Case Reports in Clinical Medicine
Vol.3 No.3(2014), Article ID:43508,6 pages DOI:10.4236/crcm.2014.33032
Severe Hyperphosphatemia Resulting in Acute Renal Failure and Ischemic Encephalopathy in a Patient with Infantile Leukemia
Atsuko Watanabe1,2*, Atushi Itano3, Takeshi Koga3, Ikuma Musha3, Michio Shimizu4, Ryuhei Tanaka2
1Department of Pediatrics, Kasumigaura Medical Center, Tuchiura, Japan
2Department of Pediatric oncology/Hematology, International medical center, Saitama Medical University, Hidaka, Japan
3Department of Pediatrics, Saitama Medical University Hospital, Moroyama, Japan
4Department of Diagnostic Pathology, International Medical Center, Saitama Medical University, Hidaka, Japan
Email: *atsuko-w@gf7.so-net.ne.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 2 February 2014; revised 28 February 2014; accepted 10 March 2014
ABSTRACT
Tumor lysis syndrome (TLS), hyperleukocytosis, and disseminated intravascular coagulation (DIC) are representative oncological emergencies that overlap mutually at the beginning of therapy for aggressive leukemia. Lately recombinant urate oxidase (rUO) enables to control uric acid level and its crystallization, the most frequent risk factor for clinical TLS; therefore, hyperphosphatemia appears to be the main risk in the rUO era. We here report an infantile leukemia patient who developed severe hyperphosphatemia, resulting in acute renal failure and ischemic encephalopathy. A 9-month-old female baby was adynamic with a bulging anterior fontanel, and was diagnosed as infantile acute lymphoblastic leukemia with a mixed lineage leukemia gene rearrangement. A laboratory examination revealed leukocytosis, bicytopenia, hyperuricemia, a prolonged prothrombin time, activated partial thromboplastin time, and elevated lactate dehydrogenase level. Soon after a reduced dose of prednisolone was administered, she developed hypoxia caused by systemic inflammatory response syndrome and heart failure. Her white blood cell count decreased sharply, leading to acute renal failure due to hyperphosphatemia, which required continuous hemodiafiltration for 48 hours. Although renal function subsequently recovered, severe ischemic encephalopathy remained. She achieved morphological remission once, however, relapsed and passed away soon after. We have to pay attention to the progression of hyperphosphatemia, hyperkakemia and DIC, although hyperuricemia was controlled using rUO. Changes in electrolyte levels must be continuously monitored, and TLS, DIC and/or hyperleukocytosis should be promptly managed especially in patients who are sensitive to therapy.
Keywords:Hyperphosphatemia; Tumor Lysis Syndrome; Hyperleukocytosis; Disseminated Intravascular Coagulation; Continuous Hemodiafiltration

1. Introduction
Tumor lysis syndrome (TLS), hyperleukocytosis, and disseminated intravascular coagulation (DIC) are lethal complications that typically occur at the beginning of therapy for aggressive leukemia.
TLS is characterized by hyperuricemia, hyperphosphatemia, hyperkalemia, and hypocalcemia due to the rapid destruction of a large number of malignant cells. These complications predispose the patient to clinical toxicities including acute renal failure (ARF), cardiac arrhythmia, seizures, and sudden death even worth. Although TLS commonly develops at the initiation of chemotherapy, it may also exist at diagnosis. The incidence of TLS secondary to hyperuricemia is expected to decrease with the increasing use of recombinant urate oxidase (rUO), while that normouricemic TLS may increase [1] .
Hyperleukocytosis leads to an increase in blood viscosity and is associated with the aggregation of leukemic cells in the microcirculation, resulting in brain hemorrhage and pulmonary infarction. Patients with acute lymphoblastic leukemia and a peripheral white blood cell count > 200,000/μL are at higher risk of such events [2] . Hyperleukocytosis for infants is initially managed by vigorous hydration and the exchange transfusion has been considered in some cases. However, volatile changes in blood pressure during exchange transfusion increase the risk of brain hemorrhage in infantile patients.
