American Journal of Plant Sciences
Vol.5 No.14(2014), Article
ID:47555,10
pages
DOI:10.4236/ajps.2014.514232
Growth and Biomass Allocation of Muhlenbergia schreberi
Pedro Valério Dutra de Moraes1, Patricia Rossi1, Willian W. Witt2, Luis Eduardo Panozzo3
1Federal Technologic University of Paraná, UTFPR, Dois Vizinhos, Brazil
2University of Kentucky, Lexington, USA
3Federal University of Pelotas, Pelotas, Brazil
Email: pvdmoraes@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 18 May 2014; revised 15 June 2014; accepted 29 June 2014
ABSTRACT
The main goal of this research was to evaluate the growth of nimblewill (Muhlenbergia schreberi), an emerging threat to forage grass. Our initial experiment was arranged in a completely randomized design, with 17 treatments (i.e., weeks) and 4 repetitions. Seventeen growth evaluations (i.e., treatments) were carried out every 7 days, totaling a 119-day cycle, followed by an analysis of the variables such as stolon number, length, number of leaves, dry matter, biological productivity, growth rate, and relative growth rate. A second experiment was conducted over a 5-week period to compare the growth variable between nimblewill plants and pastures. For all variables, the results showed that the weed grass developed slowly within the first 5 weeks after germination, indicating that this would be the best time to implement a chemical or cultural control measure. It was also observed that cultures with a rapid growth in the first 5 weeks after emergence could easily suppress weed growth. At the end of the experiment, stabilization of all variables was observed. However, additional observations are required to obtain more accurate results.
Keywords:Nimblewill, Fescues, Kentucky Bluegrass, Weed Biology, Management Strategy, Forage

1. Introduction
A decrease in crop yield or quality resulting from the interactions between weeds and crops forms the basis of modern weed science. However, competition is one of the most debated issues in ecology [1] . Weeds can reduce the quantity and stand life of desirable forage plants in pastures and hayfields. These unwanted plants are often more aggressive than existing or desired forage species and compete for light, water, and nutrients. Weeds can also diminish the quality and palatability of the forage available for livestock grazing animals.
Nimblewill (Muhlenbergia schreberi) is an important weed in pastures and hayfields [2] . It is a native warmseason or C4 perennial grass that thrives in lightly shaded areas and easily invades lawns, waste areas, and other disturbed sites [3] . In unmowed areas, nimblewill can grow to 20 - 60 cm in height; however, it is more aggressive in shaded areas with a moist, rich soil. Nimblewill spreads by seeds and has slender culms that fall to the ground and root at nodes; however, the plants do not have true rhizomes or stolons [3] . Nimblewill is difficult to control with herbicides and poses a serious problem in pastures. The species is unpalatable to horses; thus, its presence decreases the grazing quality of bluegrass [2] and other grass pastures.
Therefore, every effort should be made to prevent the establishment of these weed grasses. However, selective control measures are often difficult. Selection of adapted turfgrass species and cultivars and the use of cultural practices are important in minimizing weed grass encroachment and competition. For the efficient management of this weed, it is necessary to investigate the factors contributing to its growth.
Knowledge of weed biology is essential for establishing weed control strategies, and it is important to recognize the natural growth and developmental strategies of major weeds known to infest crop fields [4] . Weed biology is the study of plant attributes such as morphology, seed dormancy and germination, growth physiology, competitive ability, and reproductive biology.
Investigations into the quantitative aspects of whole plants can be conducted using growth analysis techniques. Plant growth analysis is an explanatory, holistic, and integrative approach to interpreting plant form and function. Various parameters can be used to compare species growth, including leaf area, height, branch number, and dry weight. These comparisons provide an indication of the relative size, productivity, and photosynthetic capacity of a species that may influence its competitive ability [5] . Calculated parameters such as specific leaf area, leaf weight ratio, and leaf area ratio provide estimates of the photosynthetic area per unit of biomass. The proportion of biomass allocated to the leaves and an index of plant leafiness may reflect the ability of a species to obtain resources and compete with other plants [6] .
A successful weed management strategy is one that is based on biology, including information on environmental factures and their effects on plant properties [7] because plant growth requirements, characteristics, and competitive ability affect a species’ ability to survive and spread. Thus, understanding the growth and development of a species may help explain the recent success of that species. Investigating the mechanisms related to weed seedling growth rates is also important when considering weed management strategies that include the use of herbicides or cultural control measures.
The main goal of this research was to evaluate the growth of Nimblewill (M. schreberi), which poses an emerging threat to the growth of forage grass.
2. Materials and Methods
The experiment was conducted in 2 stages in a greenhouse at the University of Kentucky at Lexington (38˚5'N, 84˚44'W) from April to June in 2010.
Initially, seeds were collected from the fields and college grounds in the spring of 2008. Nimblewill were planted in 5-L pots with local soil (i.e., Maury-Bluegrass silt loam complex; fine, mixed, active, mesic Typic Paleudalfs with 7% sand, 75% silt, 18% clay, and 2.5% organic matter and a pH of 6.8. Seed germination occurred 6 days after planting. After emergence, seedlings were thinned to 1 plant per pot. Plants were maintained with daily watering in the greenhouse under natural conditions of light and a mean temperature of 25˚C.
