American Journal of Plant Sciences
Vol.4 No.5A(2013), Article ID:32252,4 pages DOI:10.4236/ajps.2013.45A007
Asplenium nidus; The Bird’s Nest Fern: Developmental Studies and Its Conservation
![]()
Department of Botany, University of Delhi, Delhi, India.
Email: *contactrsri@gmail.com
Copyright © 2013 Ruchi Srivastava, P. L. Uniyal. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 16th, 2013; revised April 20th, 2013; accepted May 5th, 2013
Keywords: Reproductive Biology; Asplenium nidus
ABSTRACT
Asplenium nidus L. commonly called as Bird’s Nest Fern, is a threatened, ornamental fern, which is widely used as novel foliage ornamental plant and local people use it in worship. The taxon is threatened due to over exploitation, habitat destruction and genetic barriers. To understand the constraints in the regeneration, reproductive biology studies are made. It is observed that more sporophytes are produced in composite population (13.3%) in comparison to isolate population (10%). This pattern is suggestive of the fact that the parental sporophyte is heterozygous for recessive sporophytic lethal. On the basis of the results obtained A. nidus was initially adapted for outbreeding with the capacity for considerable amount of inbreeding. The low potential of sporophyte production in isolate gametophyte could be the constitution of the zygotic genotype.
1. Introduction
Asplenium nidus (Bird’s Nest fern) is a threatened ornamental plant belongs to family Aspleniaceae [1]. It is commonly used in horticulture trait as decorative plant, local people use it in worship, and in folk medicine (to treat asthma, sores and weakness) and hygienically to treat halitosis (bad breath) [2]. It occurs as epiphyte, as well as terrestrial plant and prefer warm, humid areas in partial to full shade and grows on rich organic matter.
The taxon is becoming rare and threatened day by day due to climatic factors, over exploitation for nursery trade and also by genetic barriers. Present study deals the reproductive biology of Asplenium nidus, which would give an insight for conservation strategies.
2. Material and Methods
Fertile fronds with ripen spores of A. nidus were collected from Calicut, Kerala. The fronds were kept in brown paper packets and stored in desiccator for the release of the spores at room temperature. The spores were surface sterilized with 2 percent sodium hypochlorite solution for two minutes and rinsed thoroughly with double distilled water before sowing in the petriplates in sterilized Parker’s macro—and Thompson’s micronutrient culture medium (P & T) gelled with 1 per cent agar. The culture plates were kept in the culture room having light intensity ranges between 47.3 µmol·m−2·sec−1 - 56.8 µmol·m−2·sec−1 at 23˚C ± 2˚C temperature for 16 hr light photo period followed by 8 hr dark photo period.
Periodically the spore germination and subsequent gametophyte growth, differentiation and sex ontogeny were observed under Nikon trinocular Eclipse 80i microscope and the photographs were taken using a camera Nikon DS Fi 1.
Periodic observations of gametophytes of stock culture were made and ratios of gametophytes bearing male, female or bisexual conditions were recorded (Table 1). Before initiation of gametangia in stock cultures, the gametophytes were isolated and placed in different petriplates containing P & T media in the following manner:
1) 25 petriplates with single gametophyte in each (isolate culture); and 2) 10 petriplates with 25 gametophytes in each petriplates (composite culture).
After the initiation of gametangia, all the isolates and composite populations were flooded from above with sterile distilled water twice in a week for bursting the antheridia and release of antherozoids, which fertilized the egg of archegonium. Percentage of sporophytes produced at each level was recorded (Table 2).
Five plates of isolate and two of composite population were kept unwatered throughout the course of experiment to check the sexuality of the species. These populations did not produced sporophytes thus proved the sexual nature of the species. Five composite cultures were also maintained up to six months to observe the regeneration pattern of the gametophyte. Periodic sowing of spores was done to check their viability.
3. Observations
Spores of A. nidus are monolete, bilateral 22 × 37 m (at X 240) in size, polar and Vittaria-type germination is found [3] (Figures 1(a) and (b)). About 47 percent spores were germinated after 47 days of spore sowing which became 100 percent after 60 days of spore sowing. The viability of spores was totally lost after six months when kept at room temperature.