DIC in malignancies is characterized by the systemic intravascular activation of coagulation due to the rapid release of tissue factors from malignant cells, leading to the deposition of the fibrin polymers (i.e., thrombi) in the bloodstream. Thrombi that develop in blood vessels promote the secretion of tissue plasminogen activator by vascular endothelial cells, causing fibrinolysis-enhanced DIC followed by the appearance of hemorrhagic symptoms.
These conditions usually overlap at the onset of leukemia and start of initial treatments, and mutually form a vicious circle.
We here report a case of severe hyperphosphatemia that resulted in ARF and ischemic encephalopathy with the background of possible vascular endothelial disorder caused by TLS and DIC in an infantile leukemia patient.
2. Case Presentation
A 9-month-old female baby was taken to a general practitioner by her mother in November 2011 because she looked fretful and had not been doing well for 10 days. Since she was pale, adynamic, and had a bulging anterior fontanel, she was immediately transferred to a general hospital. A laboratory examination revealed leukocytosis and bicytopenia (white blood cells 560,000 /μL, hemoglobin 3.3 mg/dl, platelets 41,000/μL). Following an emergency transfusion, she transferred to our hospital the next day. She was drowsy and had tachypnea, tachycardia, a bulging anterior fontanel, puffy eyelids, and hepatosplenomegaly (4 cm and 7 cm below the costal margin, respectively). Bone marrow aspiration revealed hypercellular marrow with numerous leukemic blasts (Figure 1(a)) and the flow cytometry showed the blasts were positive for CD 19, 34, HLA-DR and negative for CD 10. Chromosomal analysis showed t (4; 11) (q21; q23), and genetic analysis using fluorescence in-situ hybridization (FISH) detected the split signal of the mixed lineage leukemia (MLL) gene (Figure 1(b)). We did not collect spinal fluid because of her poor condition. The final diagnosis was infantile acute lymphoblastic leukemia with an MLL gene rearrangement.
The results of the laboratory examination showed markedly elevated lactate dehydrogenase levels, a mildly prolonged prothrombin time, activated partial thromboplastin time, and fibrinogen was not within detectable levels. Although the patient’s serum uric acid levels were markedly elevated (11.6 mg/dl), creatinine, phosphate, and calcium levels were within normal limits. Her potassium level was under the lower limit (Table 1).
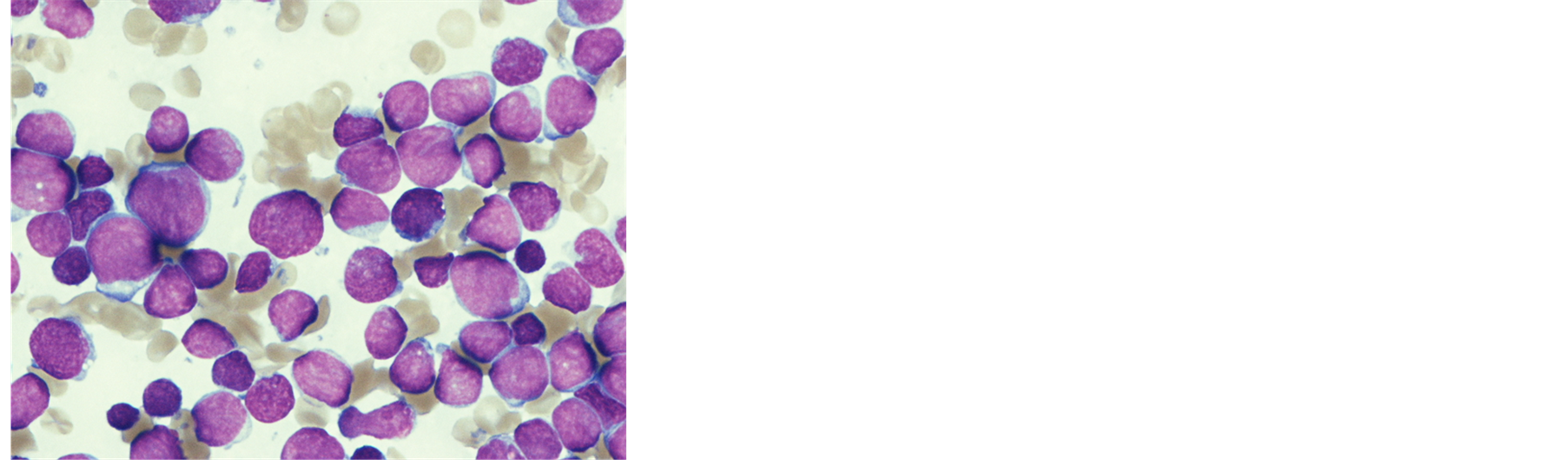 (a)
(a) (b)
(b)
Figure 1. (a) May-Grunwald-Giemsa stained bone marrow smear at diagnosis shows diffuse proliferation of small to medium-sized abnormal lymphocytes; (b) FISH study using MLL (11q23) Dual Color Break Apart Rearrangement Probe (Vysis®) reveals MLL split signals.