The pots were arranged in a completely randomized design with 17 treatments and 4 replications. Seventeen harvests were conducted over a 17-week period (i.e., 1 per week). The first harvest was conducted 1 week after emergence of the nimblewill, and the others were conducted from weeks 2 - 17.
At each harvest, the number of stolons, height, number of leaves, dry matter, biological productivity, relative growth rate, and growth rate were calculated. Plant dry mass was obtained by drying the plant material at 65˚C for 48 h.
A second experiment with the same conditions as described above was performed to compare the biological productivity, growth rate, and dry matter of nimblewill and pastures within the first 5 weeks. These 5-week evaluations were based on another experiment where the slow growth of nimblewill was shown to provide a cultural control in the air where the soil was plowed. For comparison, we used fescue seeds with or without endophytic fungi and Kentucky bluegrass. Species were sown in separate pots, taking care that all were germinated at the same time; 1 plant per pot was kept and they were collected on a weekly basis.
The growth rate (GR) of a unit area of canopy cover at any point in time was defined as the increase in plant material per unit of time. The relative growth rate (RGR; i.e., the change in total dry mass per total dry mass of plant per day) was also calculated for each harvest interval.
The following formulae were used to calculate the necessary growth parameters [8] [9] :
(1) RGR was the amount of dry matter produced per unit (W2) of dry matter present (W1) per unit of time (t) (g·g·d−1).
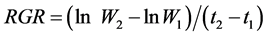
(2) GR was the amount of dry matter produced (W) per unit of time (t) (g·d−1).
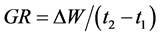
(3) Biological productivity (BP) was the amount of dry matter produced (W) per soil area (S) per unit of time (t) (g·m2·d−1).
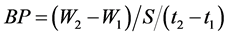
The data in both experiments were subjected to regression analyses, and a model was chosen, taking into account the logic of the biological phenomenon and the coefficient of determination.
3. Results and Discussion
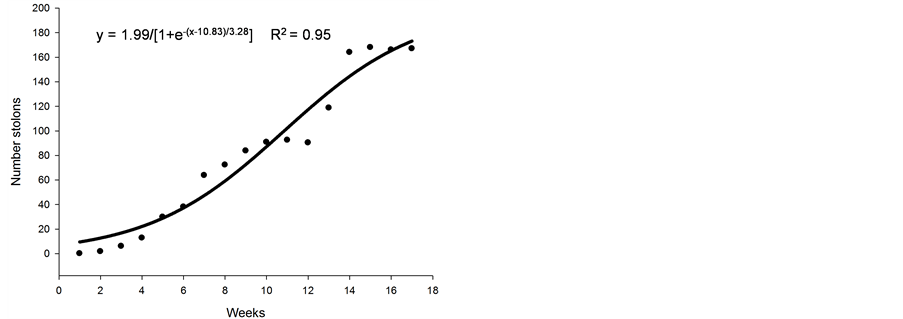
The increase in the number of stolons throughout the duration of the experiment is shown in Figure 1(a). The number of stolons per plant remained nearly constant until the fourth week after germination; subsequently, a linear increase was observed up week 7, where it was held constant during the last evaluation and appeared to
 (a)
(a)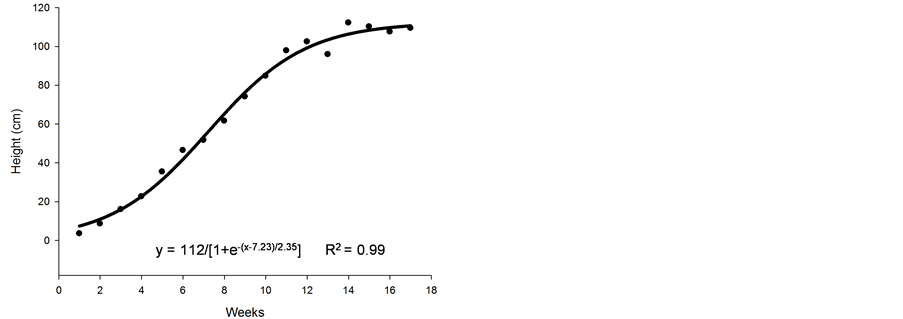 (b)
(b)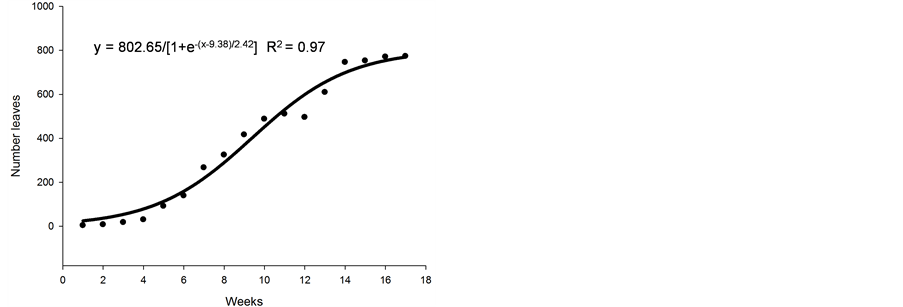 (c)
(c)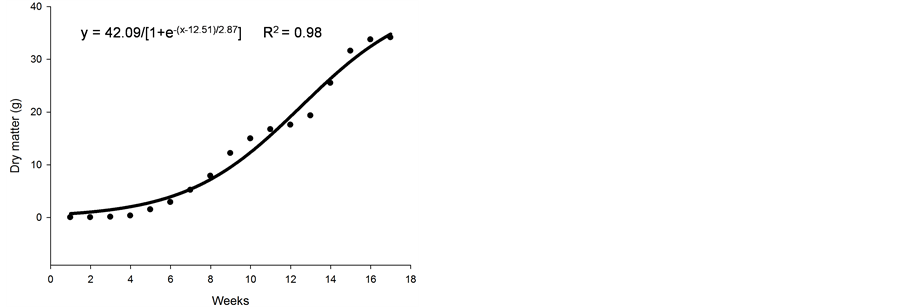 (d)
(d)
Figure 1. Average number of stolons (a), height (cm) (b), number of leaves (c), and dry matter (d) of nimblewill plants at 17 weeks of growth after germination, Lexington, KY, USA, 2010.
