearman correlations were completed for the areas of anomalous neurons within the left and right amygdala, entorhinal cortices, endopiriform area, piriform area, and temporal cortices and the relative latencies for the first trials. The only significant correlation was between the area of anomalies within right endopiriform area (0.66) but not the left (0.30) and relative latency for the first day only and between the right amygdala (0.68, p < 0.05) but not the left amygdala (0.02) and the relative latency for the first trial for the second day only.
4. Discussion
Our hypothesis that rats who sustained “mild” closed head injuries, often associated with no obvious immediate clinical symptoms, would perform poorly on the hidden platform MWM was supported. However the relationship between complex behaviours and “mild” brain injury changed over time as a function of trials and the regions of the brain involved with these transitions. Our results suggest that the effects of mild CHI are most noticeable when rats are exposed to a novel environment after the trauma. If the rats behave for a considerable time in that context, their deficits diminish. CHI rats required on average three days to improve and perform similarly to the sham animals in the Morris Water Maze (MWM). Similar cognitive slowing but within longer time frames has been reported for humans after a CHI [32].
The MWM is a hippocampal-dependent task that is sensitive to lesions of the hippocampus and cortex [8,33]. The parietal cortex is involved with proximal cues while the hippocampus is involved with processing both proximal and distal cues. Studies have shown that D1 dopamine, β adrenergic, and muscarinic receptors enhance and 5HT1A receptor inhibit retrieval of inhibitory avoidance in the hippocampus as well as the entorhinal, posterior parietal and anterior cingulate cortices [34]. Lesions of the right parietal lobe in rats interfere with spatial memory acquisition and consolidation [35]. Although no obvious cellular anomalies were observed in the hippocampus itself, it may be possible that the mild impact induced functional lesions whose resolutions were beyond the scope of measurements employed in this study. Indeed studies have shown that the hippocampus appears to be very sensitive to a wide variety of CNS insults [36-41].
In addition to the parietal cortices, “neuropathology” was observed as well in the amygdala and the piriform, perirhinal, endopiriform parietal and entorhinal cortices. The cytometric alterations were consistent with dormancy-like processes. It may be relevant that the very local forces from the pressure wave of ~3 to 20 kPa generated by the mechanical impact in this study would be within the range of adhesion forces (nN/µm2) between cells that trigger signalling molecules within the cell [42]. Some neurons were characterized by shrunken somas and nuclei as assessed through light microscopy (40×, 100×, 400×). Although areas of shrunken soma were observed bilaterally, the largest areas were most concentrated in the right hemisphere (under the impact site) both dorsally and ventrally. All of these areas play a crucial role in spatial memory. For example, in rats the entorhinal cortex plays a role in modulating attention to cues in the MWM paradigm [43]. The connection between the lateral entorhinal and the perirhinal cortices may provide access to prefrontal areas involved in attention [43,44]. The posterior parietal cortex is involved in visuospatial orientation [43,44].
Other researchers have hypothesized that the cognitive deficits may be due to heightened anxiety and stress levels in the animals produced by being exposed to a novel environment after the trauma [45,46]. After habituating to that environment (which our data shows takes on average 3 days), the CHI animals could perform similarly to the sham animals. Indeed studies with humans have shown that concussed patients performed poorly on the PASAT (paced auditory serial addition task) relative to controls when the information was presented at a fast rate. When the information was presented slowly the concussed and controlled groups performed similarly leading McMillian & Glucksman [45] and Shaw [46] to suggest that the patients’ cognitive deficits were due to abnormal elevations in stress and anxiety levels rather than due to residual organic lesions. Because concussion may result in cognitive slowing, it may be that presenting the PASAT at a slow rate allows the patient to become familiar with the material and assimilate it efficiently.
Another syndrome that has been observed in our model of traumatic brain injury is emotionality. In unpublished studies we have found that CHI female rats exhibited enhanced emotionality when exposed to a contextual fear condition paradigm after the trauma but not when they were exposed to it before the trauma. It may be that the trauma generates sensory-limbic hyperconnections that result in heightened emotional reactivity to normally neutral stimuli or context [47-51].
