American Journal of Plant Sciences
Vol.5 No.4(2014), Article ID:43260,8 pages DOI:10.4236/ajps.2014.54062
The Transfer of Cu, Zn, Mn and Fe between Soils and Allium Plants (Garlic and Onion), and Tomato in the Southwest of the Buenos Aires Province, Argentina
![]()
INQUISUR-Departamento de Química, Universidad Nacional del Sur, Bahía Blanca, Argentina.
Email: pilarmor@criba.edu.ar
Copyright © 2014 María del Pilar Moralejo, Silvia Graciela Acebal. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property María del Pilar Moralejo, Silvia Graciela Acebal. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received December 6th, 2013; revised January 8th, 2014; accepted January 22nd, 2014
KEYWORDS
Micronutrients; Soil-Plant Transfer; Garlic (Allium sativum L.); Onion (Allium cepa L.); Tomato (Solanum lycopersicum L.); Extractions
ABSTRACT
Chemical extraction methods are generally used to evaluate trace element concentrations in soils. The adequacy of these soil tests is commonly assessed by comparing the extraction results with the metal contents in the plants. In this study, soil and leaf samples were collected in the southwest area of the Buenos Aires Province, Argentina. Garlic (Allium sativum L.), onion (Allium cepa L.) and tomato (Solanum lycopersicum L.) are species of great regional economic importance. These crops need good mineral nutrition for optimum growth and sustainable production. Cu, Zn, Mn and Fe micronutrient uptake by plants was analyzed together with the trace element contents in the soil in which those plants were grown. A single EDTA-extraction procedure was performed to determine soil micronutrients. The amount of extractable-trace elements increased as the concentration of the chelating agent EDTA increased. The range of total element content in soil was: 15.68 - 31.5 mg∙kg−1 for Cu, 75.0 - 386.3 mg∙kg−1 for Zn, 542.5 - 1686 mg∙kg−1 for Mn and 28,325 - 32,675 mg∙kg−1 for Fe. Micronutrient contents in mature leaf tissue were determined by the acid digestion method. Total and available micronutrient content in soil as well as total content in leaves were measured by flame atomic absorption spectrometry (FAAS). Total micronutrient content and the available extractable-fraction in soils were below the critical values for plant growth. This was in agreement with the amount of micronutrients present in the leaf tissue. A strong relationship between the extraction data and the soil-plant transfer coefficients suggested an appropriate exchange of trace elements from soils to garlic, onion and tomato plants.
1. Introduction
Soils are complex systems, considered as multi-component systems, and can act as physical, chemical and biological reactors. Soils are the main source of nutrient elements for plants, and soil conditions play a crucial role in metal ion behavior. Soil properties, metal speciation, plant species, water regime, and especially soilplant interactions determine the bioavailability of soil metal ions [1,2].
The soil-plant transfer of metal ions is a part of the cycling of chemical elements in nature. It is a very complex process governed by several factors, both natural and human-related. In general, plants readily take up the species of trace elements that are dissolved in the soil solution in either ionic or chelated or complexed forms.
Micronutrients are defined as the elements that plants need in very small quantities, in contrast with macronutrients, which are consumed in larger quantities. Micronutrients are mostly found in soils in low quantities, with the exception of Fe. Iron is a micronutrient because it is required in small amounts by plants, and it can become deficient due to insolubility at high pH. The micronutrients considered in the present work were Cu, Zn, Mn and Fe.
Garlic (Allium sativum L.), onion (Allium cepa L.) and tomato (Solanum lycopersicum L.) are important species grown in the Southwest of the Buenos Aires Province, Argentina. These crops have great economic importance, especially as a result of changing consumer habits towards healthy eating and increased awareness of their healing properties [3]. Optimal mineral nutrition is crucial to maintain crop production. Analyses of the content of trace elements are generally carried out to evaluate plant development. The plant ability to take up micronutrients from the soil may also be evaluated by determining the ratio of the total element concentration in the plant to the total element concentration in soil, and this ratio is called “transfer coefficient” [4].
Various one-step extraction methods are frequently used to assess bioavailability because of their simplicity and ease of operation. Chelating agents and buffered salt solutions are believed to extract potentially mobile portions of metals.
EDTA (ethylenediaminetetraaccetic acid) is a powerful chelating agent that is widely used in soil sciences for the prediction of the bioavailability of trace metals. EDTA can release trace metals in non-silicate bound phases and, therefore, correlates well with plant contents and with the available fraction of micronutrients in the soil solution. Several studies on the reactions of EDTA-metal complexes in soils have been published [1,5-10].
The aim of the present study was to determine the levels of the micronutrients Cu, Zn, Mn and Fe in garlic— onion—and tomato-leaf samples, the EDTA-extractable amounts in the soils, and the soil-plant transfer coefficients. Cu, Zn, Mn and Fe content in soils and leaves were determined by flame atomic absorption spectrometry (FAAS).
The main objectives were 1) to investigate the contents of Cu, Zn, Mn and Fe in soils and leaves, and 2) to provide further information about soil-metal transfer of trace elements in these agricultural soils as a matter of local interest, and by extension, contribute to other systems with the same characteristics.
2. Materials and Methods
2.1. Soil and Plant Sample Sites
Soil and plant samples were collected from different zones of the department of Villarino. These zones are irrigated lands from the Low Valley of the Colorado River located in the Southwest of the Buenos Aires Province (Figure 1).