stabilize (Figure 1(a)). Within 14 weeks of growth, the nimblewill plants developed ~160 stolons, on average. Additional weekly observations would be required to accurately determine when the plants ceased to produce additional stolons. Nimblewill spreads by its seeds and stolons; investigating these dispersal mechanisms is important in determining effective control strategies for this weed.
Figure 1(b) demonstrates that the growth of plants by the length of their stolons experienced a linear increase in the first week and continued up until ~12 weeks after germination. Following week 12, the growth of the stolons appeared to be stabilized. At 12 weeks after germination, the runner was, on average, 1 m in length. However, as shown in Figure 1(a), the number of stolons increased up until the end of the period. Subsequently, the plants ceased to extend into the runner length, perhaps due to an accumulation of reserves.
From weeks 1 - 6, the increase in the number of leaves on nimblewill plants was not significant (Figure 1(c)). After week 6, however, the nimblewill plants experienced a rapid and linear increase up until week 14, at which point its growth appeared to stabilize. After 14 weeks, a nimblewill plant can develop an average of 700 - 800 photosynthetically active leaves. The leaves are ~1 - 2 inches long and have a blue-green coloration, with many visible veins on the upper surfaces of the leaves. The leaves are rolled in the bud. There is a very short, membranous ligule with a slightly jagged top.
It can be supposed that species with high leaf production have higher photosynthetic rates, which improves a plant’s ability to compete with other plants [7] .
Figure 1(d) shows that the shoot dry matter experienced a period of slow growth up until 6 weeks after germination. At 7 weeks, there was a rapid and linear accumulation in the shoot dry matter of nimblewill plants, probably because, at this time, the plant also experienced an increase in the number of leaves and stolons. The maximum accumulation of shoot dry matter occurred just before flowering (i.e., at 16 weeks), with a value of ~33 g per plant. It is believed that, at this time, the chemical control of nimblewill may be limited; thus, chemical control measures for this weed should be applied when it is still young and actively growing in the late spring to early summer.
Dry weight accumulation is the typical parameter used to characterize growth because it usually has great economic significance. The total dry matter yield of crops depends on the size of the leaf canopy, the rate at which the leaf functions (i.e., it efficiency), and the length of time the canopy persists (i.e., its duration) [10] .
Observations indicated that the long slender flower stalks emerge in the late summer. Flowering begins at 16 weeks. The flowers are borne at the ends of the stems and in the leaf axils, 2 - 6 inches long, and cylindrical. Over the course of 17 weeks, the nimblewill plant can produce an average of 900 - 1000 inflorescences. Each inflorescence is capable of producing an average of 238 seeds, and seed production remains high until winter. In addition, it was observed that the average weight of 1000 seeds of nimblewill at 17 weeks was 0.0687 g per plant (data not shown).
Nimblewill plants have a high potential for vegetative and reproductive development, a feature that indicates a high degree of competition for factors that influence crop development (e.g., space, nutrients, and light). In most herbaceous annual plants, vegetative growth is terminated by reproductive growth. Developing flowers and fruits are stronger sinks for mineral nutrients, sugar, and amino acids, and there is a corresponding decrease in the amounts of these nutrients available for the growth of other plant parts [11] .