The spore coat ruptures at the laesura region leading to formation of first hyaline rhizoid and chlorophyllous protonemal initial cell (Figure 1(c)). The protonemal initial cell divided transversely repeatedly and a filamentous, unidiamensional protonema consisting of 5 - 6 cells was developed (Figure 1(d)). Further repeated transverse
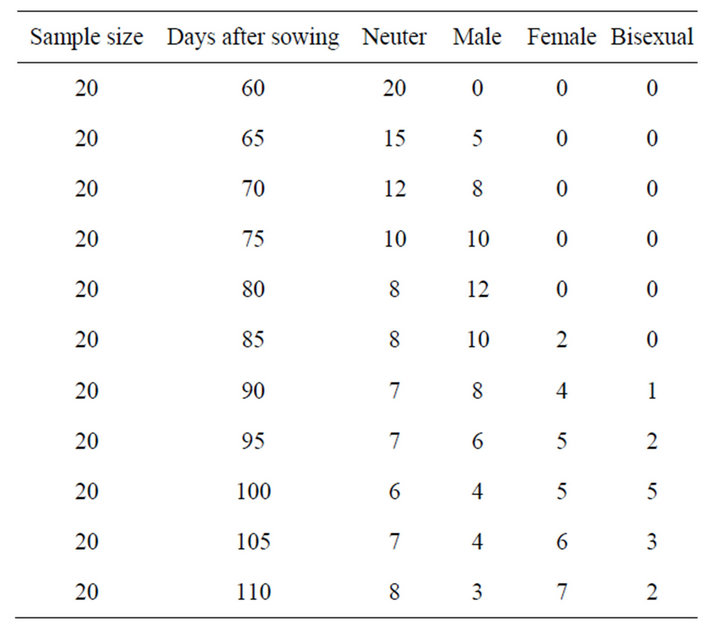
Table 1. Chronological changes in sex ratio of a composite culture in A. nidus.
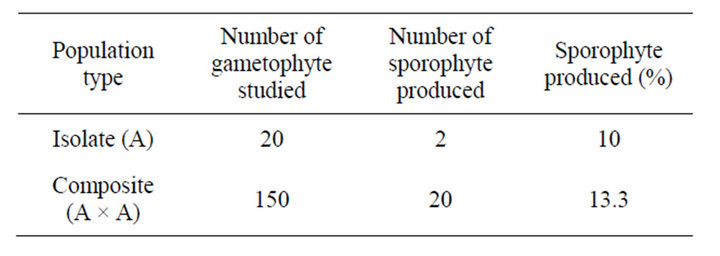
Table 2. Breeding behaviour of different population in A. nidus.
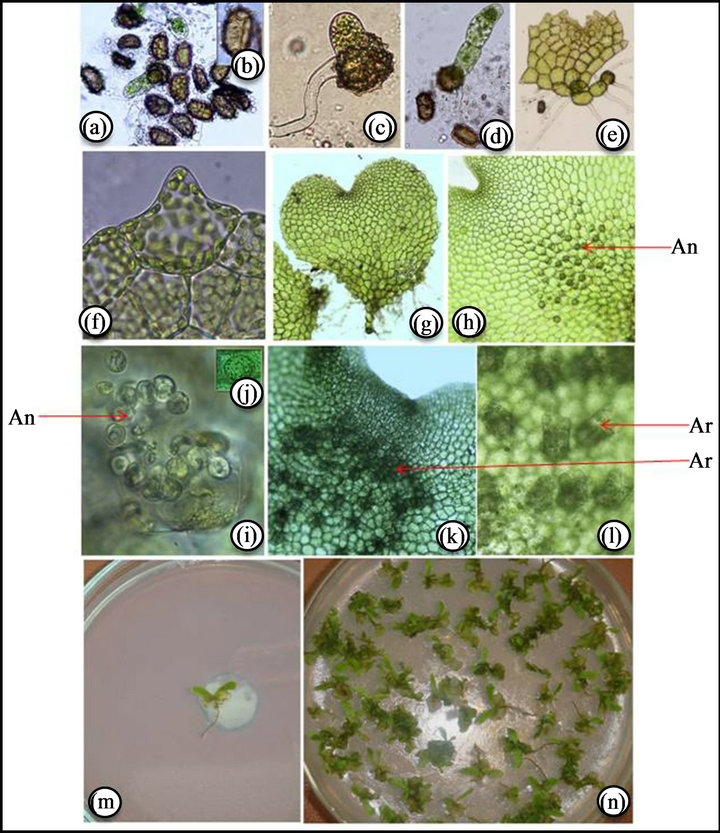
Figure 1. (a), (b) Monolete spores; (c) Emerging protonema; (d) Filamentous stage; (e) Two-diamensional stage; (f) Unicellular hair; (g) Cordate gametophyte; (h) Antheridia showing in between rhizoid of gametophyte; (i) Enlarged view of antheridia; (j) Single antheridium; (k) Archegonia below the apical notch; (l) Enlarge view of archegonia; (m) Sporophyte in isolate culture; (n) Sporophyte in composite culture.
