International Journal of Medical Physics, Clinical Engineering and Radiation Oncology
Vol.06 No.02(2017), Article ID:76357,19 pages
10.4236/ijmpcero.2017.62016
Magnetic Resonance Perfusion in Brain Tumors: Comparison of Different Evaluation Approaches in Dual-Echo and Multi-Echo Techniques
Volker Hietschold1, Andrij Abramyuk2, Tareq Juratli3, Kerim Hakan Sitoci-Ficici3, Michael Laniado1, Jennifer Linn2
1Radiology, University Hospital Carl Gustav Carus, Dresden, Germany
2Neuroradiology, University Hospital Carl Gustav Carus, Dresden, Germany
3Neurosurgery, University Hospital Carl Gustav Carus, Dresden, Germany

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: March 6, 2017; Accepted: May 20, 2017; Published: May 23, 2017
ABSTRACT
Dynamic measurements of T1 shortening (dynamic contrast enhanced―DCE) as well as of 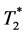 shortening (dynamic susceptibility contrast―DSC) as two separate measurement strategies are widely used to quantitatively describe tumor perfusion and vascularity. Dual-echo approaches allow for the simultaneous assessment of both effects. The extension to multi-echo sequences should inhere the advantage of improved signal-to-noise ratios and more precise sampling of the
shortening (dynamic susceptibility contrast―DSC) as two separate measurement strategies are widely used to quantitatively describe tumor perfusion and vascularity. Dual-echo approaches allow for the simultaneous assessment of both effects. The extension to multi-echo sequences should inhere the advantage of improved signal-to-noise ratios and more precise sampling of the 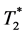 decay. The aim of our study is to investigate, if an extension of the dual-echo approach to the multi-echo approach allows for more stable quantitative determination of pharmacokinetic parameters in brain tumors. This study applies a multi-echo approach to obtain different estimations of a vascular input function and analyzes various combinations of vascular input functions and pharmacokinetic models. Perfusion measurements were performed with 52 consecutive patients with different brain tumors using a 10-echo gradient echo sequence. Our findings show that the extension to multi-echo sequences leads to an 11%-improvement of the Contrast-to-Noise ratio. Compared to other combinations, an application of Extended Tofts model using the
decay. The aim of our study is to investigate, if an extension of the dual-echo approach to the multi-echo approach allows for more stable quantitative determination of pharmacokinetic parameters in brain tumors. This study applies a multi-echo approach to obtain different estimations of a vascular input function and analyzes various combinations of vascular input functions and pharmacokinetic models. Perfusion measurements were performed with 52 consecutive patients with different brain tumors using a 10-echo gradient echo sequence. Our findings show that the extension to multi-echo sequences leads to an 11%-improvement of the Contrast-to-Noise ratio. Compared to other combinations, an application of Extended Tofts model using the 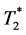 -related venous output function or an output function estimated in the tumor tissue enables the most reliable determination of perfusion parameters, reducing the reproducibility range by a factor of 1.2 to 10 for Ktrans and of 1.2 to 5.5 in the case of rBV calculation. Determination of Ktrans within repeated measurements within about 3 days results as most stable, if AIF from tumor pixels is used as vascular input function, meaning that the scatter is reduced by a factor of 1.2 compared to the next best VIF and by a factor of 10 compared to the worst of the tested approaches. In addition, this study shows that signal decomposition into two components with different Larmor frequencies might provide additional information concerning tissue composition of brain tumors.
-related venous output function or an output function estimated in the tumor tissue enables the most reliable determination of perfusion parameters, reducing the reproducibility range by a factor of 1.2 to 10 for Ktrans and of 1.2 to 5.5 in the case of rBV calculation. Determination of Ktrans within repeated measurements within about 3 days results as most stable, if AIF from tumor pixels is used as vascular input function, meaning that the scatter is reduced by a factor of 1.2 compared to the next best VIF and by a factor of 10 compared to the worst of the tested approaches. In addition, this study shows that signal decomposition into two components with different Larmor frequencies might provide additional information concerning tissue composition of brain tumors.
Keywords:
MRI, Brain Tumors, Perfusion

