American Journal of Plant Sciences
Vol.3 No.3(2012), Article ID:17917,7 pages DOI:10.4236/ajps.2012.33042
Cucurbit Host Range of Myrothecium roridum Isolated from Watermelon
![]()
1Wes Watkins Agricultural Research Laboratory, USDA-ARS, Lane, USA; 2USDA-ARS, Stillwater, USA.
Email: wfish-usda@lane-ag.org
Received December 15th, 2011; revised January 6th, 2012; accepted January 29th, 2012
Keywords: Myrothecium roridum; watermelon; leaf and stem blight; cucurbits
ABSTRACT
In 2010, a foliar and stem-lesion disease that produced moderate to severe defoliation of watermelon was observed in the southern Great Plains. The disease was ultimately determined to be caused by Myrothecium roridum. The objective of this study was to compare the susceptibility of the vegetation and fruit of a broad range of commercially important cucurbits to three isolates obtained from these foliar lesions on watermelon. In greenhouse foliar inoculation experiments, cantaloupe, honeydew, cucumber, squash, and watermelon were susceptible to the fungus with cantaloupe and honeydew being the most susceptible and watermelon the most resistant. Furthermore, greenhouse inoculations supported earlier field observations as differential resistance was exhibited among the watermelon cultivars as well as the cucurbit types. All tested cucurbit fruit exhibited interior lesions when inoculated sub-epidermally with M. roridum isolates. However, natural infection of watermelon and pumpkin fruit has never been reported.
1. Introduction
Myrothecium roridum Tode ex Fr. is a common soilinhabiting fungus with a relatively wide host range that includes such agronomic crops as cotton, tomato, cocao, coffee, potato, soybean, and cucurbits, as well as various ornamental plants [1-3]. M. roridum has been demonstrated to be seed-transmitted in numerous cases, including cucurbits [4-7], and has been evaluated for biocontrol of water hyacinth [3,8]. Diseases caused by M. roridum are generally thought to be associated most frequently with warmer environments during wet conditions [1,9, 10]. In contrast, M. roridum was recently reported as an endophyte of the gymnosperm, Pinus albicaulis, at high elevation in Oregon [11].
The first known record of M. roridum on cucurbits appears to be from Mexican cantaloupe (Cucumis melo) intercepted at the Texas border in 1950 and later in the Rio Grande Valley of Texas in 1961 [10]. Although watermelon (Citrullus lanatus) has been reported as a host for M. roridum in greenhouse testing [3,6,12], the first report of M. roridum causing a leaf spot in watermelon under field conditions was from Korea in 2003 [13]. The disease was subsequently reported in Georgia (USA) in 2005 [14] and in Oklahoma (USA) in 2012 [15].
Greenhouse studies have demonstrated that isolates of M. roridum from diseased field plants of cucumber (Cucumis sativus), gherkin (Cucumis anguria), and squash (C. moschata) caused disease symptoms on watermelon, pumpkin, and cantaloupe as well as the original host plants [12]. Cabral et al. [12] noted that cucurbit isolates demonstrated differential aggressiveness when inoculated onto cucumber, squash, watermelon, and cantaloupe. They further stated that watermelon was the least susceptible of the cucurbits tested. Bean et al. [16] reported that a M. roridum isolated from cantaloupe fruit produced the trichothecene, roridin E, and later demonstrated that the presence of this mycotoxin was related to lesion size [17]. A correlation between sensitivity to roridin E produced by some isolates of M. roridum and level of resistance exhibited by cucurbits has been reported [17,18].
Three distinct phases of disease caused by M. roridum have been observed in cucurbits: leaf spot, crown and stem canker, and fruit rot, also known as crater rot [1]. In 2010 at the Wes Watkins Agricultural Research Laboratory, Lane, OK, an outbreak of leaf spot and stem canker occurred in a 0.5 hectare experimental field in which were growing twenty different cultivars of watermelon. This disease was ultimately determined to be caused by Myrothecium roridum [15]. The objective of this study was to determine the susceptibility of selected cucurbits to the fungus, specifically M. roridum isolates from these diseased watermelon plants.
2. Materials and Methods
2.1. Isolation and Determination of M. roridum
Isolations were performed from leaf lesions of three watermelon plants that exhibited moderate to severe disease symptoms in the field [15]. Diseased tissue was plated out on potato dextrose agar (PDA) and incubated for 5 days under laboratory conditions. Mycelia from colonies that emerged from the plated leaf tissue were hyphaltipped and transferred to PDA. The M. roridum isolates from watermelon vegetation were designated 46-100117, 46-100137, and 46-100138.