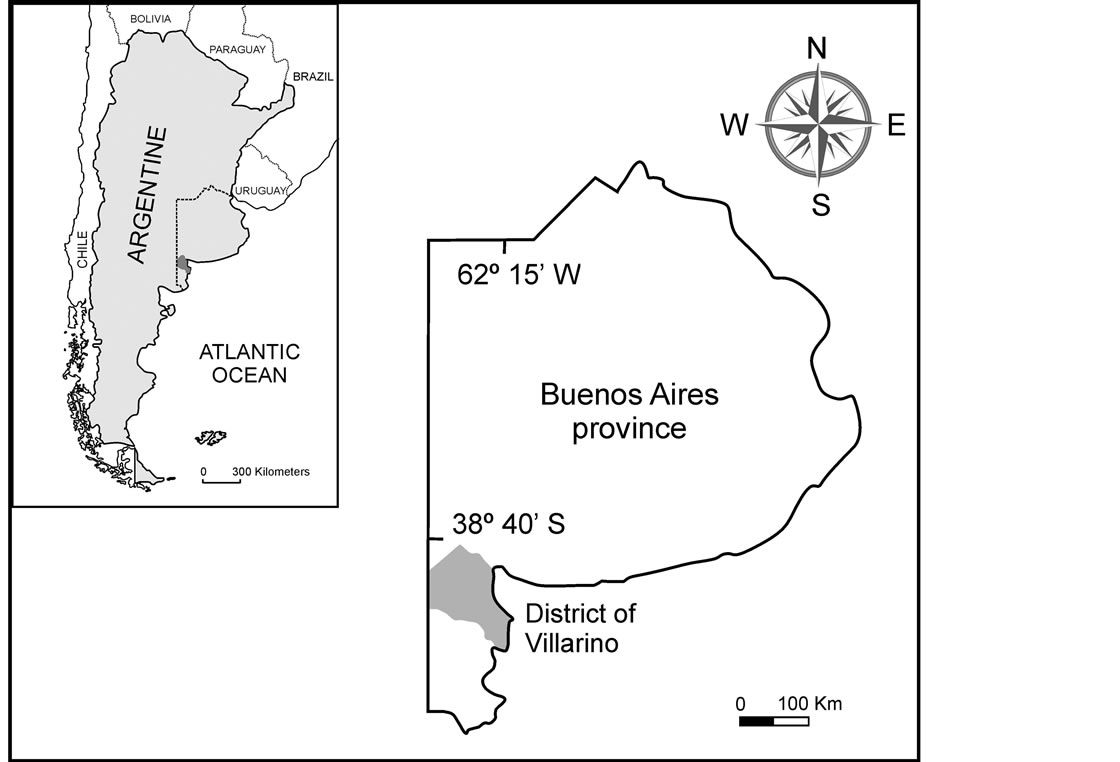
Figure 1. Location of the studied area.
Seven agriculture soil samples were used. Surface soils (0 - 20 depth) representing the Ap horizon were collected from Ascasubi (denoted as A1 and A2), San Adolfo (denoted as B1 and B2), Los Alfalfares (C), Santa Blanca (D) and Ombucta (E).
A and B were sampling sites for soil, onion and tomato plants, while C, D and E were sampling sites for garlic and soil.
Plants and soils were sampled in November 2009 (springtime).
2.2. Soil Samples
On each site, 20 samples of topsoil were taken randomly on an area of about 0.1 ha and mixed to form a composite topsoil sample, and then stored in polyethylene bags at 20˚C. Soils of this region are characterized as sandy soils with low organic matter, containing mainly quartz, Narich feldspars and plagioclase in the sand fraction, and illite, interstratified illite-smectite, quartz, Na-rich feldspars and mica in the silt and clay fraction, being Ca2+ and Mg2+ the main exchangeable cations [11,12]. Soils were classified following the Soil Taxonomy system [13] into four Aridisols (Typic Camborthid: A1, A2, B1 and B2) and three Entisols (Typic Ustipsament: C, D, and E). Soil samples were air-dried at room temperature, ground and passed through a 2 mm stainless steel sieve to obtain a <2 mm size fraction.
Particle size distributions were evaluated using the hydrometer method [14]. Soil pH was determined in a 1:2.5 soil/deionised water suspension and in a soil/KCl suspension using a glass pH electrode in an Orion digital Ionalyzer (Model 701A). Total organic carbon (TOC) was measured by dry combustion using a LECO CR-12 Carbon System Analyzer (model 781 - 600). Soil samples were selected to cover a rather wide TOC range (from 0.52% to 1.76%). Cation exchange capacity (CEC) of each soil sample was obtained by exchanging the samples with 1 mol∙L−1 NH4OAc at pH 7 [15]. Detailed soil analysis procedures were described in [16].
All the reagents were commercial products of the highest available purity (Merck, Darmstadt, Germany). Calibrants and reagent solutions were stored in polyethylene containers.
Some relevant physico-chemical properties of the studied soils are presented in Table 1.
2.3. Plant Samples
For each species, four mature plants were selected and tagged for soil and plant collection in November 2009. Plant samples were collected together with soil samples. In each tagged plant, adult leaves (third and fourth) with a healthy appearance were collected and pooled. No plant presented deficiency symptoms.
Leaf samples were separated, carefully rinsed in bidistilled water, air-dried, cut in small pieces, sieved through a 2 mm sieve and ground to fine powder prior to performing the analysis. A total of three pooled leaf samples were analyzed for each plant species.
2.4. Soil and Leaf Analysis
Elemental analyses of soils and plants are commonly conducted after bringing the sample into solution using acid digestion.
Total concentrations of trace elements (Cu, Zn, Mn and Fe) in soil samples were determined after the decomposition of 0.1 - 0.5 g sample of finely ground soil (<2 mm fraction) with an acid mixture of HNO3 (70%) + HClO4 (70%), and then HF (48%) at 200˚C to 225˚C [17]. Total concentrations of trace elements in leaf samples were determined after decomposition by acid mixture of HNO3 (65%) + HClO4 (70%) (4:1 ratio), and HF (40%) in an open system at 200˚C [1]. A blank digestion solution was made for comparison.