Pregnancy and rearing of offspring following a mechanical impact with no stunning or stunning of <30-sec interacted deleteriously. Relative to the stunned CHI and sham animals, this group displayed lengthy escape latencies. It seems that the neurophysiological processes associated with no stunning inhibited rats’ normal behaviour in the MWM after a CHI. Current results are consistent with our previous findings that the rats exhibiting no stunning after an impact lost as much weight as those that did display stunning after the impact. It may be relevant that in our clinical populations those patients who sustained an impact to the skull without suspension of consciousness developed significantly more epileptic-like experiences and symptoms that complicated their life difficulties during the subsequent three years than those whose were unconscious for more than 10 min.
5. Conclusion
Often individuals with mild CHI are misdiagnosed because many professionals employ criteria that brain injury requires a loss of consciousness or Glasgow Coma Scale scores of 10 or less. The results of our studies suggest these patients may actually sustain covert “brain damage” manifested by scattered shrunken neurons that without concomitant oedema would not be evident on MRI or CT scans. These people would be expected to display slowed information processing and the presence of post-traumatic stress disorders, post concussive syndrome, and personality changes. Our data suggest that individuals who sustain mechanical pressure waves to the skull and exhibit mild or no stunning should be accommodated with the same concern as those who displayed a conspicuous loss of consciousness.
REFERENCES
- D. R. Oppenheimer, “Microscopic Lesions in the Brain Following Head Injury,” Journal of Neurology and Neurosurgical Psychiatry, Vol. 31, No. 4, 1968, pp. 299-306. doi:10.1136/jnnp.31.4.299
- A. Peru, A. Beltramellow, V. Moro, L. Sattibaldi and G. Berlcchi, “Temporal and Permanent Signs of Interhemispheric Disconnection after Traumatic Brain Injury,” Neuropsychologica, Vol. 41, No. 5, 2003, pp. 634-643. doi:10.1016/S0028-3932(02)00203-8
- B. C. Albensi, “Models of Brain Injury and Alterations in Synaptic Plasticity,” Journal of Neuroscience Research, Vol. 65, No. 4, 2001, pp. 279-283. doi:10.1002/jnr.1151
- A. Finset, A. W. Anke, E. Hofft, K. D. Roaldson, J. Pillgram-Larson and J. K. Stranghelle, “Cognitive Performance in Multiple Trauma Patients 3 Years after Injury,” Psychosomatic Medicine, Vol. 61, No. 4, 1999, pp. 576- 583.
- R. J. Hamm, B. G. Lyeth, W. M. Jenkins, M. J. O’Donovan and B. R. Pike, “Selective Cognitive Impairment Following Traumatic Brain Injury in Rats,” Behavioural Brain Research, Vol. 59, No. 1-2, 1993, pp. 169-173. doi:10.1016/0166-4328(93)90164-L
- S. Marguilies, “The Postconcussion Syndrome after Mild Head Injury: Is Brain Damage over Diagnosed?” Journal of Clinical Neuroscience, Vol. 7, No. 5, 2002, pp. 400- 408. doi:10.1054/jocn.1999.0681
- T. W. McAlister, “Mild Traumatic Brain Injury and the Postconcussive Syndrome,” In: J. M. Silver, S. C. Yudofsky and S. C. Hales, Eds., Neuropsychiatry of Traumatic Brain Injury, American Psychiatric Press, New York, 1994, pp. 361-400.
- O. Zohar, G. V. Schreiber, J. P. Schwartz, P. G. Mullins and C. G. Pick, “Closed-Head Minimal Traumatic Brain Injury Produces Long-Term Cognitive Deficits in Mice,” Journal of Neuroscience, Vol. 118, No. 4, 2003, pp. 949- 955. doi:10.1016/S0306-4522(03)00048-4
- H. Miller, “Accident Neurosis,” British Medical Journal, Vol. 1, 1961, pp. 992-998. doi:10.1136/bmj.1.5231.992
- J. T. Povlishock and T. H. Colburn, “Morphopathological Change Associated with Mild Head Injury,” In: H. S. Levin, M. D. Eisenberg and A. L. Benton, Eds., Mild Head Injury, Oxford University Press, New York, 1989, pp. 37-53.