1. Introduction
Dynamic contrast enhanced (DCE) magnetic resonance imaging and dynamic susceptibility contrast (DSC) magnetic resonance imaging are widely used techniques for the assessment of tumor perfusion and tumor vascularity. In DCE- MRI, the measured T1 shortening is mainly caused by contrast medium (CM) distributed in the interstitial space, but partly also by intravascular CM [1] [2] . 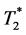 shortening measured in DSC-MRI is predominantly determined by CM in the capillary space. However, interstitial CM accumulation has some effect on
shortening measured in DSC-MRI is predominantly determined by CM in the capillary space. However, interstitial CM accumulation has some effect on  shortening as well.
shortening as well.
Since the diffusion of CM into the interstitial space is much slower compared to the passage of a CM bolus through the capillaries, T1-related dynamic measurements can be performed with a temporal resolution of about 45 seconds to 3 minutes [3] [4] . For this reason, spin echo or gradient echo volume sequences with short echo times (TE) can be used to minimize the influence of 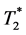 changes. In contrast,
changes. In contrast, 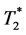 -related measurements usually are performed with EPI sequences to cover a sufficient volume with a temporal resolution of 1 to 2 seconds [5] . Here, long repetition times (TR) assure minimal influence of T1 shortening.
-related measurements usually are performed with EPI sequences to cover a sufficient volume with a temporal resolution of 1 to 2 seconds [5] . Here, long repetition times (TR) assure minimal influence of T1 shortening.
Using a dual-echo approach, temporal distribution of CM in both interstitial and capillary compartments can be assessed during only one CM administration [6] . The idea of simultaneous measurement and separation of T1 and 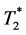 -related signal changes was firstly proposed in the 1990s [6] [7] [8] . The basic principle of this approach is the use of a gradient dual-echo sequence in order to simultaneously obtain images with equal T1 weighting but different
-related signal changes was firstly proposed in the 1990s [6] [7] [8] . The basic principle of this approach is the use of a gradient dual-echo sequence in order to simultaneously obtain images with equal T1 weighting but different 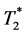 weighting. The structure of the gradient echo signal intensity formula allows the calculation of the time course of
weighting. The structure of the gradient echo signal intensity formula allows the calculation of the time course of 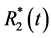 and at least of a hypothetical signal S0 for TE = 0 (being independent of
and at least of a hypothetical signal S0 for TE = 0 (being independent of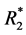 ).
).
However, to achieve a sufficient temporal resolution, this dual-echo approach is restricted to a few slices only. This major drawback might be solved by the application of more sophisticated sequence designs (e.g., parallel imaging, keyhole, segmented EPI etc.).
Another disadvantage of the dual-echo approach is the compromise between temporal resolution and signal to noise ratio (SNR) of the calculated images, which does not always produce sufficient accuracy of results.
In addition, the determination of a vascular input function (VIF) still is a critical step in MR perfusion measurements. In vessels apart from the tumor, the time course of the CM concentration is known to be different from that in the intratumoral vasculature in terms of temporal position (i.e., delay) as well as peak broadening (i.e., dispersion). This might result in systematic errors of estimated perfusion parameters [9] .
These problems can be diminished when applying the concept of simultaneous dynamic registration of T1 and 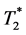 -related signals. We hypothesize that:
-related signals. We hypothesize that:
・ The multi-echo approach results in a significant better contrast-to-noise ratio (CNR) of the calculated 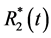 and S0(t) compared to the dual-echo approach. This is because the acquisition of more than two echoes within a given TR samples the
and S0(t) compared to the dual-echo approach. This is because the acquisition of more than two echoes within a given TR samples the 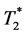 decay curve with more than two data points.
decay curve with more than two data points.
・ Simultaneous dynamic T1 and 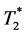 -based measurement enables estimation of a
-based measurement enables estimation of a  based VIF from the tumor pixels in addition to the DCE perfusion properties. Thus, location related VIF bias might be avoided.
based VIF from the tumor pixels in addition to the DCE perfusion properties. Thus, location related VIF bias might be avoided.
Moreover, there exists still some hidden potential of the multi-echo approach. Especially when we express the TE dependent signal intensity as a complex sum of two components with different Larmor frequencies (e.g., water and fat), we can model the signal dependence on TE more correctly with the multi-echo approach.
Therefore, the aim of this paper is to reveal the potential benefits of the multi-echo approach in dynamic imaging of brain tumors.
More precisely, we aim to assess alterations of CNR using higher numbers of echoes within a given TR. For this purpose, different correction methods for the calculation of S0(t) and 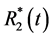 are compared.
are compared.
Additionally, different estimations of a vascular input function are compared quantitatively and various combinations of vascular input functions and pharmacokinetic models are tested.
Finally, assuming tumor tissue to consist of two components with different Larmor frequencies, we aim to obtain more information about the tumor structure by differentiating those substances. We think that at least rough spectroscopic differences are identifiable with multi-echo measurements. Therefore, we analyze the potential clinical value of the signal decomposition into two parts with different Larmor frequencies in a population of brain tumor patients.
2. Material and Methods
2.1. Patients
52 consecutive patients with different, newly diagnosed brain tumors were included in the study (25 glioblastoma, 12 meningioma, 12 metastasis, 3 lymphoma patients). For 21 patients, MRI examination was performed twice with a time interval of 1 to 4 days. 18 patients received preoperatively dexamethasone (10 glioblastoma, 2 meningeoma, 5 metastasis, 1 lymphoma patients). We excluded five patients who received an MRI examination twice and eight patients with single-time MRI examinations from further analysis due to non-plausible vascular input functions or movement artifacts.
2.2. MRI Measurements
MRI was performed on a 3 Tesla MR scanner (Siemens Magnetom Verio). In addition to the standard tumor protocol, a dynamic 10 echo FLASH sequence (TR = 44 ms, α = 70˚, TE = 1.2, 2.2, 3.1, 4.1, 5.5, 6.4, 8.0, 10.0, 12.0 and 13.0 ms, matrix 256 × 208 (acquisition matrix: 128 × 73), GRAPPA acceleration factor = 3) with a temporal resolution of 2 seconds was run during the application of CM (0.1 ml/kg body weight of Gadovist, (Bayer Schering) at a flow of 4 ml/s followed by 10 ml of saline). The sequence includes 3 slices of each 5 mm thickness. One slice was positioned in the neck region for measurement of an Arterial Input Function (AIF), two others were placed in the tumor region, identified with native T1 and T2 weighted images. After 10 of 60 dynamic scans, the CM was administered.
2.3. Multi-Echo Correction Methods (Calculation of 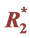 and S0)
and S0)
For calculation of 
The quality of the multi-echo corrections (i.e., the determination of S0 and 