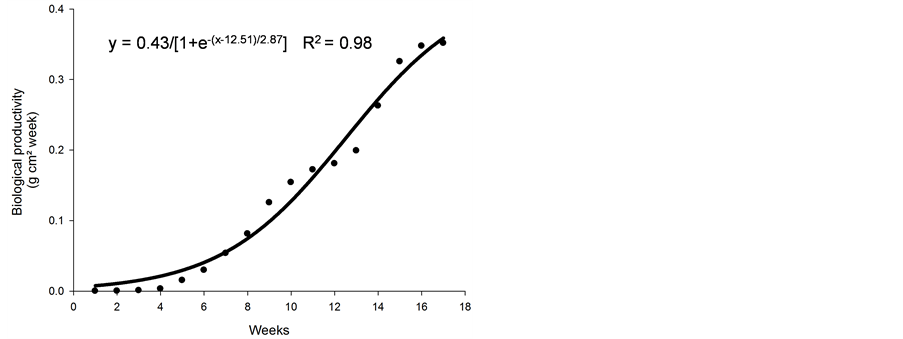
The biological productivity (Figure 2(a)) showed increasing values from the fifth week after germination and continued up to week 17. Before the fifth week, the values of biological productivity were low and constant. Figure 2(a) shows that biological productivity was low prior to week 5, probably due to low leaf area index and light absorption.
The relative growth rate (RGR) represents the amount of dry matter production with respect to the unit weight of the current dry matter of a plant over time [5] . This measure was used to evaluate plant growth, which depends on the amount of material that has accumulated gradually.
The RGR (Figure 2(b)) is the most appropriate measure for assessing plant growth and is dependent on the amount of plant material that has been accumulated. The RGR, which expresses the increase in shoot dry matter in relation to pre-existing shoot dry matter, decreased with time, with a mean of 0.082 g·g−1·d−1. At week 9, the RGR appeared to stabilize.
The decrease in the RGR with plant age resulted, in part, in the gradual increase of non-photosynthetic tissues [12] . With an increase in dry mass accumulation in plants, there is an increase in assimilates for the maintenance of structures already formed; thus, the amount of assimilates available for growth is typically low and the RGR decreases with time [13] .
 (a)
(a)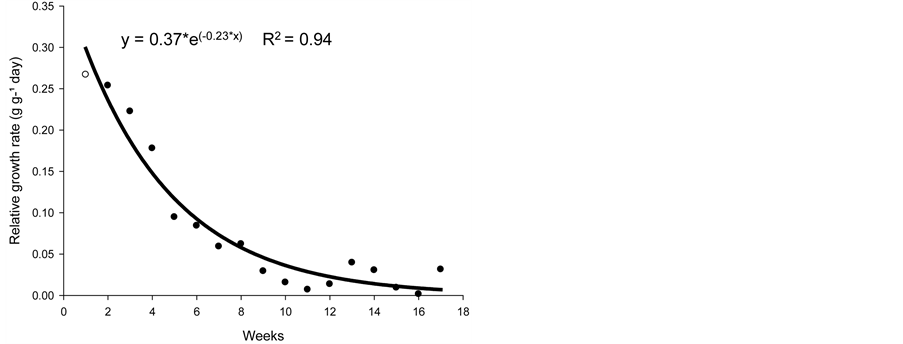 (b)
(b)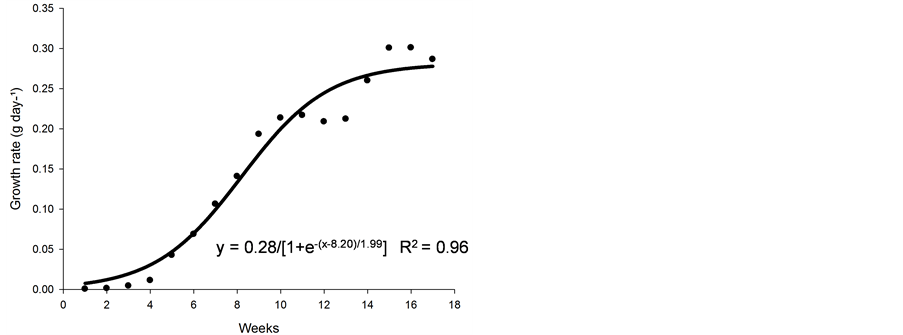 (c)
(c)
Figure 2. Biological productivity (g·cm2·week) (a), relative growth rate (g·g−1·d−1) (b), and growth rate (g·d−1) (c) of nimblewill plants at 17 weeks of growth after germination, Lexington, KY, USA, 2010.
From weeks 1 - 4, the rate of growth (Figure 2(c)) was slow, probably because the plant was invested in the growth of its root system. Following this period, there was an increase in the number and length of the stolons and the number of leaves. The rate of growth of nimblewill plants increased up to week 13, and typically stabilized thereafter.
Figures 2(a)-(c) demonstrates that nimblewill plants experience slow growth, initially, which can affect competition with other species via suppression by faster-developing annual species. In considering the slower developmental stages of the nimblewill plants, the ideal time for implementing a control strategy could be extended up to 4 weeks to avoid the negative effects of vegetative propagation and seed production. In addition, this slow growth rate over a 4-week period suggests the possibility of its control by a species that can quickly cover the ground.
Initially, a seedling depends on the reserves contained in its seeds and growth is slow. Following development of the root system and emergence of the leaves, rapid growth occurs due to the removal of water and nutrients from the substrate for the photosynthetic activity of the plant [14] .