divisions in terminal and subterminal cells developed a two-diamensional protonema after 15 days of spore germination (Figure 1(e)). Subsequently repeated longitudinal and transverse divisions in the daughter cells of the two diamensional protonema formed a broad spatulate prothallus. In some case the anterior region of the germ filament may not take part in the formation of an expanded thallus and may be sluggish. In such cases a prothallial plate is formed from the intercalary cell. Prothallial development of Asplenium mainly of the Aspidiumtype, with most of the germ filaments developing an obconical meristematic cell in one of the daughter ceils of the terminal cell and the terminal cell developing a hair before initiating plate formation. In such cases the prothalli are hairy from the early stages of development onwards [3]. The adult prothallus was cordate, thalloid in nature (Figure 1(f)). The prothalli have papillate protrusion on the peripheral walls of the marginal cells. These are simple, unicellular hairs and are mammillate with a conspicuously swollen base and suddenly narrowed to short, subconieal apex (Figure 1(g)) [3,4].
Gametangial development is of common leptosporangiate type. After 60 days of spore sowing, antheridia developed at the posterior zone of gametophyte between rhizoids (Figures 1(h)-(j)) and after 80 days of spore sowing archegonia, were developed below the apical notch (Figures 1(k) and (l)). Within three months the gametophyte became bisexual and maximum bisexual condition were observed after 100 days of sowing. Later the antheridia degenerated and only archegoniate gametophytes were observed which ultimately became neuter. The details of sex ontogeny are appended in Table 1.
In composite populations sporophyte production was 13.3 percent (Figures 1(n) and 2) (Table 2) while in isolate population sporophyte production was 10 percent (Figures 1(m) and 2). No sporophyte was produced in unwatered population, proving the sexual nature of the taxon.
4. Discussion
Asplenium nidus is a threatened taxon due to over exploitation, habitat destruction and genetic barriers [5]. The reproductive biology studies are essential to understand the cause of rarity and success of reproduction.
Greater local adaptation was detected in outcrossers than in selfers when accounting for environmental differences [6], but the difference was not significant when species was included in their model. A significant difference would have indicated that population genetic processes that dominate selfing population or species, such as genetic drift, may limit local adaptation more often than gene flow, which should be more limiting in outcrossing species.
During studies it was observed that more sporophytes were produced in composite population in comparison to isolate population. e.g., in A. nidus, 13.3 percent sporophytes were produced in composite population (Figure 2) (Table 2).
Our observation indicates that A. nidus initially adapted for outbreeding with the capacity for considerable amount of inbreeding. The possible explanation for the lack of sporophyte production in isolate gametophyte could be the constitution of the zygotic genotype. This pattern is suggestive of the fact that the parental sporophyte were heterozygous for recessive sporophytic lethal [7-9].
For the colonization of open or barren habitat, if spores land sufficiently in distance only the chances of intragametophytic selfing are possible. It has been observed that
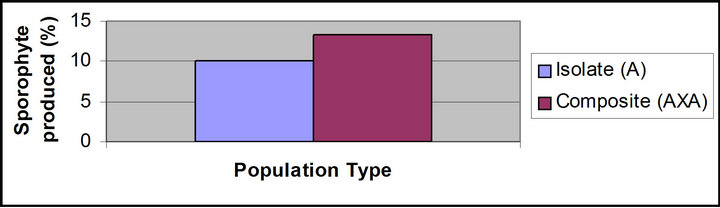
Figure 2. Precentage sporophyte produced in isolate and composite population of Asplenium nidus.
the majority of taxa having intragametophytic selfing and crossing were successful colonizers and rich in population density [10-18].
Since A. nidus have capacity to form sporophyte through intragametophytic selfing thus may colonise the barren land. Thus during the early stages of colonization of new habitat intragametophytic selfing predominates and at later stages mating occurs at the level of intergametophytic selfing. After a number of growing seasons the mode of sexuality will be transformed into almost obligatorily intergametophytic selfing.
5. Acknowledgements
Authors are thankful to Director, National Botanical Research Institute, Lucknow and Council of Scientific and Industrial Research (CSIR), New Delhi for providing providing necessary facilities. We are grateful to the University of Delhi for financial support. The help provided by Manju Nair, Calicut University, Kerala for the collection of plant samples during this study is gratefully acknowledged.