Table 1. Sum of contrast-to-noise ratios over the ROIs in tumors, arteries and venes and sum of Euclidean distances between the median values of glioblastoma, meningioma, metastases and lymphoma.
2.4. Vascular Input Function
For estimation of the vascular input function, we implemented several time courses: S0(t) and 









The degree of nonlinearity in S0(CM concentration) was qualitatively eva- luated by comparing the curve shape of S0(t) with that of
2.5. Comparison of Pharmacokinetic Model―Vascular Input Function Combinations
The following models were combined with the above-described vascular input functions:
For S0,Tumor(t) we applied the Patlak and Extended Tofts models [10] [11] [12] [13] to calculate Ktrans, rBV and ve. The curve fits were performed with precalculated rBV as well as with rBV as free parameter.
For 

Additionally, we evaluated simple empiric parameters like maximum relative and maximum absolute enhancement in S0,Tumor(t) and maximum temporal increase of
Based on the perfusion parametric images, lacunarity parameters were calculated as published elsewhere [17] .
Figure 1. Examples for reproducibility diagrams: patient by patient course of parameters calculated from the first and second investigation (loglinearmultiecho correction). Left: relative enhancement of S0 in the tumor, normalized to contralateral tissue. Good reproducibility, mean normalized deviation


To identify model/VIF-combinations allowing the most stable perfusion parameter estimation, the reliability and reproducibility of the calculated parameters were evaluated visually and quantitatively. For the visual evaluation of each parameter, the values of the first and second investigation were drawn on a patient-by-patient basis (see Figure 1). For the quantitative assessment, a normalized mean difference between the consecutive MRI sessions was calculated to be used as reproducibility parameter

(n = number of patients, x1,i or x2,i = parameter x for patient i at first or second measurement resp.). This parameter normalizes the mean of difference of measures between the two time points to the median of the corresponding parameter and defines in this way a relative measure of stability of that parameter. For plausibility reasons, 
2.6. Data Analysis
Statistical analysis was done using Excel with user functions written in VBA (Visual Basic for Applications, Microsoft Corporation). The image analysis was performed with a program written in IDL (“Interactive Data Language”, Exelis Visual Information Solutions Inc.).
3. Results
3.1. Comparison of Multi-Echo Correction Methods (Calculations of 
The CNR values averaged over all measurements for 
Most reliable pharmacokinetic parameters occurred to be Ktrans and rBV. In Table 2, their reproducibility is shown depending on different models (Tofts, Patlak or fit to a Gaussian or Gama Variate peak) and VIF selected for calculation.
3.2. Vascular Input Function
In general, the overall signal intensity in the slice aimed for the AIF estimation was significantly lower than in the tumor covering slice(s).
For all kinds of multi-echo correction, the CNR of the VOF was better than that of the AIF by a factor of 1.75 to 3.5, where the superior sagittal sinus allowed most plausible curves. For the preferred log-linear correction these factors amount to 2.96 for S0 and 2.10 for 