2.5. Single Extraction Procedure
All the experiments were performed at two concentrations and pH 7 as a single extraction procedure. The ex-
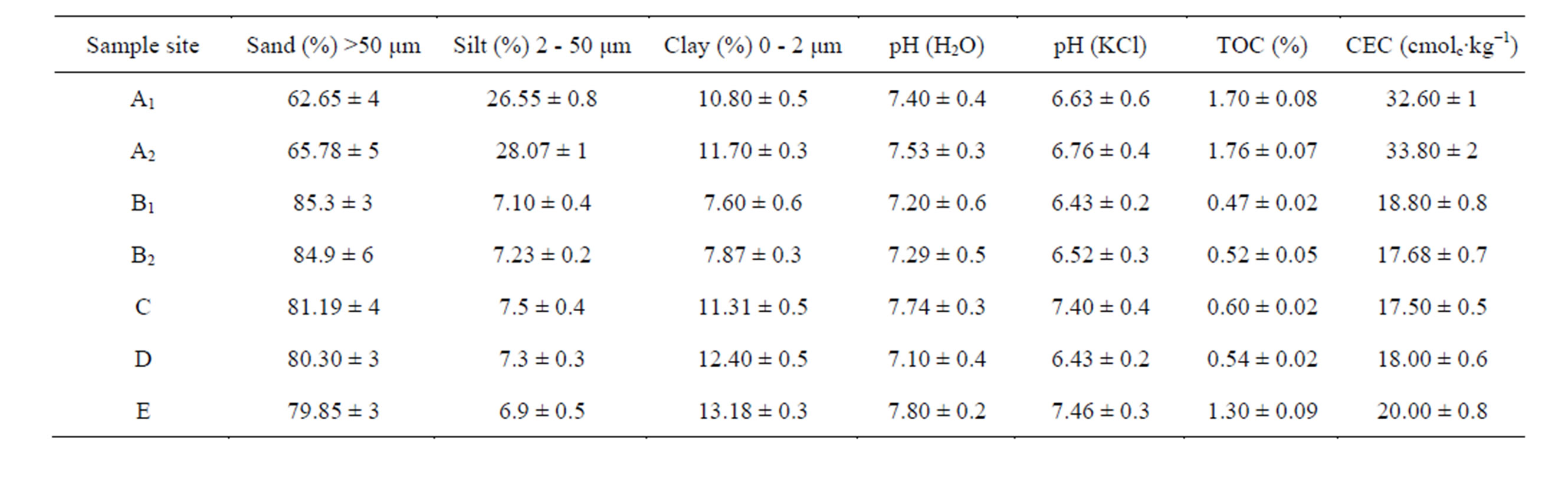
Table 1. Physico-chemical properties of the soil samples.
tractant solutions were prepared in hydroxymethyl-aminomethane (TRIS), maleic acid and NaOH buffer system. The buffer solution is described in Table 2. Solutions of 10−3 mol∙L−1 and 3 × 10−2 mol∙L−1 EDTA were prepared at pH 7. After dissolving the chelating agent, the final pH was adjusted using maleic acid or NaOH.
Separately in triplicate, 5 g of dry soil samples were extracted with 50 mL of each extractant solution (soil/ solution ratio 1:10) by shaking in a horizontal shaker at 150 rpm for 2 h at room temperature. After extraction, soil-EDTA suspensions were centrifuged for 15 min at 3500 rpm, and then filtered through Millipore filter membranes (pore diameter 0.45 μm). The supernatants were used for analysis by quantitative determination by FAAS.
2.6. Instrumentation
The atomic absorption spectrometry (AAS) technique is widely used for the determination of metal elements. This technique features a high accuracy and precision of the micro and trace determinations of elements on the condition that the analyte sample is adequately prepared. Wet digestion is commonly used for the preparation of samples of vegetable materials [18].
Cu, Zn, Mn and Fe contents in soil and leaf samples were determined by FAAS in an air-acetylene flame using a GBC spectrometer (Model 932B, Australia) with a deuterium (D2) lamp for background correction.
2.7. Statistical Analysis
All data are presented as mean values of triplicate experiments. Statistical analysis of the data was carried out using analysis of variance (ANOVA). The regression and other statistical analyses were conducted using Statistical software.
3. Results
3.1. Total Content of the Elements
Total content of micronutrients in the surface soils and leaves are given in Table 3. Total concentrations of the trace metals in leaf and soil samples followed the order:
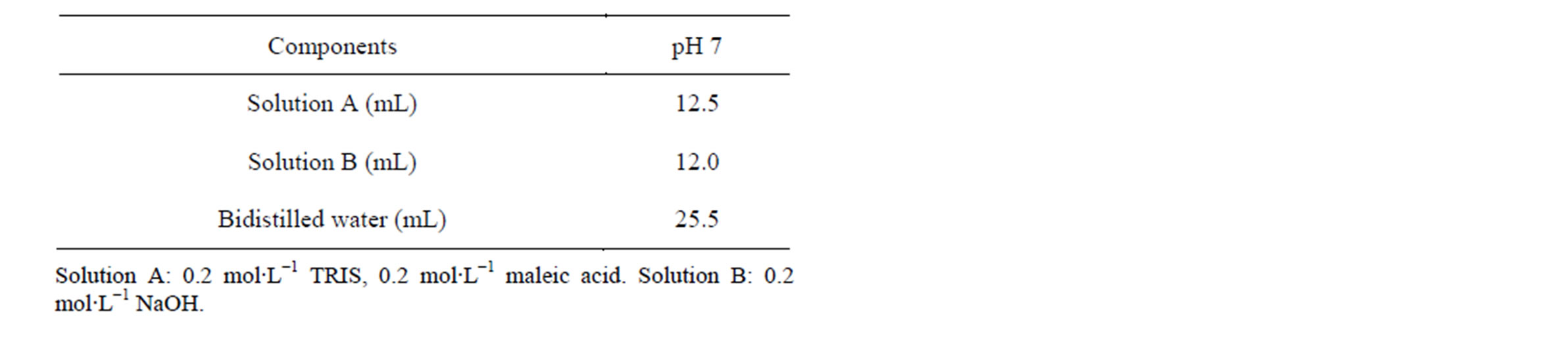
Table 2. Composition of the buffer solution.