Therefore, it appears that perennials invest more in biomass allocation than annuals, even at the beginning of their growth cycle. The distribution of dry matter between the aboveand below-ground parts of a plant does not account for these differences at the seedling stage, however, which is also the case for annual and perennial plants [8] .
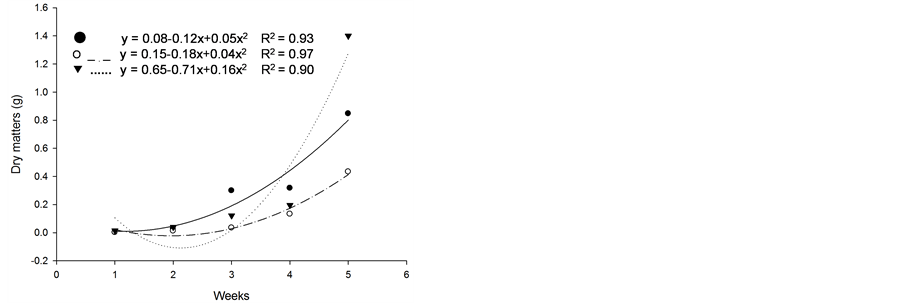
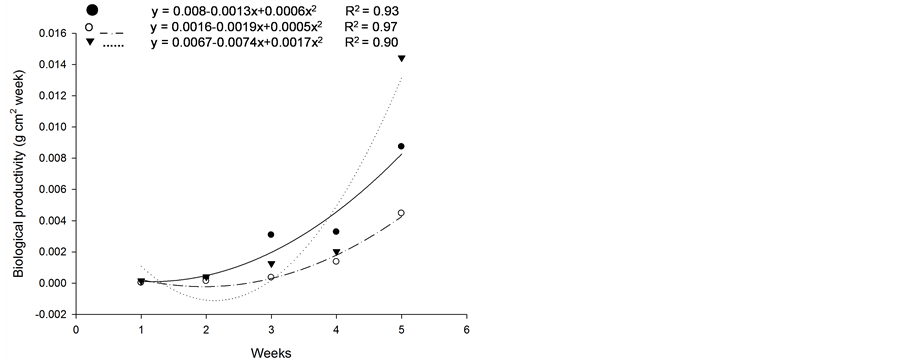
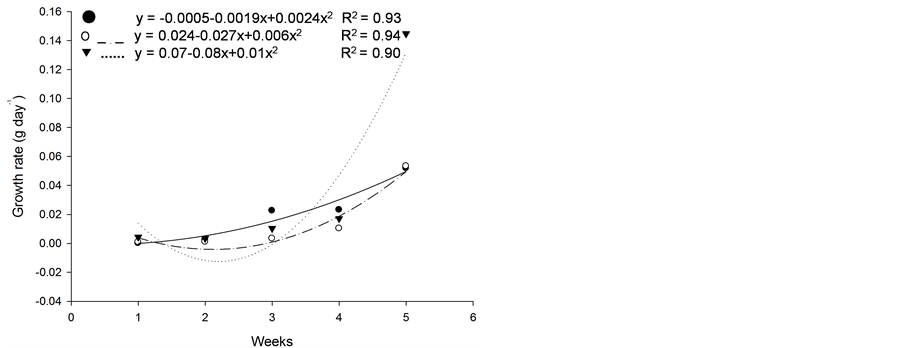
Based on initial seedling growth, all fescues (with and without endophytic fungi), with the exception of Kentucky bluegrass, showed a greater accumulation of dry matter, biological productivity and growth rate within the first 5 weeks when compared to the nimblewill, showing that nimblewill has a slower developmental stage and can be suppressed by pastures in field conditions (Figure 3, Figure 4, and Figures 5(a)-(c)). In general, the first plant to take root has a better chance in competing for environmental resources. However, bluegrass showed an accumulation of dry matter that was less than that of the weed, indicating that the bluegrass and pasture may suffer from competition with the nimblewill. In another experiment, [15] found that bluegrass grows much slower than other species.