In addition, high reproducibility of perfusion parameters was achieved by using vascular input function from 
3.3. Comparison of Pharmacokinetic Model―Vascular Input Function Combinations
The frequency of occurrence of the different models and vascular input functions in the list of the 30 best model-VIF combinations is summarized in Table 3. In this summary, we excluded the lacunarity parameters for reasons of plausibility (Apparently good lacunarity parameters mostly were based on parameter images calculated with bad performing VIFs. Hence, their information content probably consists on the tumor shape rather than on the inhomogeneity
Table 2. Reproducibility parameter 
Table 3. Percentage of models and vascular input functions in the list of 30 best parameters relative to the number of their occurrence in the list of all calculations performed. Middle column: plain parameters. Right column: parameters normalized to healthy tissue. Fractal parameters are not included. The type “---” refers to model free simple parameters (like maximum enhancement e.g.) or parameters which were calculated independent of a vascular input function resp.
properties). The most reliable calculations are based on the Extended Toftsmodel, Gaussian fit and model-free calculations (like maximum enhancement e.g., which showed minimum 
The results of IDT calculations are rather dissatisfactory. None of the IDT- based parameters was found either in the 30 best normalized or the best non- normalized parameters.
3.4. Signal Decomposition
The lowest mean “fat-to-water” signal ratio was found for malignant glioma and the highest mean for meningioma. Besides this, higher “fat-to-water” signal ratios were detected in gliomas after dexamethasone application, compared to those without such medication. But due to the small sample size, these differences were not statistically significant.
4. Discussion
Dual-echo perfusion measurements have already been proposed in the 1990s. They allow calculation of the time course of 

Compared to the dual-echo approach, the multi-echo approach that is proposed in this paper allows improvements concerning signal-to-noise ratio and estimation of vascular input functions. Furthermore, it allows at least rough estimations of the proportion of components with different Larmor frequencies.
4.1. Comparison of Multi-Echo Correction Methods (Calculation of 
In our study, the loglinear curve fit was found to suit best among all multi-echo correction algorithms tested. Remarkable, loglinear correction showed better results than the exponential one. From the physical point of view, however, the exponential fits correspond to the data more exactly than fits on logarithmized signal intensities (by taking the logarithms, the statistical uncertainty of higher values is compressed. Thus, linear fitting of logarithms overweighs the low-in- tensity data points corresponding to the late echoes). Otherwise, for numerical reasons, they are less stable compared to the linear fit on the logarithmized data. In other words, if the weighting of data points influences the curve fit to an important way, then the exponential fits should give better results than the loglinear ones. But, if numerical robustness is the essential objective of the multi- echo correction method, then the loglinear model should work better than the exponential ones. Therefore, in our data the stability aspect underwent more influence than the biased weighting of the data points.
We also found the biexponential fat-water models resulting in higher Euclidean distances between tumor entities and CNR―at least compared to the corresponding monoexponential models. But we want to stress, that models based on loglinear and monoexponential calculations are actually identical, the only differences are the different weighting of data points and the different numeric stability.
An extended discussion of the biexponential fat-water model can be found in the “signal decomposition” paragraph.
Testing simple dual-echo corrections, Euclidean distances between tumor entities based on Ktrans and rBV, appeared to be surprisingly good for the first and second echo only ? in contrast to the CNR evaluation. This, however, seems to be a statistical artifact due to the high data noise and the small sample size. Obtained parameters were not significantly biased compared to the ones from the other correction methods, but they were noisier.
4.2. Vascular Input Function
A prerequisite for quantitative perfusion measurements is the determination of VIF. Several approaches of manual, semi-automatic or automatic VIF determinations for brain perfusion measurements are available. There are for example measurements of a special slice caudal to the tumor for deriving of an AIF [22] , automatic selection of pixels within the brain with highest and/or fastest enhancement [23] or with clustering algorithms [24] , reference to the venous output function [25] or VIF estimation from averaging over several patients [13] [26] .
In the present study, we drew the ROIs manually in order to control their influence on the time courses of S0 and


In our data, nonlinearity of S0 (CM concentration) as well as biased curve shapes in the venous ROIs were less noticeable than in the arterial ones. This subjective impression was confirmed by CNR analysis as well as by rating of perfusion parameters with different vascular input functions. According to Yuan [25] , the advantage of venous time courses can be explained by lower flow velocity as well as more steady flow in veins compared to arteries. Additionally, in their work, VIFs derived from veins occurred to be less dependent on the selected slice than that derived from arteries.
Earlier studies [27] [28] demonstrated the crucial influence of the temporal shift of vascular input function on the estimation of perfusion parameters. Applying multi-echo sequences, this problem can be avoided by the measurement of 