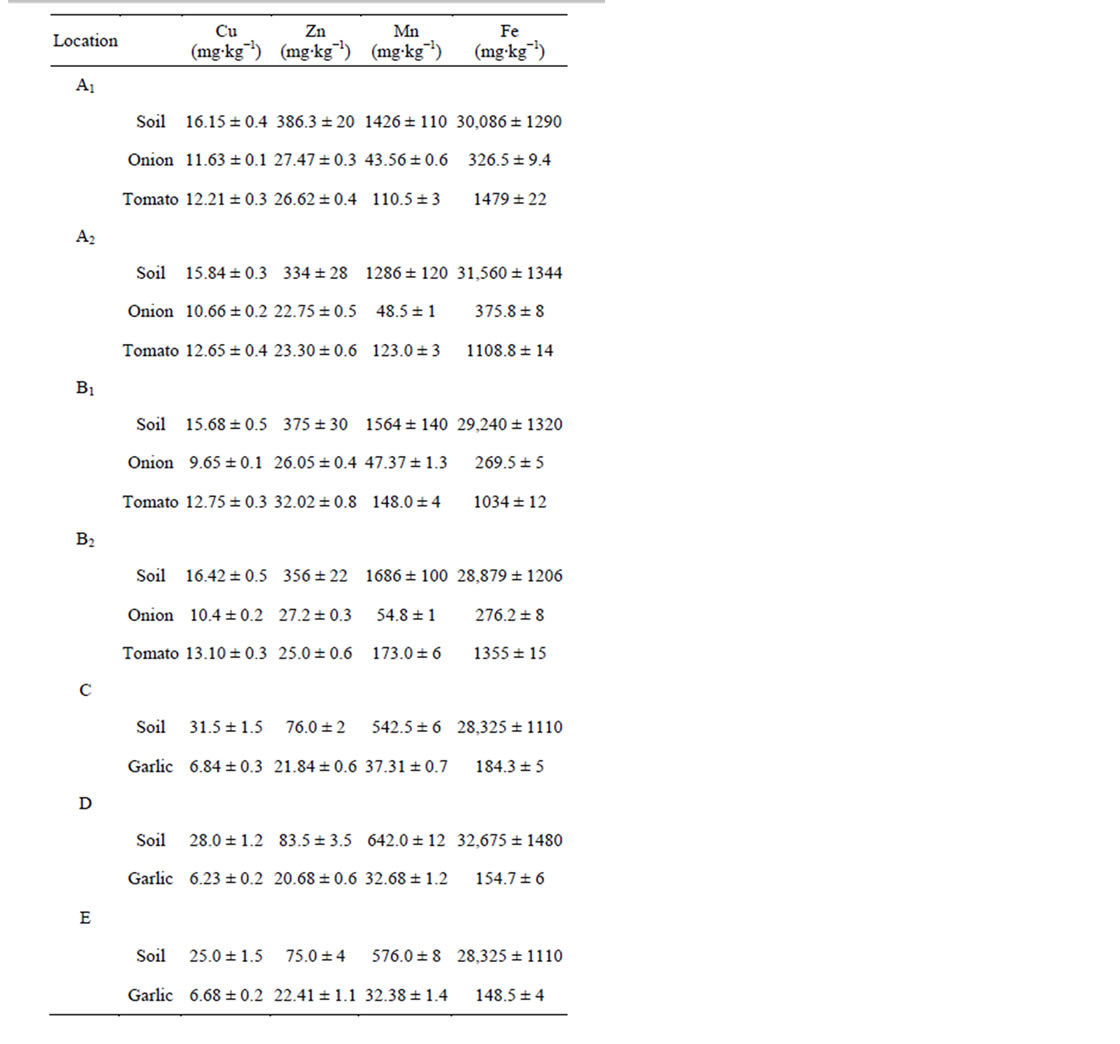
Table 3. Total content of micronutrients in topsoils and leaves (mean ± standard deviation).
Fe > Mn > Zn > Cu.
The obtained values were similar to those of the chemical composition of the earth’s crust, and the levels fell within the range found for other agriculture soils of different regions of the world, thus the critical values for plant growth were not exceeded [4].
The analysis of the leaf and soil samples showed that soil samples contained relatively high levels of micronutrients, since soils play an important role in the accumulation and availability of metal species for plant uptake.
3.2. Single Extraction Procedure
Amounts of Cu, Zn, Mn and Fe extracted using 10−3 mol∙L−1 and 3 × 10−2 mol∙L−1 EDTA are shown in Table 4. Total contents are also included for easy comparisons.
EDTA-extractable amounts of trace elements were as follow: Fe > Mn > Cu > Zn. EDTA solutions extracted several chemical pools that include plant available form, exchangeable cation, labile and complexed species.
The single extraction procedure with EDTA (10−3 mol∙L−1 and 3 × 10−2 mol∙L−1) was validated by good inter-laboratory reproducibility for Cu, Zn, Mn and Fe. Moreover, this technique was readily available, economically practical, and provided quantitative results rapidly. The percentage of extractable amounts is shown in Table 5.
3.3. Soil-Plant Transfer Study
The soil-plant transfer coefficients were calculated as the ratio of the total metal concentration in the plant to the total metal concentration in the corresponding surface soil horizon where the plant had grown in order to establish a relative sequence between Cu, Zn, Mn and Fe mobility in the different sites.