2.2. Host Range of Commercially Important Cucurbits
Pathogenicity tests, using M. roridum isolates 46-100117, 46-100137, and 46-100138 from watermelon vegetation were carried out on healthy seedlings of various cucurbits. Watermelon cultivars tested were “AC 7177”, “Dixie Lee”, “Sangria”, and “Sugar Baby”. Cantaloupe cultivars tested were “Bella Tuscana”, “Caravelle”, and “Magnum 45”. Cucumber cultivars tested were “Dasher II” and “Poinsett 76”. The squash (Cucurbita pepo) cultivar tested was “Lemon Drop”. The honeydew cultivar tested was “TamDew Improved”. Seedlings were planted, grown, and tested in Speedling trays (Speedling Inc., Sun City, FL) (8 rows of 16 cells). Every other 16 cell row in the tray was left empty so that a flat contained up to 64 seedlings at the two true-leaf stage. Rows of seedlings of each cultivar were randomly grown among the flats so that a total of 34 - 40 plants (samples) of each of the eleven cucurbit cultivars were tested. A suspension of 1 x 106 conidia per ml was applied to leaves and stems with a Nalgene aerosol spray bottle (Thermo Scientific, Rochester, NY). Control plants (17 - 23 plants of each of the eleven cultivars) were sprayed with sterile distilled water. After treatment, flats of plants were sealed inside a plastic dew chamber at 21˚C - 29˚C and 100% humidity for 17 hr. Flats were then removed from the chamber and placed in the greenhouse. PDA plates were sprayed with the same suspension and placed in the plastic dew chambers along with the inoculated plants to make sure the environmental conditions and 17 hr time-frame were conducive to conidial germination. To this end, more than 95% of the spores had germinated and a germ-tube at least 3 times longer than the spore diameter had formed after 17 hr. Temperatures in the greenhouse ranged between 30˚C ± 6˚C during the day and 21˚C ± 2˚C at night. Plants were watered twice daily. The experiment was conducted twice for each of the three M. roridum isolates.
2.3. Disease Ratings and Statistical Analyses
Disease ratings were made on treated plants and their controls 7 days after inoculation. The disease rating system employed was an interval scale of 0 to 4 with 0 being healthy; 1 = 1% to 25% of the leaf or cotyledon exhibiting leaf spot, 2 = 26% to 50%, 3 = 51% to 75%, and 4 = 76% to 100%. Separate disease ratings were made on the cotyledons and the first two true-leaves. The experimental design was a factorial with two replications of each treatment combination of fungal isolate and cucurbit host and 34 - 40 samples (plants) for each treatment combination. Analyses of variance were performed to determine significance of main effects and interaction for ratings of cotyledons and true-leaves using the Kenward-Roger method to compute denominator degrees of freedom. Least squares means were computed and compared at P ≤ 0.05 using Tukey’s adjustment. Normally, more than 2 replications are desirable. The interaction of isolates with cultivars was significant at P ≤ 0.05 with just 2 replications in an experiment designed to determine if there were effects of isolate, cultivar or the interaction of isolates with cultivars.
2.4. Inoculation of Various Cucurbit Fruit with the Fungal Isolates from Watermelon
The host range of various cucurbit fruit to Myrothecium roridum was examined by inoculation with the three fungal isolates. Watermelon (“Jubilee”), cantaloupe (“Caravelle”), and pumpkin (“Fall Splendor”) (Cucurbita pepo), grown at the Lane Research Center, were used for fruit inoculations. Cucumber fruit (cv. unknown) were purchased from a local retail grocery. The fruit were washed using warm water and dish soap and then allowed to dry. Each of 4 to 6 fruit representing the various cucurbits was inoculated at multiple sites (3 to 5) with one of the three fungal isolates by a procedure previously described [19]. Fruit were inoculated by surface disinfesting with 80% ethanol and removing a cylinder of tissue aseptically (1 cm deep) with a cork borer (0.7 cm diameter). The isolates were grown on PDA for 7 days at 25˚C prior to inoculation. A PDA disc (0.5 cm diameter) colonized by the fungus was placed into each inoculation site, covered with a small autoclaved cotton ball, and sealed with Kwik Seal Caulk (DAP Inc., Dayton, Ohio). Fruit inoculated with PDA discs without the fungus served as controls. The inoculated fruit were maintained on the laboratory bench at 25˚C ± 2˚C. After 6 days, the fruit were cut perpendicular to the inoculation sites and the resulting lesions were traced onto transparent film. The area of fungal decay was calculated using an area meter (Li-Cor, Lincoln, NE, USA) and analyzed as a completely randomized factorial experiment, with factors being type of cucurbit and pathogen isolate.
3. Results
Fungal colonies obtained from the diseased watermelon leaf lesions reached 40 - 60 mm in diameter after 14 days at 25˚C. The fungal colonies were white, floccose, wrinkled, and somewhat raised in the center. Sporulation occurred throughout the colony in concentric greenishblack zones. These zones consisted of groups of conidiophores forming sporodochia. Conida were rod-shaped (1.5 - 2 μm × 5 - 10 μm). The characteristics of the fungus were consistent with those reported for M. roridum [9,20]. When sections of symptomatic tissue from test seedlings were plated out (see below), they yielded only M. roridum colonies, thereby fulfilling Koch’s Postulates.