- G. Lynch and M. Baudry, “The Biochemistry of Memory: A New and Specific Hypothesis,” Science, Vol. 224, No. 4, 1984, pp. 1057-1063. doi:10.1126/science.6144182
- A. C. Conti, R. Raghupathi, J. Q. Trojanowski and T. K. McIntosh, “Experimental Brain Injury Induces Regionally Distinct Apoptosis during the Acute and Delayed PostTraumatic Period,” Journal of Neuroscience, Vol. 18, No. 4, 1998, pp. 5663-5672.
- A. A. Farooqui, S. E. Haunand and L. A. Horrocks, “Ischemia and Hypoxia: Basic Neurochemistry,” Raven Press, New York, 1993, pp. 867-883.
- C. Iadecola, “Bright and Dark Sides of Nitric Oxide in Ischemic Brain Injury,” Trends in the Neurosciences, Vol. 20, No. 3, 1997, pp. 132-139. doi:10.1016/S0166-2236(96)10074-6
- S. M. Rothman and J. W. Olney, “Excitotoxicity and the NMDA Receptor,” Trends in the Neurosciences, Vol. 10, No. 7, 1987, pp. 299-302. doi:10.1016/0166-2236(87)90177-9
- Y. Shapira, A. M. Lam, A. A. Artu, C. Eng and L. Sotow, “Ketamine Alters Calcium and Magnesium in Brain Tissue Following Experimental Head Trauma in Rats,” Journal of Cerebral Blood Flow and Metabolism, Vol. 13, 1993, pp. 962-968. doi:10.1038/jcbfm.1993.120
- A. H. Dickenson, “Plasticity: Implications for Opioid and Other Pharmacological Interventions in Specific Pain States,” Behavioral and Brain Sciences, Vol. 20, No. 3, 2000, pp. 392-403.
- A. Dray, L. Urban and A. Dickenson, “Pharmacology of Chronic Pain,” Trends in Pharmaceutical Sciences, Vol. 15, No. 6, 1994, pp. 190-197. doi:10.1016/0165-6147(94)90147-3
- M. A. Persinger, “Neuropsychologica Principia Brevita: An Application to Traumatic (Acquired) Brain Injury,” Psychological Reports, Vol. 77, No. 3, 1995, pp. 707-724. doi:10.2466/pr0.1995.77.3.707
- C. J. Woolf and T. P. Doubell, “The Pathophysiology of Chronic Pain-Increased Sensitivity to Low Threshold A Beta-Fibre Inputs,” Current Opinions in Neurobiology, Vol. 4, No. 4, 1994, pp. 525-534. doi:10.1016/0959-4388(94)90053-1
- B. E. McKay, W. E. Lado, L. J. Martin and N. M. Fournier, “Learning and Memory in Agmatine-Treated Rats,” Pharmacology, Biochemistry and Behavior, Vol. 72, No. 3, 2002, pp. 551-557. doi:10.1016/S0091-3057(02)00724-4
- K. R. Magnusson, B. Scruggs, J. Aniya, K. C. Wright, T. Ontl, Y. Xing and L. Bai, “Age-Related Deficits in Mice Performing Tasks in a Water Maze,” Behavioral Neuroscience, Vol. 117, No. 3, 2003, pp. 485-495. doi:10.1037/0735-7044.117.3.485
- W. E. Lado and M. A. Persinger, “Mechanical Impacts to the Skulls of Rats Produce Specific Deficits in Maze Performance and Weight Loss: Evidence for Apoptosis of Cortical Neurons and Implications for Clinical Neuropsychology,” Perceptual and Motor Skills, Vol. 97, No. 3, 2003, pp. 1115-1127.