Table 1. Laboratory findings at the onset.
The patient was treated with 1.5 mg/day (0.18 mg/kg) rUO for laboratory TLS, 3 μg/kg/min of dopamine hydrochloride to prevent heart failure, and recombinant thrombomodulin for progressive DIC prior to chemotherapy.
A reduced dose of prednisolone (PSL: 5 mg/dose) was administered the day after her admission. Although these supportive care, she developed hypoxia due to systemic inflammatory response syndrome and heart failure. She was intubated immediately and managed with a respirator. A marked decrease in her leukocyte count and phosphate and creatinine levels rapidly increased to 13.6 and 1.52 mg/dl, respectively, three days after her admission (Figure 2). The clinical condition of the patient was getting deteriorated by the DIC and ARF associated with hyperphosphatemia. Continuous hemodiafiltration (CHDF) was started and PSL was transiently discontinued. Because both phosphate and creatinine levels were normalized after 48 hours, CHDF was discontinued and PSL at 30 mg/sqm/day was resumed. Intermittent tonic convulsion appeared at 8 days after her admission, and her brain computed tomography (CT) revealed an edematous, low-absorption area in the cerebrum and microhemorrhage in the cerebral parenchyma (Figure 3). Phenobarbital and glycerin was administered to control the brain pressure. The disappearance of peripheral blood blasts was noted ten days after her admission (total PSL administration period: 7 days), which suggested a favorable response to PSL. Unfortunately, extensive and irreversible cerebral dysfunction occurred, therefore we decided the intramedullary administration and vincristine which have nourotoxicity were avoided during induction therapy. A bone marrow aspiration following bone marrow recovery showed morphological remission 66 days after her admission. Single photon emission CT (SPECT) showed that cerebral cortex blood flow was markedly reduced (Figure 4). Because of her dismal prognosis, the best support of care was given instead of cytotoxic aggressive therapy with her parents’ consent. Peripheral blood blasts appeared again 73 days after her admission. Bone marrow failure gradually progressed, and the patient died 95 days after being admitted.