Healthy seedlings of several cucurbits were subjected to the M. roridum isolates to examine the vegetative host range of the fungus. The progress of the disease after 7 days consisted of small tan lesions 1 - 3 mm across on cotyledons and leaves; all five types of cucurbits exhibited some disease symptoms. For cotyledons, there was a significant interaction (P ≤ 0.05) between cultivars and fungal isolates (Table 1). However, when grouping the cultivars by cucurbit type, the interaction of fungal isolate and cucurbit type were not significant. Isolates 46-100137 and 46-100138 were significantly (P ≤ 0.05) more aggressive on cotyledons as compared to 46- 100117. “Bella Tuscana” cantaloupe was the most highly susceptible cucurbit tested; it ranked in the top 10 of all treatment combinations (Table 1). On the other hand, “Sangria” watermelon was the least susceptible cucurbit tested; it ranked in the bottom 5 of all treatment combinations. The average cotyledon disease ratings ranged from 0.18 to 1.51 over the five cucurbit types with cantaloupe having the highest rank and watermelon the lowest.
Analysis of the disease rating on true leaves showed a significant (P ≤ 0.05) interaction for cucurbit type, isolate, and cucurbit type by isolate. The average true-leaf disease ratings ranged from 0.11 to 1.67 (Table 2). Cantaloupe and squash occupied the first 7 ranks of the average disease rating on true leaves. Fungal isolate 46- 100137 tended to be the most aggressive on all cucurbit types. Although the leaf ratings were slightly different, they followed a pattern similar to that observed on the cotyledons. Again, “Bella Tuscana” exhibited some of the highest levels of disease and “Sangria” watermelon exhibited some of the lowest (Table 2).
Table 3 presents the host range of a selection of cucurbit fruit inoculated with M. roridum. In contrast to foliar inoculations, watermelon fruit were more susceptible than cantaloupe fruit. All fruit tested exhibited lesions; the average lesion size ranged from 1.19 cm2 in cantaloupe to 3.23 cm2 in watermelon. There was a significant interaction between isolate and cucurbit type. There was no significant (P ≤ 0.05) isolate effect in the fruit.
4. Discussion
The recent plethora of first reports of M. roridum causing disease on plants here-to-fore not observed to be susceptible [11,13,14,21-23] suggests that either regional change in weather resulting in local growing conditions more conducive to M. roridum infection has occurred or an evolution of M. roridum sub-species or races has occurred that facilitates a broader host range.
Even though there are numerous reports of M. roridum as a pathogen of cucurbits, little is known about the environmental conditions most conducive to disease development. Several reports [1,9,10] suggest that relatively high temperatures and frequent rain events are prerequisite. In contrast, Chase and Poole [24] noted that 21˚C to 27˚C was optimum for disease development in Dieffenbachia maculate and temperatures of 30˚C or higher inhibited lesion formation. Although most inoculation studies have used temperatures in the range of 25˚C, Fitton and Holliday [9] reported the optimum temperature for conidial germination was 28˚C.
Duration of leaf wetness is another relatively unknown requirement for infection and disease development. Incubation periods of 100% RH following inoculation of cucurbits have ranged between 18 - 72 hr [3,6,12,14]. In the present study, a 17 hr incubation period in the dew chamber was maintained before placing the flats in the greenhouse. Within 2 to 3 days, small leaf spots about 1 to 2 mm in diameter could be observed. Once the plants were placed in the greenhouse at about 30˚C and ≤ 50% RH, lesions ceased to enlarge. (The average relative humidity recorded in the month during the field disease outbreak was 76% ± 9%) If previously inoculated plants were re-introduced into the dew chamber (100% RH), misted, and allowed to stay an additional 17 hr, the diameter of the lesions increased to 2 - 5 mm (data not presented).
Foliar disease, caused by M. roridum, is a relatively easy disease to control with broad spectrum fungicides used at a frequency dictated by the weather [25]. Many of the fungicides normally used in watermelon production should have good activity against M. roridum which may be why it has been reported only twice on watermelon in the US [14,15].