- D. M. Geddes, M. C. LaPlaca and R. S. Cargill, “Susceptibility of Hippocampal Neurons to Mechanically Induced Injury,” Experimental Neurology, Vol. 184, 2003, pp. 420- 427. doi:10.1016/S0014-4886(03)00254-1
- Z. Cai, F. Xiao, B. Lee, I. A. Paul and P. G. Rhodes, “Prenatal Hypoxia-Ischemia Alters Expression and Activity of Nitric Oxide Synthase in the Young Brain and Causes Learning Deficits,” Brain Research Bulletin, Vol. 49, No. 5, 2000, pp. 359-365. doi:10.1016/S0361-9230(99)00076-3
- W. Fu, J. Lee, Z. Guo and M. P. Mattson, “Seizures and Tissue Injury Induce Teleomerase in Hippocampal Microglial Cells,” Experimental Neurology, Vol. 178, No. 2, 2002, pp. 294-300. doi:10.1006/exnr.2002.8030
- W. P. Gray, K. May and L. E. Sundstrom, “Seizure-Induced Dentate Neurogenesis Does Not Diminish with Age in Rats,” Neuroscience Letters, Vol. 330, 2002, pp. 235-238. doi:10.1016/S0304-3940(02)00810-8
- T. J. Shors, G. Meisegaes, A. Beylin, M. Zhao, T. Rydel and E. Gould, “Neurogenesis in the Adult Is Involved in the Formation of Trace Memories,” Nature, Vol. 410, 2001, pp. 372-376. doi:10.1038/35066584
- T. Shingo, C. Gregg, E. Enwere, H. Fujikawa, R. Hassam, C. Geary, J. C. Cross and S. Weiss, “Pregnancy-Stimulated Neurogenesis in the Adult Female Forebrain Mediated by Prolactin,” Science, Vol. 299, No. 5603, 2003, pp. 117-200. doi:10.1126/science.1076647
- H. Jeltsch, F. Betrand, C. Lazarus and J. C. Cassell, “Cognitive Performances and Locomotor Activity Following Dentate Granule Cell Damage in Rats: Role of Lesion Extent and Type of Memory Tests,” Neurobiology of Learning and Memory, Vol. 76, 2001, pp. 81-105. doi:10.1006/nlme.2000.3986
- G. F. Lafreniere, O. Peredery and M. A. Persinger, “Progressive Accumulation of Large Aggregates of Calcium Containing Polysaccharides and Basophilic Debris within Specific Thalamic Nuclei after Lithium/Pilocarpine Induced Seizures,” Brain Research Bulletin, Vol. 28, No. 5, 1992, pp. 825-830. doi:10.1016/0361-9230(92)90268-3
- F. R. Ferraro, “Cognitive Slowing after Closed Head Injury,” Brain and Cognition, Vol. 32, No. 3, 1996, pp. 429-440. doi:10.1006/brcg.1996.0075
- D. H. Lowenstein, M. J. Thomas, S. H. Smith and T. K. McIntosh, “Selective Vulnerability of Dentate Hilar Neurons Following Traumatic Brain Injury: A Potential Mechanistic Link between Head Injuries and Disorders of the Hippocampus,” Journal of Neuroscience, Vol. 12, No. 12, 1992, pp. 4846-4853.
- D. M. Barros, T. Mello Souza, T. De David, H. Choi, A. Aguzzoli, C. Madche, P. Ardenghi, J. H. Medina and I. Izquierdo, “Simultaneous Modulation of Retrieval by Dopaminergic D(1), Beta-Noradrenergic, Serotonergic 1-A and Cholinergic Muscarinic Receptors in Cortical Structures of the Rat,” Behavioral Brain Research, Vol. 124, 2001, pp. 1-7. doi:10.1016/S0166-4328(01)00208-X
- O. Piot-Grosjean, F. Wahl, O. Gobbo and M. Stutzmann, “Assessment of Sensorimotor and Cognitive Deficits Induced by Moderate Traumatic Brain Injury in the Right Parietal Cortex of the Rat,” Neurobiological Disorders, Vol. 8, No. 6, 2001, pp. 1082-1093. doi:10.1006/nbdi.2001.0450
- S. Hogg, D. J. Sanger and P. C. Moser, “Mild Traumatic Lesions of the Right Parietal Cortex in the Rat: Characterization of a Conditioning Freezing Deficit and Its Reversal by Dizoilpine,” Behavioral Brain Research, Vol. 93, 1998, pp. 157-165. doi:10.1016/S0166-4328(97)00145-9
- M. R. Foy, M. E. Stanton, S. Levin and R. F. Thompson, “Behavioural Stress Impairs Long-Term Potentiation in Rodent Hippocampus,” Behavioral Neural Biology, Vol. 48, No. 1, 1987, pp. 138-149.