In addition to the correct position on the time axis, 
However, due to downscaling by the volume fraction of capillaries in the tumor voxels, 
4.3. Comparison of Pharmacokinetic Model―Vascular Input Function Combinations
Although models with fewer numbers of free parameters like the Patlak approach are supposed to be more stable, we found better reproducibility with the Tofts model. This is a contradictory finding to previously published data [26] [29] . In [30] , owing to more fitting parameters, the two-compartment exchange model occurred to give less stable results at given acquisition duration compared to the Tofts and Extended Tofts models as well. The better reproducibility found in our data applying the Extended Tofts model might be explained by the fact, that in contrary to the Extended Tofts, in Patlak model one or two boundaries on the time axis (depending on the implementation) have to be chosen. The uncertainty during manual selection of these boundaries might overcompensate the a-priori better stability of fits to model functions with fewer degrees of freedom. It has to be evaluated, to what degree automatic determination of the upper (later) boundary based on statistical methods could reduce this uncertainty.
Calculations based on the Indicator Dilution Theory lead to quite instable results. This could be caused by the limited influence of smaller, even good vascularized tumors on the Mean Transit Time (MTT) of the CM bolus due to the comparatively short pathway of the blood through the tumor. Another reason could be the less stable rBV estimation by means of the fit of a Gamma Variate Function compared to the well performing Gaussian fit.
Diagnostics based on pharmacological parameters is one way to achieve comparability of results between different sites. Another way to reach this aim is standardization of measurement conditions allowing for comparison of simple model free parameters. In MR mammography, a qualitative description of the time course of contrast enhancement under mildly standardized measurement conditions occurred to meet the clinical needs [31] . In our data, among all parameters normalized to healthy tissue, the best reproducibility was obtained for the signal increase extrapolated to TE = 0, which is influenced by vessel permeability, perfusion and extravascular extracellular space.
4.4. Signal Decomposition
The assumption for brain and tumor tissue to consist of two components with different Larmor frequency appears to improve the quality of curve fits of signal intensities such as a function of TE, compared to monoexponential fits without differentiation between fat and water signals. This is clearly seen in the evaluation of CNRs, but does not transfer to better accuracy of perfusion parameter based differentiation between tumor entities (Table 1). However, we demonstrated decreased lower-frequency-compartment (with a chemical shift in the range of fatty tissue) in gliomas after pretreating with dexamethasone compared to the untreated group (Table 4, Figure 2). Thus, the multi-echo perfusion approach has the potential to provide additional physiological information beyond the description of blood supply. The background of these findings has to be analyzed comparing such measurements with in-vivo MR spectroscopy.
4.5. Limitations
Some limitations of this study are to be mentioned:
First of all, our data was not compared to any reference method. To our knowledge, no real “gold standard” for the perfusion measures exists. Hence, for the evaluation of the reliability of our data, we introduced the reproducibility parameter defined in Equation (1).
Second, evaluations were performed with in-house software. Although carefully tested during the development process, there remains a higher risk of incorrect calculations or numeric instabilities compared to commercial and/or widespread software solutions. No reference calculations with standard software were performed.
Finally, in the literature, DCE MR studies of brain tumors usually are performed for about 5 minutes at a temporal resolution of 5.3 s [32] resulting in about 55 time points. In our study, we needed a temporal resolution of 2 seconds or higher for the DSC evaluations. At the same time, this denser sampling of 60 time points within two minutes should at least partly compensate the lack of very late data points needed for determination of Ktrans and ve or k2 resp.
5. Summary
The extension of the well-known dual-echo perfusion measurement to multi- echo sequence leads to the advantage of improved signal-to-noise ratios. The most reliable determinations of perfusion parameters (especially Ktrans and rBV) were achieved with Extended Tofts model applying the 
Temporal distribution of contrast concentration within the tumor vessel bed from multi-echo data seems to be advantageous for the estimation of a vascular
Table 4. Mean values of fat to water signal ratios for glioma without vs. with pretreatment with dexamethasone.
Figure 2. Box plot for fat-water ratios estimated with Equation (8).
input function compared to potentially dispersed and time-shifted functions derived from arterial or venous signals.
As potential added value, multi-echo FLASH MRI allowing signal decomposition into two components with different Larmor frequencies might provide additional information concerning tissue composition of brain tumors. The clinical value of these findings and particularly the exact biochemical composition of the so-called “fat” component are fruitful areas for of future research.