3. Discussion
One of the causes of hyperphosphatemia results from the rapid release of intracellular phosphate from malignant cells, which may contain up to four times that of normal cells [3] [4] . The kidneys are initially able to increase excretion of the phosphate and decrease its tubular reabsorption. However, the transport mechanism becomes saturated, which leads to an increase in serum phosphate levels. The development of hyperphosphatemia may be further exacerbated by acute renal insufficiency associated with other complications such as uric acid precipitation. The precipitation of calcium phosphate in renal tubules may lead to ARF, and secondary hypocalcemia can result in tetany, cardiac arrhythmias, seizures, and death. On the other hand, the direct influence of hyperphos-
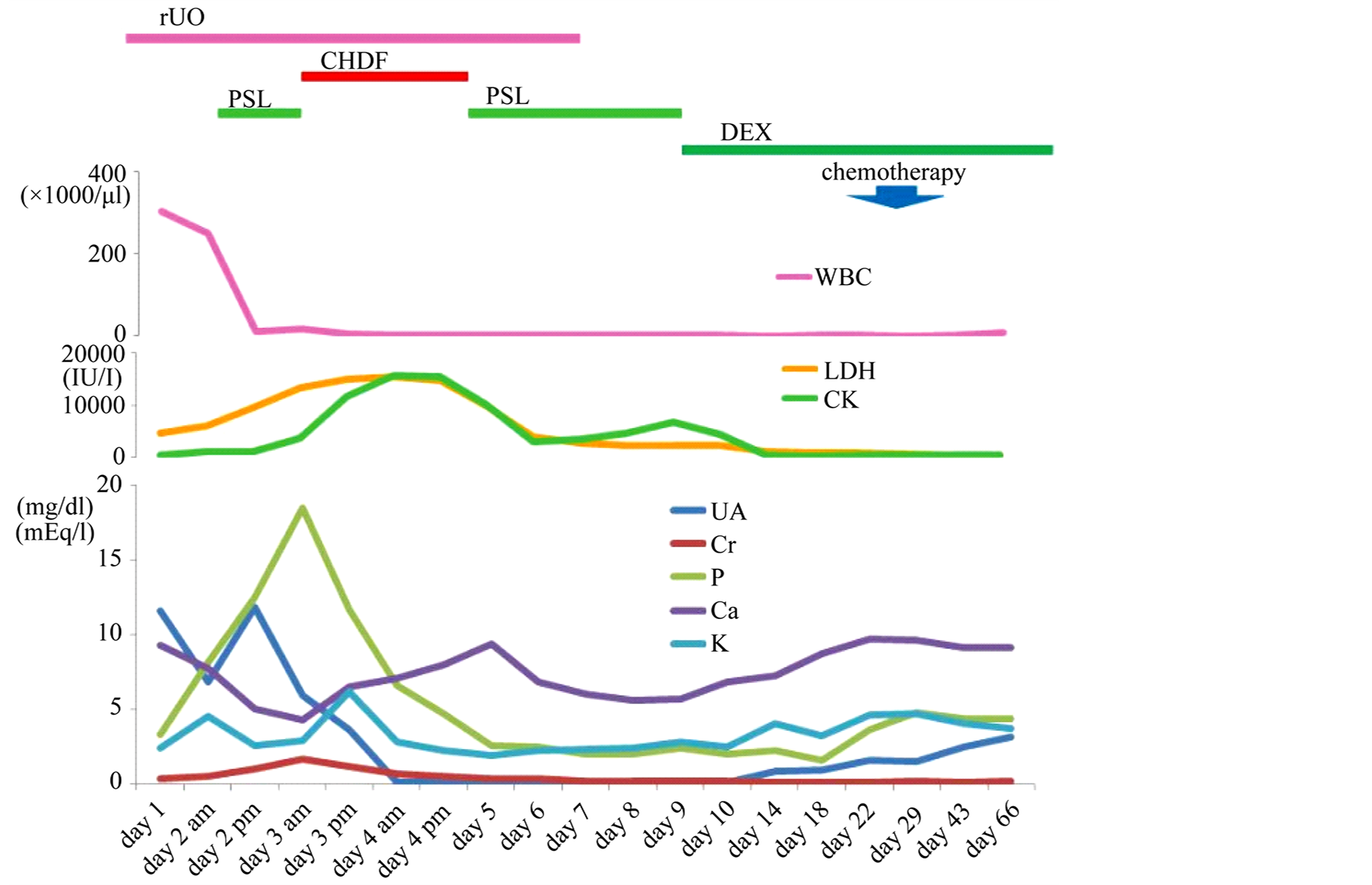
Figure 2. Clini+cal course of induction therapy.

Figure 3. Brain CT on 9 days after her admission revealed edematous cerebrum and microhemorrhage in the cerebral parenchyma.

Figure 4. Cerebral blood flow SPECT after morphological remission. Cerebral cortex blood flow was markedly reduced compared with cerebellum.
phatemia on the brain has not yet been established.
In the present case, the leukocyte count at onset exceeded 500,000/μl, and a poor general condition with bulging anterior fontanel were noted. Based on her poor condition at onset, we have speculated that the central infiltration may have been present, and the brain-cerebrospinal fluid barrier may have been affected. The patient may also have been underlying vascular endothelial disorder associated with TLS and DIC, and hyperviscosity syndrome-related microinfarction of the organs. Her leukocyte count decreased from 250,000 to 10,000 /μl in 12 hours, therefor hyperphosphatemia progressed much more rapidly than expected. Calcium phosphate or microthrombus formation may have impaired perfusion of her organs, including the brain and kidneys, lead to extensive cerebral edema ultimately cerebral atrophy.