The results (listed in Table 6) were in agreement with other reports [2,4,19]. These studies pointed out the plant ability to uptake metal species from soil solutions preferably in exchangeable forms. Their bioaccessibility through overground parts of plants, mainly leaves, is significant.
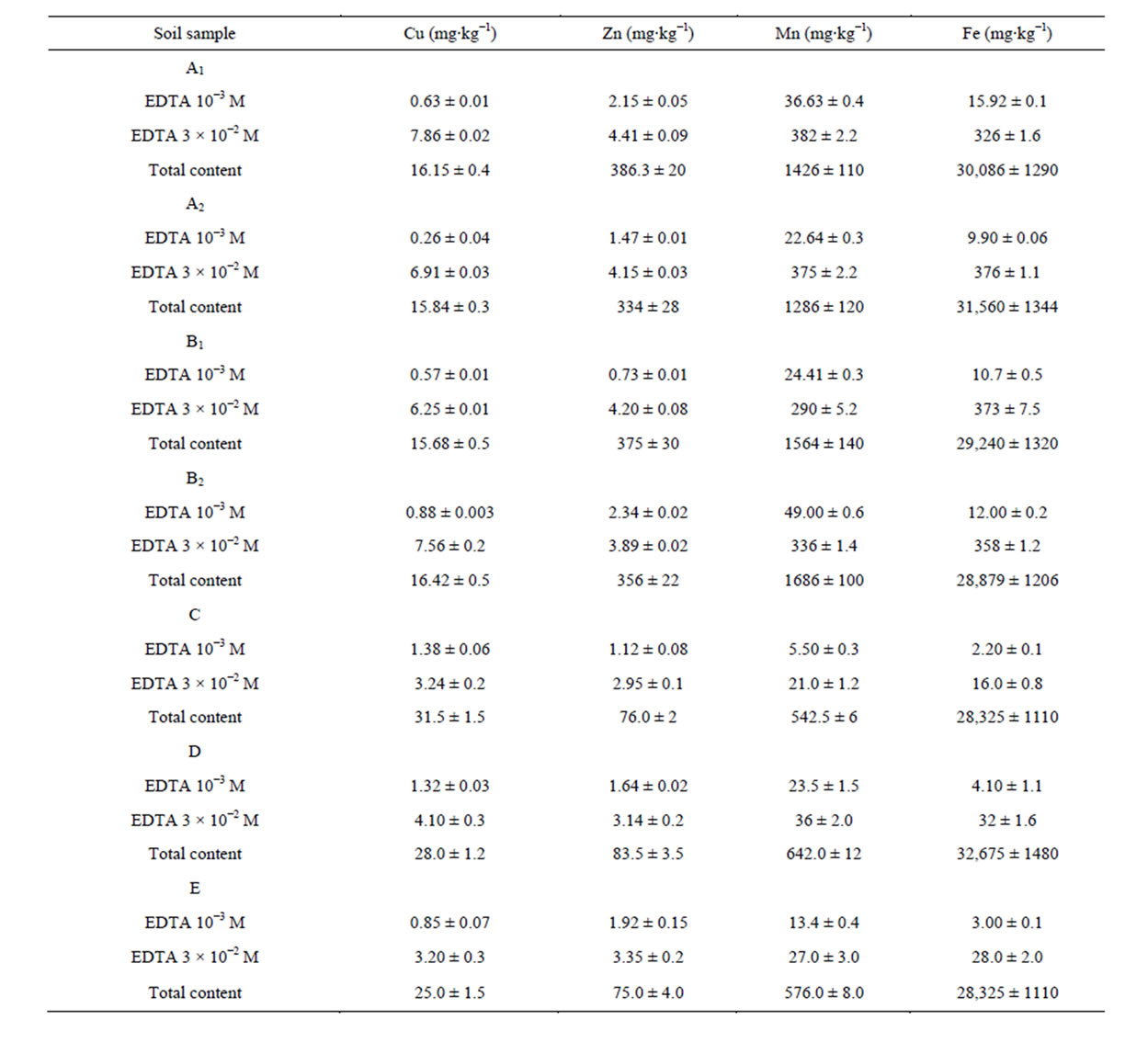
Table 4. EDTA-extractable Cu, Zn, Mn and Fe. Total contents are included (mean ± standard deviation).