All of the cucurbit fruit, including watermelon, that were inoculated in the present study exhibited decay. However, the inoculum was introduced into the fruit following the removal of the epidermal layer. In the Rio Grande Valley of Texas, up to 30% of cantaloupe fruit in the field exhibited the crater rot symptom in one report [26]. Seebold et al. [14] did not observe lesions on watermelon fruit in a mildly affected field in Georgia. Although the disease severity in Oklahoma was more severe, fruit lesions also were not observed [15]. Fruit le-
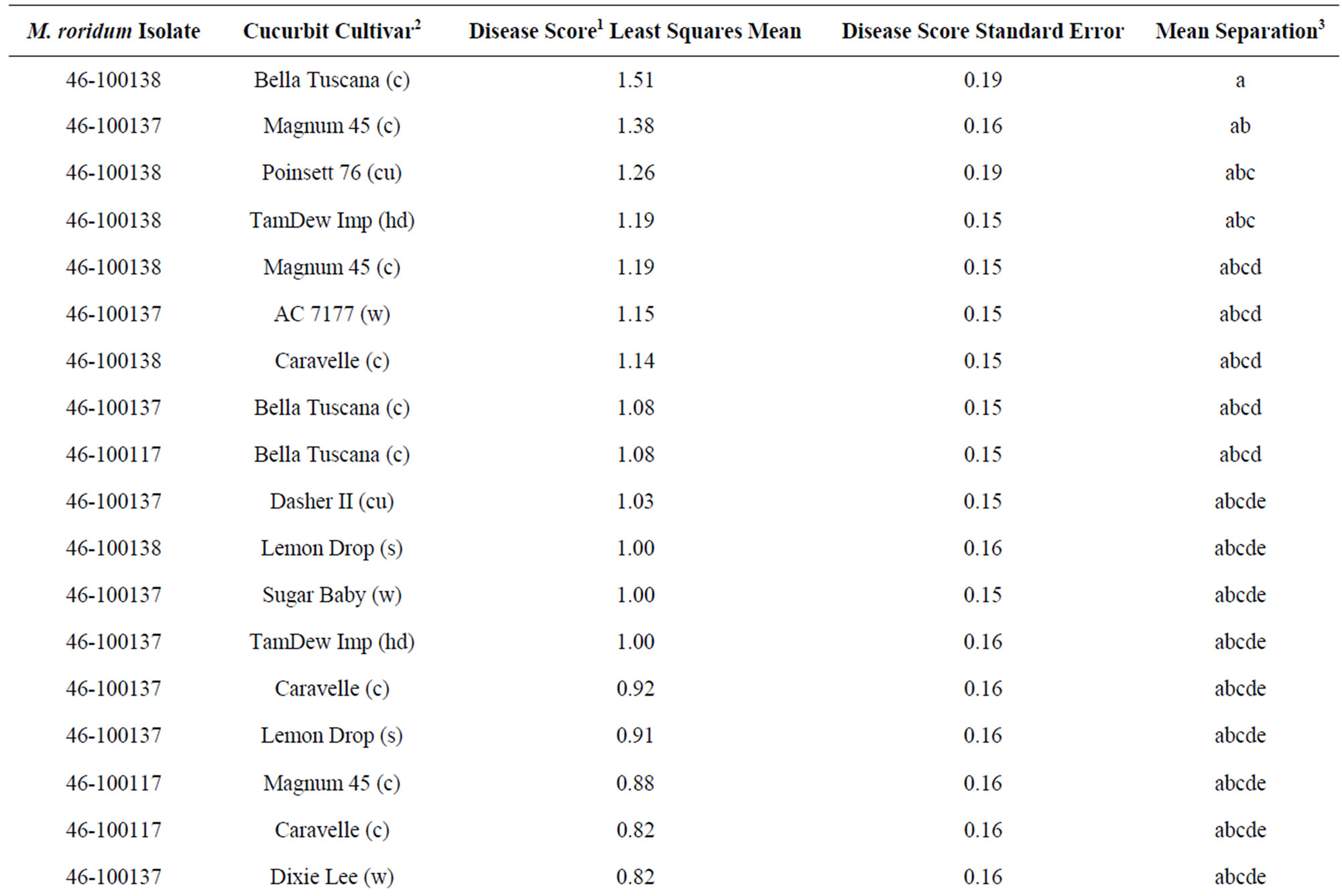
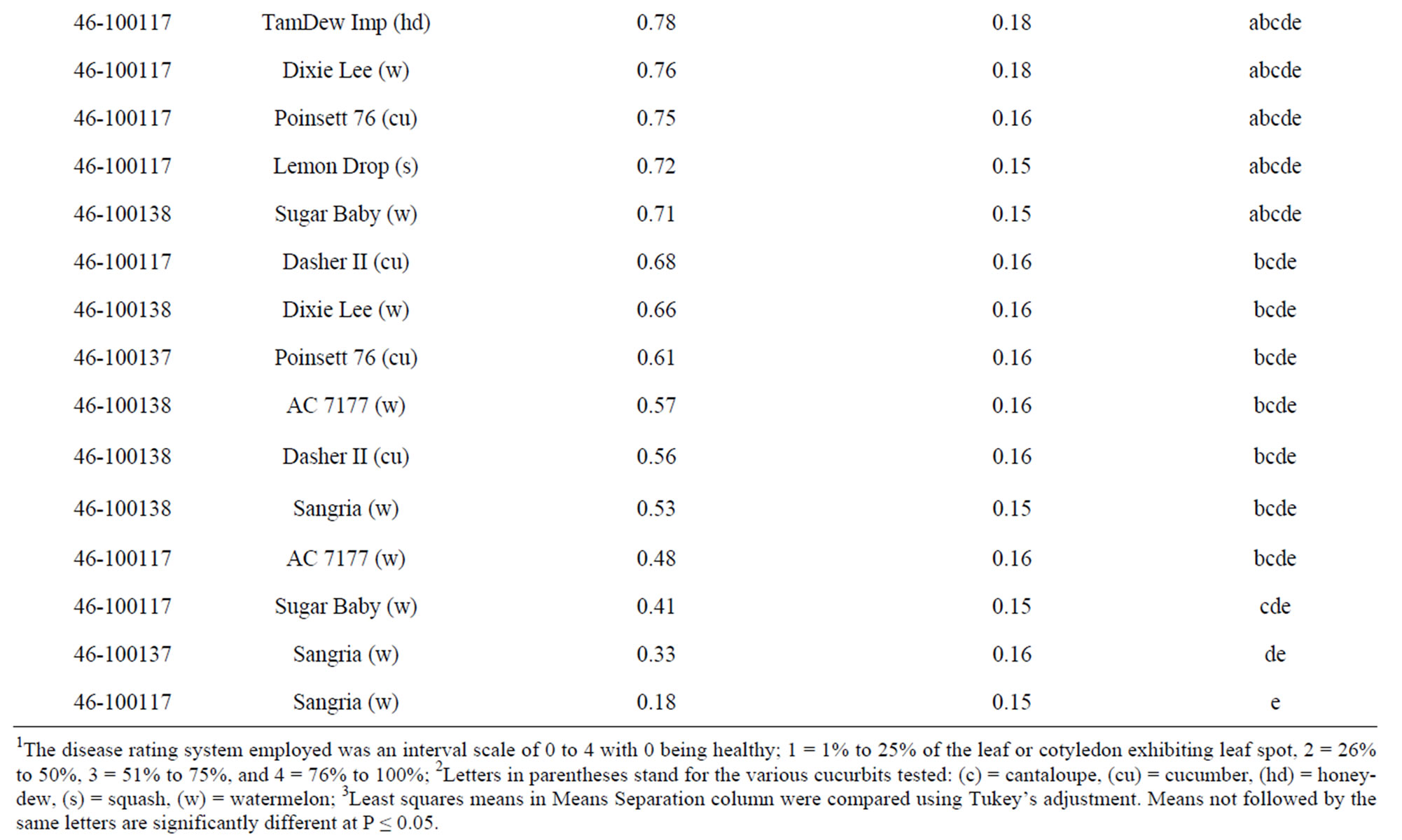
Table 1. Disease ratings of cotyledons of selected cucurbit seedlings inoculated with M. roridum isolates from watermelon leaf lesions.