Clinical TLS caused by hyperphosphatemia has rarely been reported; however, serum phosphate concentrations appear to be the main risk factor associated with TLS in the rUO era [1] . Intracellular phosphorous concentrations are high especially in Burkitt’s lymphoma and B-cell acute lymphoblastic leukemia, with the incidences of TLS being previously reported as 14.9% and 26.4%, respectively [5] . Darmon et al. reported that serum phosphate levels, the tumor burden as assessed by LHD and DIC, were associated with clinical TLS, and a 1 mmol increase in serum phosphate levels was associated with a 5-fold increase in the risk of clinical TLS [1] . Although hyperphosphatemia usually occurs after the start of anticancer therapy, there are also case reports of leukemia in which pretreatment with steroids alone caused hyperphosphatemia [6] . The onset of hyperphosphatemia must be considered in patients with leukemia who are hypersensitive to steroids.
Studies that did not use rUO reported that 10% - 20% of patients with hematological malignancies required hemodialysis; however, a more recent study found that the use of rUO decreased the frequency of hemodialysis to just a few percent [7] . Although rUO only affects uric acid levels, progression to hyperphosphatemia, hyperkalemia and DIC should be considered. Optimal supportive care to prevent the hypertphosphatemiarelated organ damage should be established.
4. Conclusion
We here reported a case of infantile leukemia that required hemodialysis because of ARF due to hyperphosphatemia. Although hyperuricemia was controlled using rUO, the patient could not be saved because hyperphosphatemia leads to irreversible and broad brain damage.
References
- Darmon, M., Vincent, F., Camous, L., Canet, E., Bonmati, C., Braun, T., Caillot, D., Cornillon, J., Dimicoli, S., Etienne, A., Galicier, A., Giraut, S., Hunault-Berger, M., Marolleau, J.P., Moreau, P., Raffoux, E., Recher, C., Thebaud, A., Theblemont, C. and Azoulay, E. (2013) Tumor Lysis Syndrome and Acute Kidney Injury in High-Risk Haematology Patients in Rasbricase Era. A Prospective Multicenter Study from the Groupe de Recherche en Reanimation Respiratoire et Onco-Hemetologique. British Journal of Haematology, 162, 489-497. http://dx.doi.org/10.1111/bjh.12415
- Rheingold, S.R. andLangen, B.J. (2006) Oncologic Emergency. In: Pizzo, P.A. and Poplack, D.G. Eds., Principle and Practice of Pediatric Oncology, 5th Edition, Lippincott Williams and Wilkins, Philadelphia, 1202-1230.
- Cario, M.S. and Bishop, M. (2004) Tmorlysis Syndrome: New Therapeutic Strategies and Classification. British Journal of Haematology, 127, 3-11. http://dx.doi.org/10.1111/j.1365-2141.2004.05094.x
- Tazi, I., Nafil, H., Elhoudzi, J., Mahmal, L. and Harif, M. (2011) Management of Pediatric Tumor Lysis Syndrome. Arab Journal of Nephrology and Transplantation, 4, 147-154. http://dx.doi.org/10.4314/ajnt.v4i3.71027
- Wilson, F.P. and Berns, J.S. (2012) Onco-Nephrology: Tumor Lysis Syndrome. Clinical Journal of American Society of Nephrology, 7, 1730-1739. http://dx.doi.org/10.2215/CJN.03150312
- Milionis, H.J. and Elisaf, M.S. (1999) Severe Life-Threatening Hyperphosphatemia Associated with Tumor Lysis in a Patient with Acute Lymphoblastic Leukemia. American Journal of Hematology, 60, 248-254. http://dx.doi.org/10.1002/(SICI)1096-8652(199903)60:3<252::AID-AJH23>3.0.CO;2-C
NOTES

*Corresponding author.