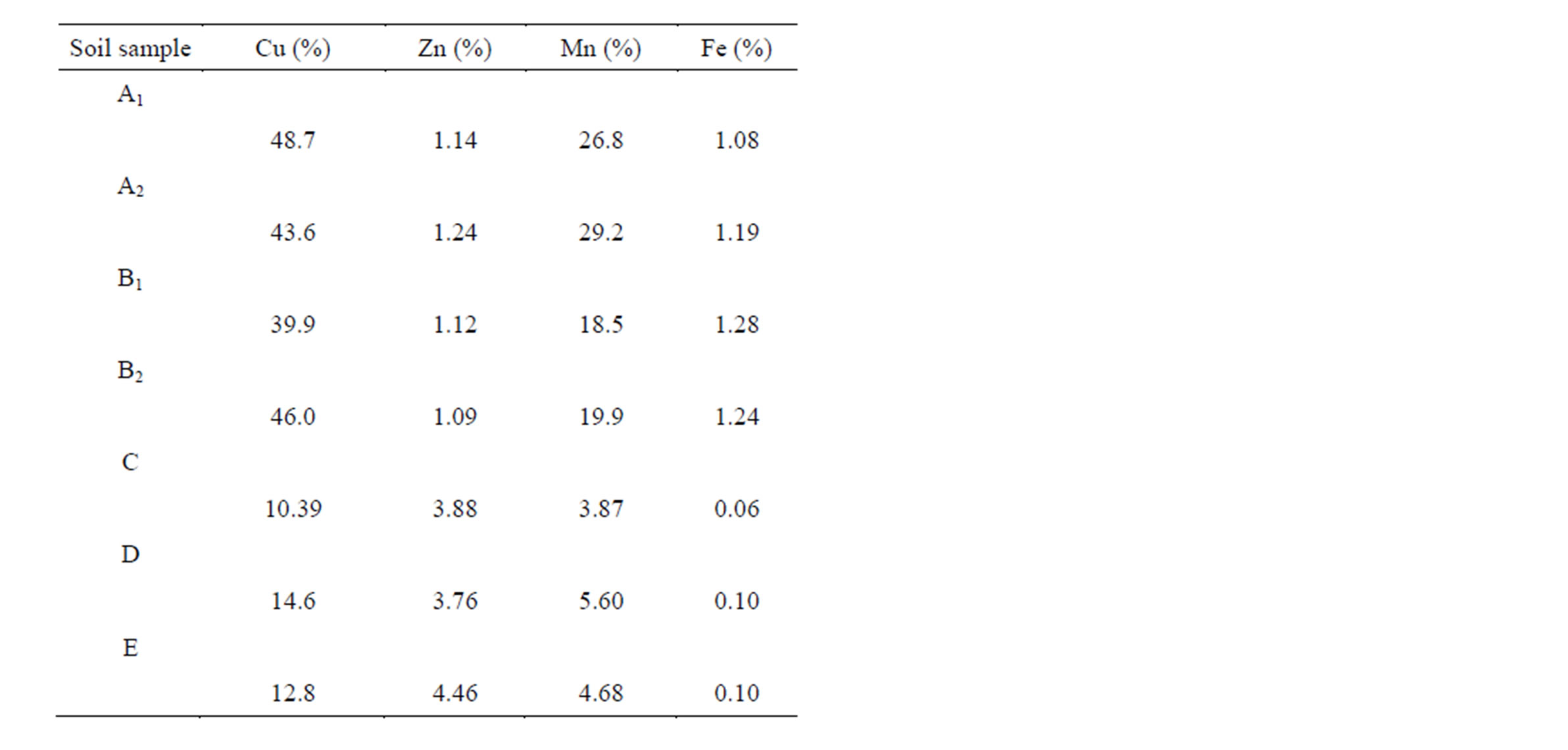
Table 5. Percentage of EDTA (3 × 10−2 mol∙L−1) extractable amounts.
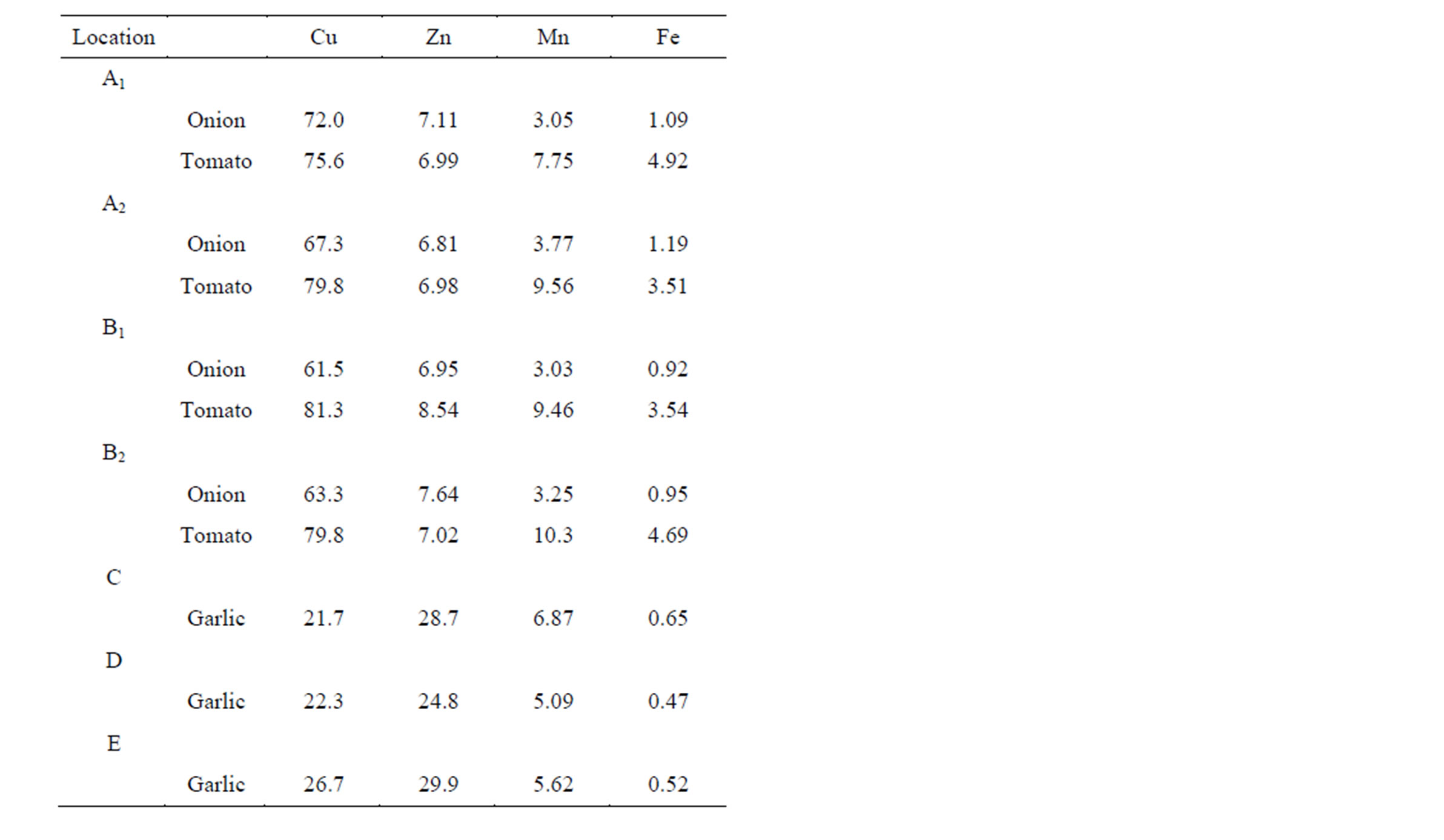
Table 6. Soil-plant transfer coefficients in percentages (%).