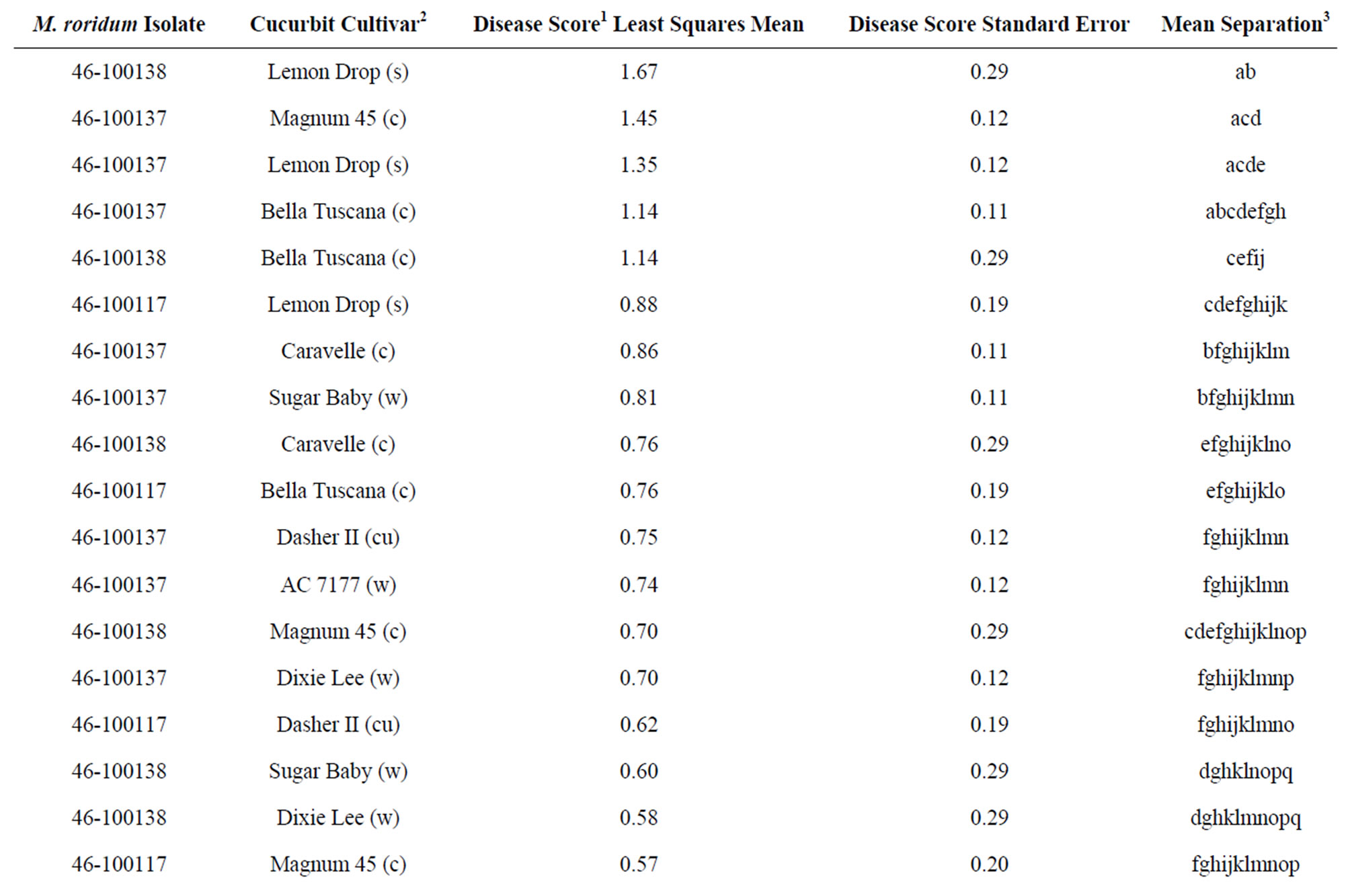

Table 2. Disease ratings of first two leaves of selected cucurbit seedlings inoculated with M. roridum isolates from watermelon leaf lesions.
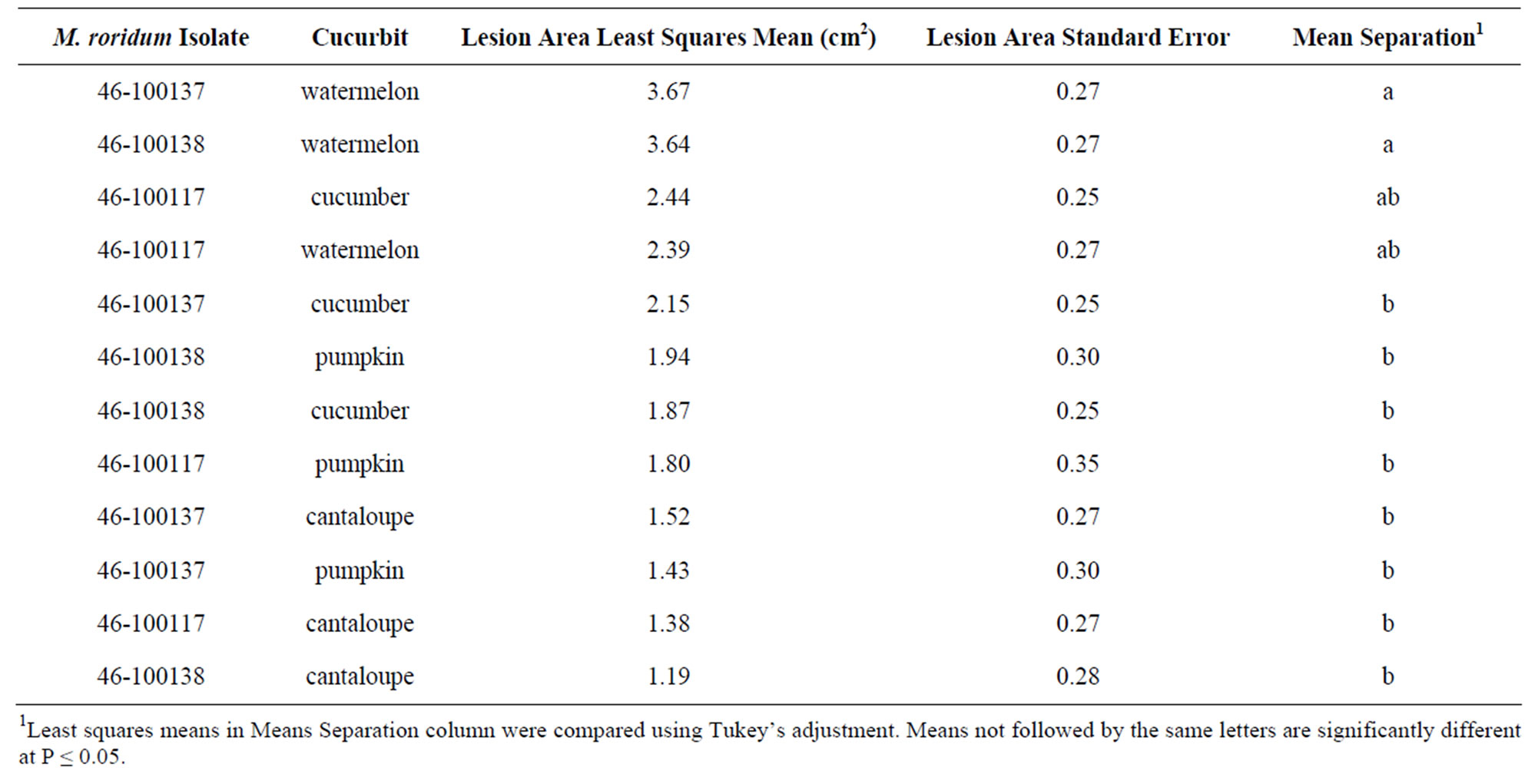
Table 3. Area of fruit lesion cross sections on selected cucurbit fruit inoculated with M. roridum isolates from watermelon leaf lesions.