Acknowledgements
We gratefully thank Nadine Hietschold for her helpful recommendations and linguistic support during the writing of this manuscript.
Cite this paper
Hietschold, V., Abramyuk, A., Juratli, T., Sitoci-Ficici, K.H., Laniado, M. and Linn, J. (2017) Magnetic Resonance Perfusion in Brain Tumors: Com- parison of Different Evaluation Approaches in Dual-Echo and Multi-Echo Techniques. International Journal of Medical Physics, Clinical Engineering and Radiation Onco- logy, 6, 174-192. https://doi.org/10.4236/ijmpcero.2017.62016
References
- 1. Rosen, B.R., Belliveau, J.W., Vevea, J.M. and Brady, T.J. (1990) Perfusion Imaging with NMR Contrast Agents. Magnetic Resonance in Medicine, 14, 249-265.
https://doi.org/10.1002/mrm.1910140211 - 2. Hietschold, V. (2004) Aspekte einer quantitativen Gewebecharakterisierung mittels Kontrastmitteldynamik in der Kernspintomographie. Cuvillier, Göttingen.
- 3. El Khouli, R.H., Macura, K.J., Barker, P.B., Habba, M.R., Jacobs, M.A. and Bluemke, D.A. (2009) Relationship of Temporal Resolution to Diagnostic Performance for Dynamic Contrast Enhanced MRI of the Breast. Journal of Magnetic Resonance Imaging, 30, 999-1004.
https://doi.org/10.1002/jmri.21947 - 4. Gutierrez, R.L., Strigel, R.M., Partridge, S.C., DeMartini, W.B., Eby, P.R., Stone, K.M., Peacock, S. and Lehman, C.D. (2012) Dynamic Breast MRI: Does Lower Temporal Resolution Negatively Affect Clinical Kinetic Analysis? American Journal of Roentgenology, 199, 703-708.
https://doi.org/10.2214/AJR.11.7836 - 5. Grøvik, E., Bjørnerud, A., Storås, T.H. and Gjesdal, K.I. (2014) Split Dynamic MRI: Single Bolus High Spatial-Temporal Resolution and Multi Contrast Evaluation of Breast Lesions. Journal of Magnetic Resonance Imaging, 39, 673-682.
https://doi.org/10.1002/jmri.24206 - 6. Miyati, T., Banno, T., Mase, M., Kasai, H., Shundo, H., Imazawa, M. and Ohba, S. (1997) Dual Dynamic Contrast-Enhanced MR Imaging. Journal of Magnetic Resonance Imaging, 7, 230-235.
https://doi.org/10.1002/jmri.1880070136 - 7. Hietschold, V., Klengel, S. and Köhler, K. (1993) Simultaneous Dynamic Measurement of Tissue Contrast Enhancement and Perfusion at 0.5 Tesla: Method and Post-processing. SMRM 11th Annual Meeting, New York, 16-20 August 1993, Book of Abstracts 619.
- 8. Heiland, S., Benner, T., Rempp, K., Reith, W., Forsting, M. and Sartor, K. (1997) Ein Verfahren zur simultanen Beurteilung der Hämodynamik und der dynamischen Kontrastmittelanreicherung bei zerebralen Läsionen mit gestörter Blut-Hirn-Schranke. Fortschr Röntgenstr, 166, S137.
- 9. Calamante, F. (2013) Arterial Input Function in Perfusion MRI: A Comprehensive Review. Progress in Nuclear Magnetic Resonance Spectroscopy, 74, 1-32.
https://doi.org/10.1016/j.pnmrs.2013.04.002 - 10. Patlak, C.S., Blasberg, R.G. and Fenstermacher, J.D. (1983) Graphical Evaluation of Blood-to-Brain Transfer Constants from Multiple-Time Uptake Data. Journal of Cerebral Blood Flow and Metabolism, 3, 1-7.
https://doi.org/10.1038/jcbfm.1983.1 - 11. Tofts, P.S. (1997) Modeling Tracer Kinetics in Dynamic Gd-DTPA Imaging. JMR, 7, 91-101.
https://doi.org/10.1002/jmri.1880070113 - 12. Tofts, P.S., Brix, G., Buckley, D.L., Evelhoch, J.L., Henderson, E., Knopp, M.V., Larsson, H.B.W., Lee, T.Y., Mayr, N.A., Parker, G.J.M., Port, R.E., Taylor, J. and Weisskoff, R.M. (1999) Estimating Kinetic Parameters From Dynamic Contrast-Enhanced T1-Weighted MRI of a Diffusable Tracer: Standardized Quantities and Sym-bols. Journal of Magnetic Resonance Imaging, 10, 223-232.
https://doi.org/10.1002/(SICI)1522-2586(199909)10:3<223::AID-JMRI2>3.0.CO;2-S - 13. Bergamino, M., Bonzano, L., Levrero, F., Mancardi, G.L. and Roccatagliata, L. (2014) A Review of Technical Aspects of T1-Weighted Dynamic Contrast-Enhanced Magnetic Resonance Imaging (DCE-MRI) in Human Brain Tumors. Physica Medica, 30, 635-643.
https://doi.org/10.1016/j.ejmp.2014.04.005 - 14. Meier, P. and Zierler, K.L. (1954) On the Theory of the Indicator-Dilution Method for Measurement of Blood Flow and Volume. Journal of Applied Physiology, 6, 731-744.
- 15. Zierler, K.L. (1962) Theoretical Basis of Indicator-Dilution Methods for Measuring Flow and Volume. Circulation Research, 10, 393-407.
https://doi.org/10.1161/01.RES.10.3.393 - 16. Clough, A.V., Manuel, A.J., Haworth, S.T. and Dawson, C.A. (2000) Application of Indicator Dilution Theory to Time-Density Curves Obtained from Dynamic Contrast Images. Proc. SPIE 3978, Medical Imaging 2000: Physiology and Function from Multidimensional Images, Location, 20 April 2000, 457.
- 17. Abramyuk, A., Hietschold, V., Appold, S., von Kummer, R. and Abolmaali, N. (2015) Radiochemotherapy-Induced Changes of Tumour Vascularity and Blood Supply Estimated by Dynamic Contrast-Enhanced CT and Fractal Analysis in Malignant Head and Neck Tumours. The British Journal of Radiology, 88, 20140412.
https://doi.org/10.1259/bjr.20140412 - 18. Yoruk, U., Saranathan, M., Loening, A.M., Hargreaves, B.A. and Vasanawa, S.S. (2016) High Temporal Resolution Dynamic MRI and Arterial Input Function for Assessment of GFR in Pediatric Subjects. Magnetic Resonance in Medicine, 75, 1301-1311.