REFERENCES
- S. Chandra, “The Ferns of India (Enumerations, Synonyms & Distributions),” International Book Distributors, Dehradun, 2000.
- http://en.wikipedia.org/wiki/Asplenium_nidus
- B. K. Nayar and S. Kaur, “Gametophytes of Homosporous Ferns,” Botanical Review, Vol. 37, 1971, pp. 295- 396. doi:10.1007/BF02859157
- T. N. Prataptosuwiryo, “Gametophytes of the Bird Nest Fern Asplenium nidus L. (Aspleniaceae) from the West Kalimantan,” Buletin Kebun Raya, Vol. 13. No. 1, 2010, pp. 1-7.
- S. S. Bir, “Pteridophytic Flora of India: Rare and Endangered Elements and Their Conservation,” Indian Fern Journal, Vol. 4, 1987, pp. 95-201.
- J. Hereford, “Does Selfing or Outcrossing Promote Local Adaptation?” American Journal of Botany, Vol. 97, No. 2, 2010, pp. 298-302.
- E. J. Klekowski Jr., “Reproductive Biology of the Pteridophyta III. A Study of the Blechnaceae,” Botanical Journal of Linnean Society, Vol. 62, 1969, pp. 153-169. doi:10.1111/j.1095-8339.1969.tb01973.x
- E. J. Klekowski Jr., “Reproductive Biology of the Pteridophyta II. Theoretical Considerations,” Botanical Journal of Linnean Society, Vol. 62, 1969, pp. 347-359. doi:10.1111/j.1095-8339.1969.tb01972.x
- F. R. Ganders, “Heterozygosity for Recessive Lethals in Homosporous Fern Populations: Thelypteris palustris and Onoclea sensibilis,” Botanical Journal of Linnean Society, Vol. 65, No. 2, 1972, pp. 211-221. doi:10.1111/j.1095-8339.1972.tb00934.x
- P. W. Hedrick, “Genetic Load and the Mating System in Homosporous Ferns,” Evolution, Vol. 41, No. 6, 1987, pp. 1282-1289. doi:10.2307/2409093
- D. Soltis and P. Soltis, “The Distribution of Selfing Rates in Homosporous Ferns,” American Journal of Botany, Vol. 79, 1992, pp. 97-100. doi:10.2307/2445202
- H. Korpelainen, “Intragametophytic Selfing Does Not Reduce Reproduction in Dryopteris filix-max,” Sexual Plant Reproduction, Vol. 9, No. 2, 1996, pp. 117-122. doi:10.1007/BF02153059
- E. A. Hooper and A. H. Haufler, “Genetic Diversity and Breeding System in a Group of Neotropical Epiphytic Ferns (Pleopeltis; Polypodiaceae),” American Journal of Botany, Vol. 84, No. 12, 1997, pp. 1664-1674. doi:10.2307/2446464
- M. S. Lott, J. C. Volin, R. W. Pemberton and D. F. Austin, “The Reproductive Biology of the Invasive Ferns Lygodium microphyllum and L. japonicum (Schizaeaceae): Implications for Invasive Potential,” American Journal of Botany, Vol. 90, No. 8, 2003, pp. 1144-1152. doi:10.3732/ajb.90.8.1144
- P. B. Khare, S. K. Behera, S. Ruchi and S. P. Shukla, “Studies on Reproductive Biology of a Threatened Tree Fern, Cyathea spinulosa Wall. ex Hook,” Current Science, Vol. 89, No. 1, 2005, pp. 173-177.
- R. Srivastava, J. Srivastava, S. K. Behera and P. B. Khare, “In-Vitro Studies on Development of Gametophyte, SexOntogeny and Reproductive Biology of a Threatened Fern, Microsorium punctatum (L.) Copel,” Indian Journal of Biotechnology, Vol. 7, 2008, pp. 266-269.
- R. Srivastava and P. B. Khare, “Development of Gametophyte and Reproductive Biology of a Homosporous Fern; Coniogramma indica from Kumaon Himalaya,” In: M. Singh and A. K. Paliwal, Eds., Advancement of Science and Technology, 2010, pp. 582-588.
- S. Khan, M. Raziq and A. Hammad Kayani, “In Vitro Propagation of Bird’s Nest Fern (Asplenium nidus) from Spores,” Pakistan Journal of Botany, Vol. 40, No. 1, 2008, pp. 91-97.
NOTES
*Corresponding author.