 (a)
(a)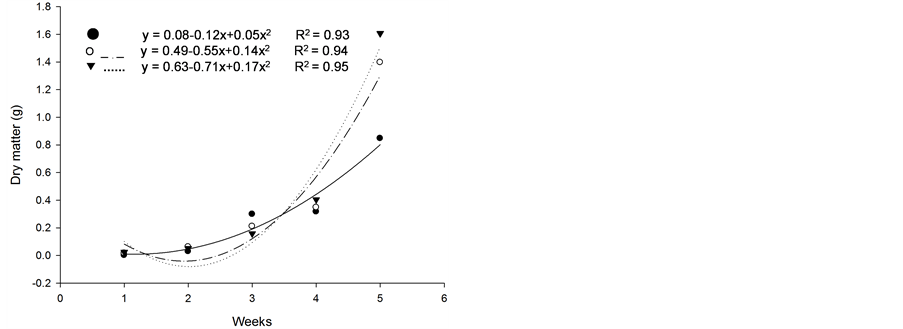 (b)
(b)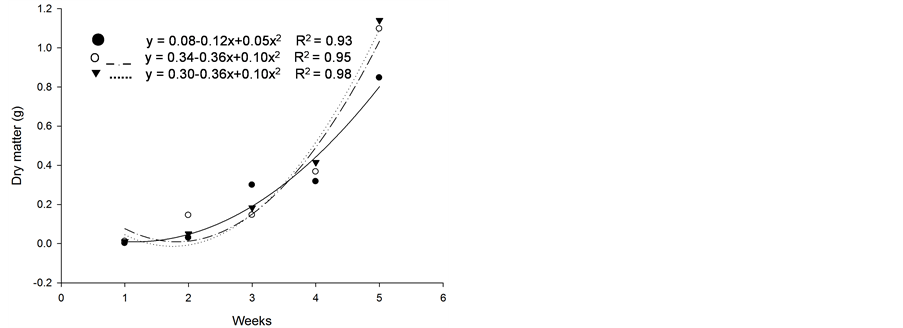 (c)
(c)
Figure 3. Dry matter of plants of (a): tall fescue “KY 31” (▼), Kentucky bluegrass (○); (b): tall fescue KYFA 9821(▼), KYFAEF 9821 (○); (c): tall fescue KYFA 9301 (▼) and KYFAEF 9301 (○); and (a), (b), and (c): nimblewill (●) at 5 weeks of growth after germination, Lexington, KY, USA, 2010.
 (a)
(a)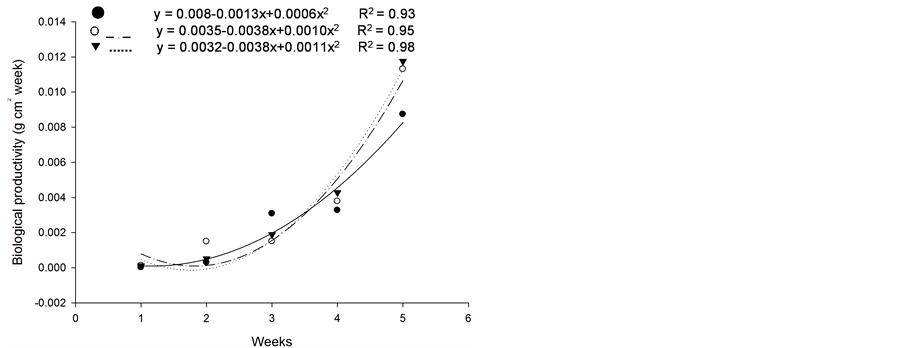 (b)
(b)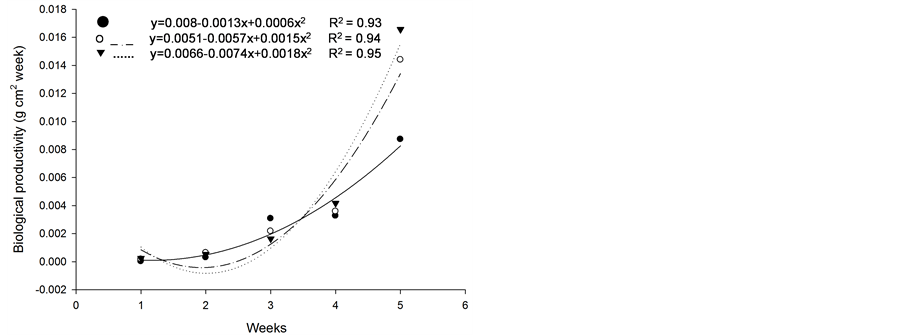 (c)
(c)
Figure 4. Biological productivity (g·cm2·week) of plants of (a): tall fescue “KY 31” (▼), Kentucky bluegrass (○); (b): tall fescue KYFA 9821(▼), KYFAEF 9821 (○); (c): tall fescue KYFA 9301 (▼) and KYFAEF 9301 (○); and nimblewill (●) at 5 weeks of growth after germination, Lexington, KY, USA, 2010.