- M. Maroun and G. Richter-Levin, “Exposure to Acute Stress Blocks the Induction of Long Term Potentiation of the Amygdala-Prefrontal Cortex Pathway in Vivo,” Journal of Neuroscience, Vol. 23, No. 11, 2003, pp. 4406- 4409.
- J. Wang, I. Akirav and G. Richter-Levin, “Short-Term Behavioural and Electrophysiological Consequences of Underwater Trauma,” Physiology and Behavior, Vol. 70, 2000, pp. 327-332. doi:10.1016/S0031-9384(00)00274-2
- L. Xu, R. Anwyl and M. J. Rowan, “Behavioural Stress Facilitates the Induction of Long-Term Depression in the Hippocampus,” Nature, Vol. 387, 1997, pp. 497-500. doi:10.1038/387497a0
- E. Moor, R. Kohen, R. J. Reiter and E. Shohami, “Closed Head Injury Increases Extracellular Levels of Antioxidants in Rat Hippocampus in Vivo: An Adaptive Mechanism,” Neuroscience Letters, Vol. 316, 2001, pp. 169-172. doi:10.1016/S0304-3940(01)02394-1
- A. D. Bershadsky, N. Q. Balaban and B. Geiger, “Adhesion-Dependent Cell Mechanosensitivity,” Annual Review of Cell and Developmental Biology, Vol. 19, 2003, pp. 677-695. doi:10.1146/annurev.cellbio.19.111301.153011
- C. J. P. Oswald, D. M. Bannerman, B. K. Yee, J. N. P. Rawlins, R. C. Honey and M. Good, “Entorhinal Cortex Lesions Disrupt Transition between the Use of Intra and Extra-Maze Cues for Navigation of the Water Maze,” Behavioral Neuroscience, Vol. 117, No. 3, 2003, pp. 588- 595. doi:10.1037/0735-7044.117.3.588
- R. D. Burwell, “The Parahippocampal Regions: Corticocortical Connectivity,” In: M. Witter, Ed., Annals of the New York Academy of Sciences, Vol. 911, 2000, pp. 25- 42.
- T. M. McMillan and E. E. Glucksman, “Neuropsychology of Moderate Head Injury,” Journal of Neurology and Neurosurgical Psychiatry, Vol. 50, 1987, pp. 393-397. doi:10.1136/jnnp.50.4.393
- N. A. Shaw, “The Neurophysiology of Concussion,” Progress in Neurobiology, Vol. 67, 2002, pp. 281-344. doi:10.1016/S0301-0082(02)00018-7
- R. Melzack, “From Gate to the Neuromatrix,” Pain, Vol. 6, 1999, pp. S121-S126. doi:10.1016/S0304-3959(99)00145-1
- D. M. Bear, “Temporal Lobe Epilepsy—A Syndrome of Sensory-Limbic Hyperconnection,” Cortex, Vol. 15, 1979, pp. 357-384.
- D. M. Bear and P. Fedio, “Quantitative Analysis of Interictal Behavior in Temporal Lobe Epilepsy,” Archives of Neurology, Vol. 34, No. 8, 1977, pp. 454-467. doi:10.1001/archneur.1977.00500200014003
- R. J. Sbordone and J. H. Jennison, “A Comparison of OBD-168 and MMPI to Assess the Emotional Adjustment of Traumatic Brain Injured Patients to Their Cognitive Deficits,” Clinical Neuropsychology, Vol. 5, 1983, pp. 87-88.
- W. E. Lado and M. A. Persinger, “Increased Conditioned Immobility and Weight Loss in Rats Following Mechanical Impacts to the Skull That Do Not Produce Loss of Consciousness,” Central European Journal of Biology Vol. 3, No. 4, 2008, pp. 422-430. doi:10.2478/s11535-008-0041-6
NOTES
*Corresponding author.