https://doi.org/10.1002/mrm.25731 - 19. Jelescu, I.O., Leppert, I.R., Narayanan, S., Araújo, D., Arnold, D.L. and Pike, G.B. (2011) Dual-Temporal Resolution Dynamic Contrast-Enhanced MRI Protocol for Blood-Brain Barrier Permeability Measurement in Enhancing Multiple Sclerosis Lesions. Journal of Magnetic Resonance Imaging, 33, 1291-1300.
https://doi.org/10.1002/jmri.22565 - 20. Pierre, E.Y., Ma, D., Chen, Y., Badve, C. and Griswold, M.A. (2016) Multiscale Reconstruction for MR Fingerprinting. Magnetic Resonance in Medicine, 75, 2481-2492.
https://doi.org/10.1002/mrm.25776 - 21. Stokes, A.M. and Quarles, C.C. (2016) A Simplified Spin and Gradient Echo Approach for Brain Tumor Perfusion Imaging. Magnetic Resonance in Medicine, 75, 356-362.
https://doi.org/10.1002/mrm.25591 - 22. Rijpkema, M., Kaanders, J.H.A.M., Joosten, F.B.M., van der Kogel, A.J. and Heerschap, A. (2001) Method for Quantitative Mapping of Dynamic MRI Contrast Agent Uptake in Human Tumors. JMRI, 14, 457-463.
https://doi.org/10.1002/jmri.1207 - 23. Chen, J., Yao, J. and Thomasson, D. (2008) Automatic Determination of Arterial Input Function for Dynamic Contrast Enhanced MRI in Tumor Assessment. Medical Image Computing and Computer-Assisted Intervention, 11, 594-601.
https://doi.org/10.1007/978-3-540-85988-8_71 - 24. Yin, J., Yang, J. and Guo, Q. (2015) Automatic Determination of the Arterial Input Function in Dynamic Susceptibility Contrast MRI: Comparison of Different Reproducible Clustering Algorithms. Neuroradiology, 57, 535-543.
https://doi.org/10.1007/s00234-015-1493-9 - 25. Yuan, J., Chow, S.K.K., Zhang, Q., Yeung, D.K.W., Ahuja, A.T. and King, A.D. (2013) The Use of Dynamic Tracer Concentration in Veins for Quantitative DCE-MRI Kinetic Analysis in Head and Neck. PLoS ONE, 8, e59885.
https://doi.org/10.1371/journal.pone.0059885 - 26. Shukla-Dave, A., Lee, N., Stambuk, H., Wang, Y., Huang, W., Thaler, H.T., Patel, S.G., Shah, J.P. and Koutcher, J.A. (2009) Average Arterial Input Function for Quantitative Dynamic Contrast Enhanced Magnetic Resonance Imaging of Neck Nodal Metastases. BMC Medical Physics, 9, 4.
https://doi.org/10.1186/1756-6649-9-4 - 27. Natsume, T., Ishida, M., Kitagawa, K., Nagata, M., Sakuma, H. and Ichihara, T. (2015) Theoretical Considerations in Measurement of Time Discrepancies between Input and Myocardial Time-Signal Intensity Curves in Estimates of Regional Myocardial Perfusion with First-Pass Contrast-Enhanced MRI. Magnetic Resonance in Medicine, 33, 1059-1065.
https://doi.org/10.1016/j.mri.2015.06.015 - 28. Hietschold, V., Gohl, P., Abramyuk, A., Koch, A., Laniado, M. and Abolmaali, N. (2007) Evaluation of CT Perfusion Results from Human Tumors: A Comparison of Four Different Two-Compartment Models. Radiological Society of North America, Scientific Assembly and Annual Meeting, Chicago, 25 November-30 November 2007. http://archive.rsna.org/2007/5003088.html
- 29. Gaens, M.E., Backes, W.H., Rozel, S., Lipperts, M., Sanders, S.N., Jaspers, K., Cleutjens, J.P.M., Sluimer, J.C., Heeneman, S., Daemen, M.J.A.P., Welten, R.J.T.J., Daemen, J.W.H., Wildberger, J.E., Kwee, R.M. and Kooi, M.E. (2013) Dynamic Contrast-Enhanced MR Imaging of Carotid Atherosclerotic Plaque: Model Selection, Reproducibility, and Validation. Radiology, 266, 271-279.
https://doi.org/10.1148/radiol.12120499 - 30. Hindel, S., Söhner, A., Maaß, M., Sauerwein, W., Baba, H.A., Kramer, M. and Lüdemann, L. (2017) Validation of Interstitial Fractional Volume Quantification by Using Dynamic Contrast-Enhanced Magnetic Resonance Imaging in Porcine Skeletal Muscles. Investigative Radiology, 52, 66-73.
https://doi.org/10.1097/RLI.0000000000000309 - 31. Neubauer, H., Li, M., Kuehne-Heid, R., Schneider, A. and Kaiser, W.A. (2003) High Grade and Non-High Grade Ductal Carcinoma in Situ on Dynamic MR Mammography: Characteristic Findings for Signal Increase and Morphological Pattern of Enhancement. The British Journal of Radiology, 76, 3-12.
https://doi.org/10.1259/bjr/14883856 - 32. Heye, A.K., Culling, R.D., Valdez Hernandez, M., Trippleton, M.J. and Wardlaw, J.M. (2014) Assessment of Blood-Brain Barrier Disruption Using Dynamic Contrast-Enhanced MRI. A Systematic Review. NeuroImage: Clinical, 6, 262-274.
https://doi.org/10.1016/j.nicl.2014.09.002 - 33. Wang, H.Z., Riederer, S.J. and Lee, J.N. (1987) Optimizing the Precision in T1 Relaxation Estimation Using Limited Flip Angles. Magnetic Resonance in Medicine, 5, 399-416.
https://doi.org/10.1002/mrm.1910050502 - 34. Dixon, W.T. (1984) Simple Proton Spectroscopic Imaging. Radiology, 153, 189-194.
https://doi.org/10.1148/radiology.153.1.6089263 - 35. Glover, G.H. (1991) Multipoint Dixon Technique for Water and Fat Proton and Susceptibility Imaging. Journal of Magnetic Resonance Imaging, 1, 521-530.
https://doi.org/10.1002/jmri.1880010504 - 36. Ma, J. (2008) Dixon Techniques for Water and Fat Imaging. JMRI, 28, 543-558.
https://doi.org/10.1002/jmri.21492 - 37. Hernando, D., Kramer, J.H. and Reeder, S.B. (2013) Multipeak Fat-Corrected Complex R2* Relaxometry: Theory, Optimization, and Clinical Validation. Magnetic Resonance in Medicine, 70, 1319-1331.
https://doi.org/10.1002/mrm.24593
NOTES
1The formal parameter “rBV” as well as Ktrans in the Extended Tofts and Patlak models have to be divided by rBV―meaning either extracting the root of the fit parameter “rBV” or applying a rBV determined by another vascular input function (e.g. the venous
Appendix: Calculation of 
According to Wang et al. [33] , for gradient echo sequences the signal intensity is given as