An annual plant has to produce a large number of seeds in a relatively short time. This can be achieved by growing rapidly in the pre-reproductive phase and converting most of the energetic and mineral resources
 (a)
(a)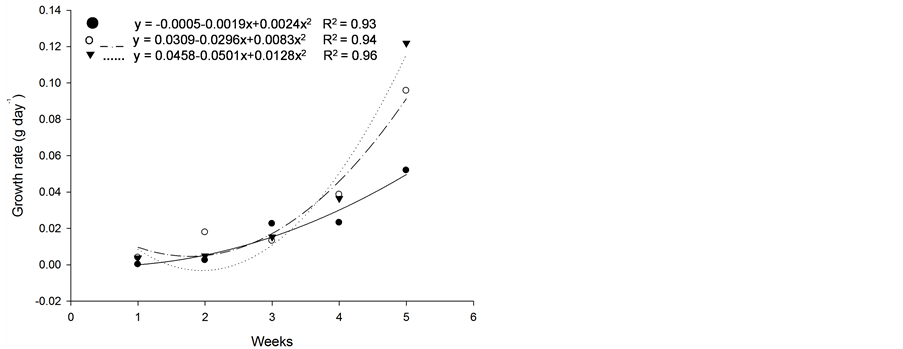 (b)
(b)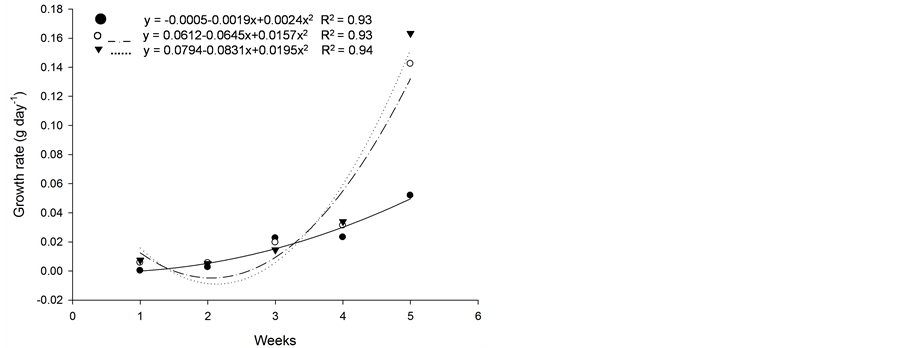 (c)
(c)
Figure 5. Growth rate (g·d−1) of plants of (a): tall fescue “KY 31” (▼), Kentucky bluegrass (○); (b): tall fescue KYFA 9821(▼), tall fescue KYFAEF 9821 (○); (c): tall fescue KYFA 9301 (▼) and tall fescue KYFAEF 9301 (○); and (a), (b), and (c): nimblewill (●) at 5 weeks of growth after germination, Lexington, KY, USA, 2010.
obtained during the vegetative period into the seed [8] .
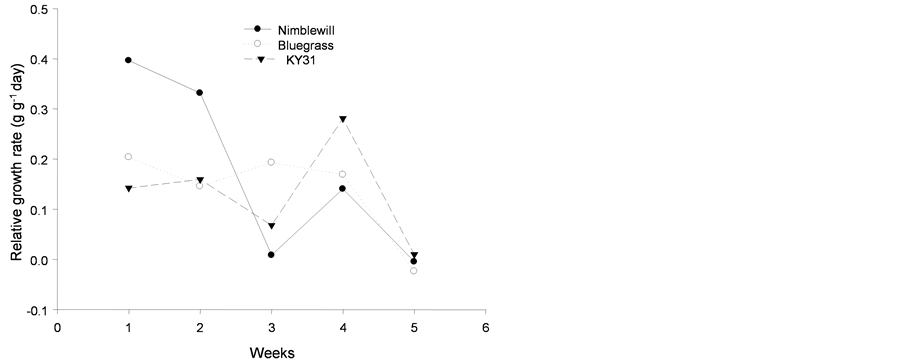
The relative growth rate showed no adjustment for any of the species studied, as shown in Figure 6.
 (a)
(a)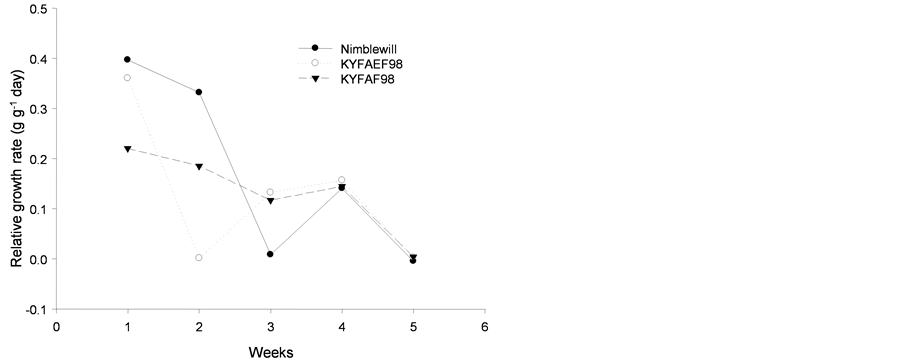 (b)
(b)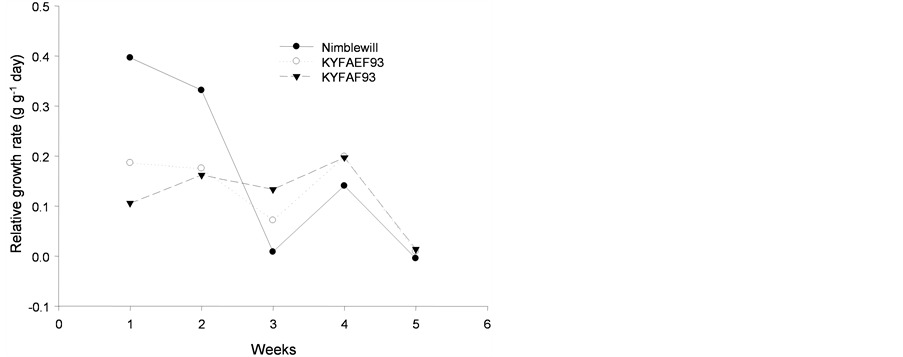 (c)
(c)
Figure 6. Tendency of relative growth rate (g·g−1 day) of plants of: (a): tall fescue “KY 31” (▼), Kentucky bluegrass (○); (b): tall fescue KYFA 9821(▼), tall fescue KYFAEF 9821 (○); (c): tall fescue KYFA 9301 (▼) and tall fescue KYFAEF 9301 (○); and (a), (b), and (c): nimblewill (●) at 5 weeks of growth after germination, Lexington, KY, USA, 2010.