sions have been reported on cantaloupe, honeydew, and cucumber [1], but there are no reports of M. roridum causing fruit lesions on watermelon or pumpkin. The thick wax layer on watermelon fruit may impede the fungus’ ability to cause infection.
Based on the research from a sizeable number of laboratories around the world, it seems likely that all the optimal conditions must come together for an outbreak of M. roridum disease to occur on watermelon. An apparently wide range of susceptibility among watermelon cultivars in the field has been observed [15] which suggests a moderately high level of resistance to Myrothecium leaf spot in some cultivars. Greenhouse inoculations support field observations by showing differential resistance within cucurbit types. Extensive epidemiological work will be required if Myrothecium leaf spot and stem canker becomes more prevalent in major watermelon production areas.
5. Acknowledgements
The authors thank Diann Baze for her valuable technical support.
6. Disclaimer
Mention of trade names or commercial products in this article is sole for the purpose of providing specific information and does not imply recommending or endorsement by the US Department of Agriculture. All programs and services of the US Department of Agriculture are offered on a nondiscriminatory basis without regard to race, color, national origin, religion, sex, age, marital status, or handicap. The article cited was prepared by a USDA employee as part of his/her official duties. Copyright protection under US copyright law is not available for such works. Accordingly, there is no copyright to transfer. The fact that the private publication in which the article appears is itself copyrighted does not affect the material of the US Government, which can be freely reproduced by the public.
REFERENCES
- B. D. Bruton, “Crater Rot,” In: T. A. Zitter, D. L. Hopkins and C. E. Thomas, Eds., Compendium of Cucurbit Diseases, The American Phytopathological Society, St. Paul, 1996, pp. 49-50.
- A. R. Chase, “Influence of Host Plant and Isolate Source on Myrothecium Leaf Spot of Foliage Plants,” Plant Disease, Vol. 67, No. 6, 1983, pp. 668-671. doi:10.1094/PD-67-668
- K. M. Ponappa, “On the Pathogenicity of Myrothecium roridum-Eichhornia crassipes Isolate,” Hyacinth Control Journal, Vol. 8, No. 1, 1970, pp. 18-20.
- B. G. Bharath, S. Likesh, B. Yashovarma, H. S. Prakash, and H. S. Shetty, “Seed-Borne Nature of Myrothecium roridum in Watermelon Seeds,” Research Journal of Botany, Vol. 1, No. 1, 2006, pp. 44-45. doi:10.3923/rjb.2006.44.45
- T. H. Nguyen, S. B. Mathur and P. Neergaard, “SeedBorne Species of Myrothecium and Their Pathogenic Potential,” Transactions of the British Mycology Society, Vol. 61, No. 2, 1973, pp. 347-354. doi:10.1016/S0007-1536(73)80156-1
- N. Sultana and A. Ghaffar, “Pathogenesis and Control of Myrothecium spp., the Cause of Leaf Spot on Bitter Gourd (Momordica charantia Linn.),” Pakistan Journal of Botany, Vol. 41, No. 1, 2009, pp. 429-433.
- A. S. A. Wahid and A. S. Shakir, “Seed-Borne Mycoflora of Sponge Gourd in Punjab,” Pakistan Journal of Agricultural Research, Vol. 12, No. 2, 1991, pp. 151-152.
- W. O. Okunowo, G. O. Gbenie, A. A. Osuntoki and A. A. Adekunie, “Media Studies on Myrothecium roridum Tode: A Potential Biocontrol Agent for Water Hyacinth,” Journal of Yeast and Fungal Research, Vol. 1, No. 4, 2010, pp. 55-61.
- M. Fitton and P. Holliday, “Myrothecium roridum,” In: Commonwealth Mycological Institute. Descriptions of Pathogenic Fungi and Bacteria, No. 253, Commonwealth Agricultural Bureau, Kew, 1970, pp. 1-2.