For T2* = TR it simplifies to the commonly used form

In principle, this simplification condition is not fulfilled for all data points in our experiments. However, in the relevant ranges of T1 and





with i = [1, 2] or i = [1, 9] (smallest or largest difference in echo times), by linear fit of 10 logarithmic signal intensities

with 

with four or all ten echoes (i = [0, 1, 4, 9] or i = [0, ×××, 9]).
In addition, according to Dixon [34] [35] [36] a signal decomposition to two components with different Larmor frequency (here called “water” and “fat” signals (W(T1) and F(T1) resp.) based on the phase differences for these components for every echo time was performed

(i = [0, 1, 4, 9] or i = [0, ×××, 9]) with

(Df ? frequency difference for the water vs. fat spins which is given by the water Larmorfrequency times the fat-water chemical shift). From Equation (8) (which is the adaption of the formula given in [35] to real numbers eliminating the influence of additional phase shifts with only slight noise penalty [37] ), a fat to water signal ratio F/W can be derived.
In the selection of the four echoes for Equations ((7) and (8)) a more or less equal signal difference between consecutive echoes for characteristic 
In the dependence of the signal intensity on TE, noise was neglected even for long TE and high CM concentration. In previous evaluations we could show, that noise was small compared to the signal, even at peak 
Submit or recommend next manuscript to SCIRP and we will provide best service for you:
Accepting pre-submission inquiries through Email, Facebook, LinkedIn, Twitter, etc.
A wide selection of journals (inclusive of 9 subjects, more than 200 journals)
Providing 24-hour high-quality service
User-friendly online submission system
Fair and swift peer-review system
Efficient typesetting and proofreading procedure
Display of the result of downloads and visits, as well as the number of cited articles
Maximum dissemination of your research work
Submit your manuscript at: http://papersubmission.scirp.org/
Or contact ijmpcero@scirp.org