Based on the measured and calculated parameters presented herein, the dry matter ranking of the species grown in a noncompetitive situation was as follows: Kentucky bluegrass > nimblewill > tall fescue KYFAEF 9821; and KYFAEF 9301 > tall fescue Kentucky 31, KYFA 9821, and KYFA 9301. The presence of endophytic fungi favored the establishment of the fescues. Those species that grow faster would be able to preempt the uptake of growth resources and, thus, would be expected to be more competitive [6] .
Control of spreading nimblewill is usually attempted with a nonselective systemic herbicide, when the weedy plants are young. The mother plants are easily killed but, oftentimes, the weed will return, growing from the stolons; thus, an additional application of the herbicide is recommended.
Control of perennial grassy weeds is a very difficult and time-consuming process. One must weigh the advantages and disadvantages before deciding whether to attempt control measures. Knowledge of the best stage during weed development to apply a control measure may increase the effectiveness of management strategies.
Information on nimblewill growth rates can be used to estimate the optimum period for post-emergence weed control with herbicides and the optimum timing of herbicide application or cultural control in conventional planting.
References
- Radosevich, S.R. (1987) Methods to Study Interactions among Crops and Weeds. Weed Technology, 1, 190-198.
- Baskin, J.M. and Baskin, C.C. (1985) Dormancy Breaking and Germination Requirements of Nimblewill (Muhlenbergia schreberi Gmel.) Seeds. Journal Range Manage, 38, 513-515. http://dx.doi.org/10.2307/3899742
- Willis, J.B., Beam, J.B., Whitney, L.B., Shawn, D.A. and McErroy, J.S. (2007) Selective Nimblewill (Muhlenbergia schreberi) Control in Cool-Season Turfgrass. Weed Technology, 21, 886-889. http://dx.doi.org/10.1614/WT-07-016.1
- Ravindra, G.M., Sridhara, S., Girijesh, G.K. and Nanjappa, H.V. (2008) Weed Biology and Growth Analysis of Celosia argentea L., a Weed Associated with Groundnut and Finger Millet Crops in Southern India. Communications in Biometry and Crop Science, 2, 80-87.
- Radosevich, S., Holt, J.S. and Ghersa, C. (1997) Weed Ecology: Implications for Vegetation Management. John Wiley and Sons, New York, 278-301.
- Horack, M.J. and Loughin, T.M. (2003) Growth Analysis of four Amaranthus Species. Weed Science, 48, 347-355. http://dx.doi.org/10.1614/0043-1745(2000)048[0347:GAOFAS]2.0.CO;2
- Salehian, H. and Eshaghi, O. (2012) Growth Analysis Some Weed Species. International Journal of Agriculture and Crop Sciences, 4, 730-734.
- Garnier, E. (1992) Growth Analysis of Congeneric Annual and Perennial Grass Species. Journal of Ecology, 80, 665-675. http://dx.doi.org/10.2307/2260858
- Hunt, R. (1982) Plant Growth Curves: The Functional Approach to Plant Growth Analysis. Edward Arnold, London.
- Tekalign, T. and Hammes, P.S. (2005) Growth and Productivity of Potato as Influenced by Cultivar and Reproductive Growth II. Growth Analysis, Tuber Yield and Quality. Scientia Horticulturae, 105, 29-44. http://dx.doi.org/10.1016/j.scienta.2005.01.021
- Salisbury, F.B. and Ross, C.W. (1992) Plant Physiology, Hormones and Plant Regulators: Auxins and Gibberellins. 4th Edition, Wadsworth Publishing, Belmont, 357-381.
- Willians, R.F. (1946) The Physiology of Plant Growth with Special Reference to the Concept of Net Assimilation Rate. Annals of Botany, 32, 41-72.
- Lambers, H., Chapin III, F.S. and Pons, T.L. (2008) Plant Physiological Ecology. Springer, New York, 321-374. http://dx.doi.org/10.1007/978-0-387-78341-3_10
- Quero, J.L., Villar, R., Maran, T., Zamora, R. and Poorter, L. (2007) Seed-Mass Effects in four Mediterranean Quercus Species (Fagaceae) Growing in Contrasting Light Environments. American Journal of Botany, 94, 1795-1803. http://dx.doi.org/10.3732/ajb.94.11.1795
- Poorter, H. and Remkes, C. (1990) Leaf Area Ratio and Net Assimilation Rate of 24 Wild Species Differing in Relative Growth Rate. Oecologia, 83, 553-559. http://dx.doi.org/10.1007/BF00317209