4. Discussion
Total content of micronutrients in soil was within the maximum allowable concentration reported in the literature [4].
Soil-plant transfer coefficients (Table 6) allowed assessing the intensity of the plant intake, phytoavailability and phytoaccumulation of Cu, Zn, Mn and Fe micronutrients for each examined site. The results showed that Cu and Zn were the most phytoavailable from all the microelements present in the ecosystems. These metal species were significantly absorbed and accumulated by the plants under study.
Copper was characterized by the highest soil-plant transfer coefficients (normalized for 100%) and the highest phytoaccumulation (Cu soil-plant transfer coefficients ranged from 21.7% to 81.3%) of all the microelements in these ecosystems.
In contrast, Fe was mostly inert to the plants ability to uptake this micronutrient in spite of the fact that Fe was found at very high concentration levels in the soils of the sampling sites.
The extraction yield data, observed as percentage of EDTA (3 × 10−2 mol∙L−1) extractable metal (Table 5), allowed assessing the mobility of these microelements for each examined site. Based on the extraction yields from the single extraction procedure, it can be concluded that Cu was the most mobile metal species of all the studied elements in soils A1, A2, B1 and B2.
Therefore, the calculated soil-plant transfer coefficients could be compared with the extraction yield data of the single extraction with EDTA. The lowest extraction yields were observed in soils C, D and E, which presented rather different properties.
Copper is an essential micronutrient and has very important biological functions in plant growth. Its extraction yield for the single extraction procedure was completely consistent with the soil-plant transfer coefficients obtained for all the examined sites.
Iron can be also considered an important plant micronutrient, but at high concentrations it can be toxic. The calculated soil-plant transfer coefficients for individual plants and the extraction yield values were very low. This indicated that the content of phytoavailable Fe and its soil-plant mobility were very low.
Manganese is an essential element for plants, but at high concentrations it can also cause toxicity. The extraction yield values for the single EDTA extraction procedure were relatively high compared with the calculated soil-plant coefficients.
Zinc is an essential element for plant nutrition, but with increasing Zn content Zn toxicity also increases. The increase in the content of available Zn forms in soil caused their higher concentration in overground parts of plants, mainly in soils C, D, and E.
The chemical composition of the leaves reflected, in general, the elemental composition of the growth media. Some ranges of trace element concentrations and their classification for the mature leaf tissue are presented in Table 7. They are general approximations. It is worth noting that concentration ranges of trace elements re-
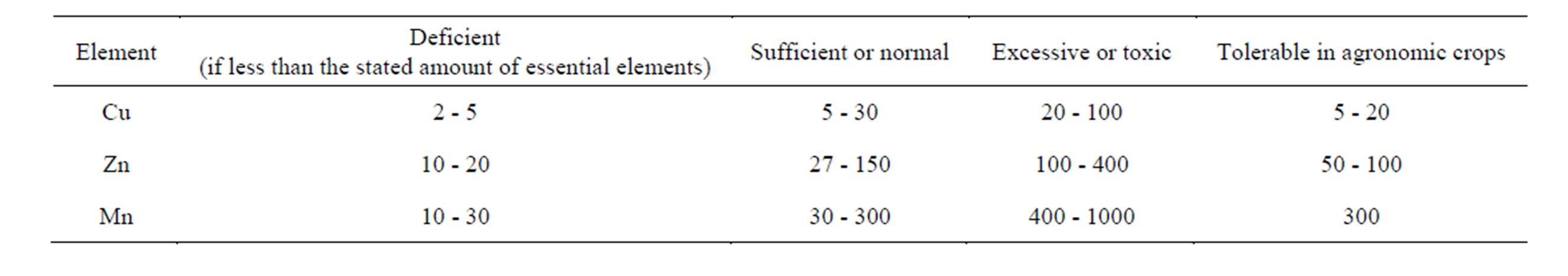
Table 7. Approximate concentrations of trace elements in mature leaf tissue generalized for various species (ppm) [4].
quired by plants are often very close to the content that exerts a harmful influence on plant metabolism. It is not easy, therefore, to make a clear division between sufficient and excessive quantities of trace metals in plants.
All the microelements (Cu, Zn, Mn and Fe) in the leaf samples were within the maximum permissible limits.
5. Conclusions
Total micronutrient content and the extractable fraction in the soils did not exceed the critical values for plant growth. These results were in agreement with the values found for the leaf samples grown in those soils.
Metal ions such as Fe2+ and Fe3+, mainly associated with the aluminosilicate lattice of soils, could not be classified as biologically available and hence they showed reduced mobility in the soil systems.
The results of this study can be useful to predict qualitative metal transfer mobility in similar soil-plant systems in different regions of the world.
It can be concluded that the extraction yield data and the soil-plant transfer coefficients confirmed that the soils in the Southwest of the Buenos Aires Province (Argentina) have an appropriate content of micronutrients that permit the satisfactory growth of important crops.Acknowledgements
The authors gratefully acknowledge the financial support of the Universidad Nacional del Sur.