- D. M. McLean and B. Sleeth, “Myrothecium Rind Rot of Cantaloupe,” Plant Disease Reporter, Vol. 45, No. 9, 1961, pp. 728-729.
- J. Worapong, J. Sun and G. Newcombe, “First Report of Myrothecium roridum from a Gymnosperm,” North American Fungi, Vol. 4, No. 6. 2009, pp.1-6. doi:10.2509/naf2009.004.006
- C. S. Cabral, G. P. Henz, A. J. A. Moreira III and A. Reis II, “New Cucurbitaceous Hosts of Myrothecium roridum in Amazonas State, Brazil,” Tropical Plant Pathology, Vol. 34, No. 6, 2009, pp. 1-6.
- D. K. Kim, D. W. Bae, S. C. Lee, K. S. Han, H. K. Kim, “Detection of Myrothecium Leaf Spot, a New Disease of Watermelon,” Plant Pathology Journal, Vol. 19, No. 4, 2003. pp. 200-202. doi:10.5423/PPJ.2003.19.4.200
- K. W. Seebold Jr., D. B. Langston Jr., R. C, Kemerait Jr. and J. E. Hudgins, “First Report of a Leaf Spot and Stem Canker Caused by Myrothecium roridum on Watermelon in the United States,” Plant Disease, Vol. 89, No.3, 2005, p. 342. doi:10.1094/PD-89-0342A
- B. D. Bruton and W. W. Fish, “Myrothecium roridum Leaf Spot and Stem Canker on Watermelon in the Southern Great Plains: Possible Factors for Its Outbreak,” Plant Health Progress, 2012. doi:10.1094/PHP-2012-0130-01-BR
- G. A. Bean, T. Fernando, B. B. Jarvis and B. Bruton, “The Isolation and Identification of Trichothecene Metabolites from a Plant Pathogenic Strain of Myrothecium roridum,” Journal of Natural Products, Vol. 47, No. 4, 1984, pp. 727-729. doi:10.1021/np50034a031
- J. O. Kuti, T. J. Ng and G. A. Bean, “Possible Involvement of a Pathogen-Produced Trichothecene Metabolite in Myrothecium Leaf Spot of Musk melon,” Physiological and Molecular Plant Pathology, Vol. 34, No. 1, 1989, pp. 41-54. doi:10.1016/0885-5765(89)90015-5
- P. Healey, T. J. Ng and F. A. Hammerschlag, “Response of Leaf Spot-Sensitive and Tolerant Muskmelon (Cucumis melo L.) Cells to the Phytotoxin Roridin E,” Plant Science, Vol. 97, No. 1, 1994, pp. 15-21. doi:10.1016/0168-9452(94)90102-3
- J. X. Jhang, B. D. Bruton and C. L. Biles, “Polygalacturonase Isozmes Produced by Phomopsis cucurbitae in Relation to Postharvest Decay of Cantaloupe Fruit,” Phytopathology, Vol. 87, No. 10, 1997, pp. 1020-1025. doi:10.1094/PHYTO.1997.87.10.1020
- M. B. Ellis, “Myrothecium,” In: Dematiaceous Hyphomycetes, Commonwealth Mycological Institute, Kew, 1971, pp. 552-556.
- J. A. Mangandi, T. E. Seijo and N. A. Peres, “First Report of Myrothecium roridum Causing Myrothecium Leaf Spot on Salvia spp. in the United States,” Plant Disease, Vol. 91, No. 6, 2007, p.772. doi:10.1094/PDIS-91-6-0772B
- M. T. Mmbaga, Y. Li and M.-S. Kim, “First Report of Myrothecium roridum Causing Leaf Spot on Garden Hydrangea in the United States,” Plant Disease, Vol. 94, No. 1, 2010, p. 266.
- Y. J. Zhao, B. J. Li, Y. X. Shi and X. W. Xie, “First Report of Myrothecioum Leaf Spot of Common Bean in China Caused by Myrothecium roridum,” Plant Disease, Vol. 94, No. 1, 2010, p.127. doi:10.1094/PDIS-94-1-0127-B
- A. R. Chase and R. T. Poole, “Development of Myrothecium Leaf Spot of Dieffenbachia maculate ‘Perfection’ at Various Temperatures,” Plant Disease, Vol. 68, No. 6, 1984, pp. 488-490. doi:10.1094/PD-69-488
- W. W. Carter, “Incidence and Control of Myrothecium roridum on Cantaloupe in Relation to Time of Fungicide Application,” Plant Disease, Vol. 64, No. 9, 1980, pp. 872-874. doi:10.194/PD-64-872
- B. D. Bruton, “Myrothecium roridum: A Potentially Devastating Pathogen of Muskmelon in South Texas,” Phytopathology, Vol. 72, No. 1, 1982, p. 355.