REFERENCES
- N. Manouchehri, S. Besancon and A. Bermond, “Major and Trace Metal Extraction from Soil by EDTA: Equilibrium and Kinetic Studies,” Analytica Chimica Acta, Vol. 559, No. 1, 2006, pp. 105-112. http://dx.doi.org/10.1016/j.aca.2005.11.050
- J. Kubová, P. Matús, M. Bujdós, I. Hagarová and J. Medved, “Utilization of Optimized BCR Three-Step Sequential and Dilute HCl Single Extraction Procedures for Soil-Plant Metal Transfer Predictions in Contaminated Lands,” Talanta, Vol. 75, No. 4, 2008, pp. 1110-1122. http://dx.doi.org/10.1016/j.talanta.2008.01.002
- K. Hohda, H. Goda, K. Itoh, K. Samejima and T. Fukuuchi, “Aged Garlic Extract Reduces ROS Production and Cell Death Induced by 6-Hydroxydopamine through Activation of the Nrf2-ARE Pathway in SH-SY5Y Cells,” Pharmacology & Pharmacy, Vol. 4, No. 1, 2013, pp. 31- 40. http://dx.doi.org/10.4236/pp.2013.41004
- A. Kabata-Pendías and H. Pendías, “Trace Elements in Soils and Plants,” 3rd Edition, CRC Press LCL, Boca Raton, 2001.
- O. Schramel, B. Michalke and A. Kettrup, “Study of the Copper Distributions in Contaminated Soils of Hop Fields by Single and Sequential Extraction Procedures,” The Science of the Total Environment, Vol. 263, No. 1-3, 2000, pp. 11-22. http://dx.doi.org/10.1016/S0048-9697(00)00606-9
- V. H. Kennedy, A. L. Sanchez, D. H. Oughton and A. P. Rowland, “Use of Single and Sequential Chemical Extractants to Assess Radionuclide and Heavy Metal Availability from Soils for Root Uptake,” Analyst, Vol. 122, No. 8, 1997, pp. 89R-100R. http://dx.doi.org/10.1039/a704133k
- A. Sahuquillo, A. Rigol and G. Rauret, “Overview of the Use of Leaching/Extraction Tests for Risk Assessment of Trace Metals in Contaminated Soils and Sediments,” Trends in Analytical Chemistry, Vol. 22, No. 3, 2003, pp. 152-159. http://dx.doi.org/10.1016/S0165-9936(03)00303-0
- K. N. Wang, R. McSorley and R. N. Gallaher, “Relationship of Soil Management History and Nutrient Status to Nematode Community Structure,” Nematropica, Vol. 34, No. 1, 2004, pp. 83-95.
- R. Pardo, M. Vega, L. Debán, C. Cazurro and C. Carretero, “Modelling of Chemical Fractionation Patterns of Metals in Soils by Two-Way and Three-Way Principal Component Analysis,” Analytica Chimica Acta, Vol. 606, No. 1, 2008, pp. 26-36. http://dx.doi.org/10.1016/j.aca.2007.11.004
- M. Nieves-Cordones, M. A. Martínez Cordero, V. Martínez and F. Rubio, “An NH4+-Sensitive Component Dominates High-Affinity K+ Uptake in Tomato Plants,” Plant Sciences, Vol. 172, No. 2, 2007, pp. 273-280. http://dx.doi.org/10.1016/j.plantsci.2006.09.003
- S. G. Acebal, A. Mijovilovich, E. H. Rueda, M. E. Aguirre and C. Saragovi, “Iron-Oxide Mineralogy of a Mollisol from Argentina: A Study by Selective-Dissolution Techniques, X-Ray Diffraction, and Mössbauer Spectroscopy,” Clays and Clay Minerals, Vol. 48, No. 3, 2000, pp. 322-330. http://dx.doi.org/10.1346/CCMN.2000.0480303
- M. Blanco and G. Stoop, “Genesis of Pedons with Discontinuous Argillic Horizons in the Holocene Loess Mantle of the Southern Pampean Landscape, Argentina,” Journal of South American Earth Sciences, Vol. 23, No. 1, 2007, pp. 30-45. http://dx.doi.org/10.1016/j.jsames.2006.08.007
- Soil Survey Staff-USDA, “Soil Taxonomy: A Basic System for Classifying Soils,” Agriculture Handbook, Vol. 436, 1999, p. 863.
- G. W. Gee and J. W. Bauder, “Particle-Size Analysis,” In: A. Klute, Ed., Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods, American Society of Agronomy and Soil Science Society of America, Madison, 1986, pp. 399-403.
- M. E. Sumner and W. P. Miller, “Cation Exchange Capacity and Exchange Coefficients,” In: D. L. Spark, Ed., Methods of Soil Analysis, Part 3, Chemical Methods, Soil Science Society of America and American Society of Agronomy, Madison, 1996, pp. 1201-1229.
- M. D. P. Moralejo, “Complejantes Fosfónicos como Agentes de Extracción de Elementos Metálicos en Suelos,” PhD Dissertation, Universidad Nacional del Sur, Argentina, 2010.
- L. R. Hossner, “Dissolution for Total Elemental Analysis,” In: D. L. Spark, Ed., Methods of Soil Analysis, Part 3, Chemical Methods, Soil Science Society of America and American Society of Agronomy, Madison, 1996, pp. 49- 65.
- E. Wieteska, A. Zióek and A. Drzewińska, “Extraction as a Method for Preparation of Vegetable Samples for the Determination of Trace Metals by Atomic Absorption Spectrometry,” Analytica Chimica Acta, Vol. 330, No. 2-3, 1996, pp. 251-257. http://dx.doi.org/10.5251/ajsir.2010.1.2.158.163
- M. A. Nkansah and C. Opoku Amoako, “Heavy Metal Content of Some Common Spices Available in Markets in the Kumasi Metropolis of Ghana,” American Journal of Scientific and Industrial Research, Vol. 1, No. 2, 2011, pp. 158-163. http://dx.doi.org/10.5251/ajsir.2010.1.2.158.163

