Pharmacology & Pharmacy
Vol.4 No.3(2013), Article ID:32563,6 pages DOI:10.4236/pp.2013.43045
An Ongoing Epidemic of Birth Defects
![]()
1Neonatology Ward and Genetics Department, Hospital del Niño de Panamá (Panama’s Children’s Hospital), Panama City, Republic of Panama; 2Department of Pediatrics, University of British Columbia, Vancouver, Canada.
Email: *gcossio@cableonda.net
Copyright © 2013 Gladys Cossio et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 20th, 2013; revised May 3rd, 2013; accepted May 17th, 2013
Keywords: Congenital Anomalies; Teratogenic Agent; Misoprostol; Pregnancy
ABSTRACT
In the 1990s, misoprostol (synthetic prostaglandin E1 analogue) was found to be an effective abortive agent when taken orally and became widely used in Latin America as a means to terminate unwanted pregnancies. A variety of congenital anomalies have been observed among the children of women who ingested misoprostol, but failed to terminate their pregnancy. We report here eight years of experience in Panama with the detection and follow-up of the malformations seen in infants associated with the use of misoprostol prostaglandin during the first trimester of pregnancy. During the period between April 1995 and March 2003, we identified 63 infants at the Panama’s Children’s Hospital who were exposed to misoprostol while in the womb and who were born with malformations. These infants were evaluated by a team of neonatologists, geneticists, cardiologists, ophthalmologists, and radiologists.
1. Introduction
Misoprostol (Cytotec) is used in the treatment of duodenal and gastric ulcers as well as gastritis due to its cellular protection and antisecretory properties. During the previous decade in Latin America, it has become practice to use misoprostol to terminate unwanted pregnancies, abortion remains illegal in Panama and misoprostol is commonly used. Health authorities have at tempted to regulate and control the sale of Cytotec for abortion purposes because of its association with congenital anomalies when used during early pregnancy. However it is sold on the black market as an abortive agent with a price ranging from US$10.00 to $20.00 for an effective dose (Personal Communication).
Various reports regarding congenital anomalies associated with the prenatal use of misoprostol during the first trimester have been published over the last 10 years [1-4], in this report, we describe the Panamanian experience in which Moebius syndrome has been the most common feature observed.
2. Methods
During the period from April 1995 through March 2003 at the Hospital del Niño de Panamá in Panama City, Panama we identified and examined 63 newborns and infants who were exposed to misoprostol and born with malformations. In this time there were a total of 446,474 children born in Panama. Infants with congenital malformation were evaluated by a multidisciplinary team of neonatologists, pediatric geneticists, pediatric cardiologists, pediatric ophthalmologists, and pediatric radiologists. A clinical history was taken, a specialized pediatric physical examination was performed in each case, and the specifics of the ingestion of misoprostol (e.g., mode, dose, and timing) were documented by interviewing the mothers. We only identified 63 cases of congenital anomalies from misoprostol exposure but this may not represent all cases of misoprostol toxicity.
3. Results
An analysis of the data is presented in Table 1. The most frequent abnormality diagnosed was Moebius in a total of 36 individuals (57%); 23 of the Moebius cases had paralysis of the VI and VII cranial nerves only; while 13 cases had involvement of the VI, VII and IX cranial nerves. Twenty-one cases of Moebius syndrome affected individuals also had either unilateral or bilateral equinovarus deformity of the foot (Figure 1). Only 3 of the
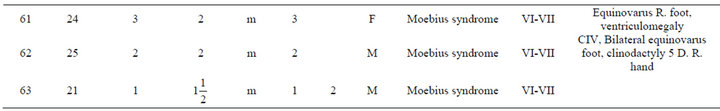
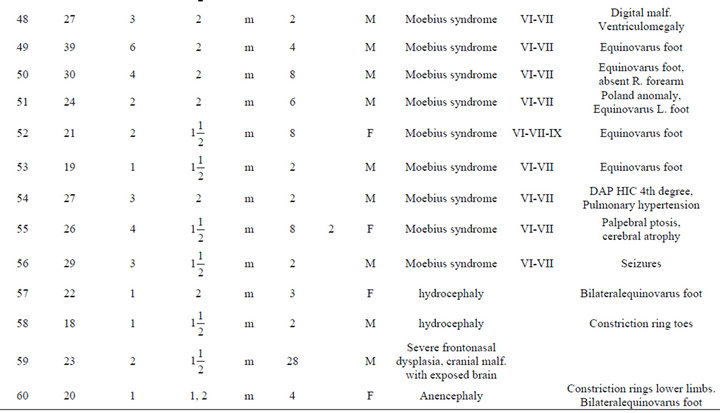
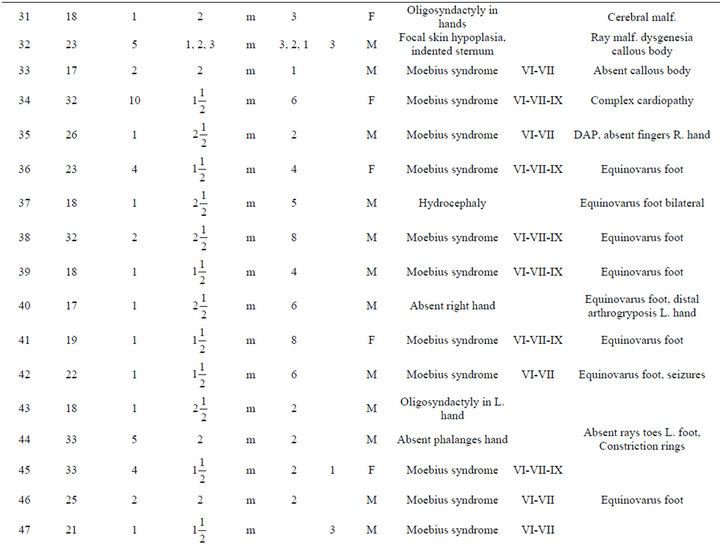
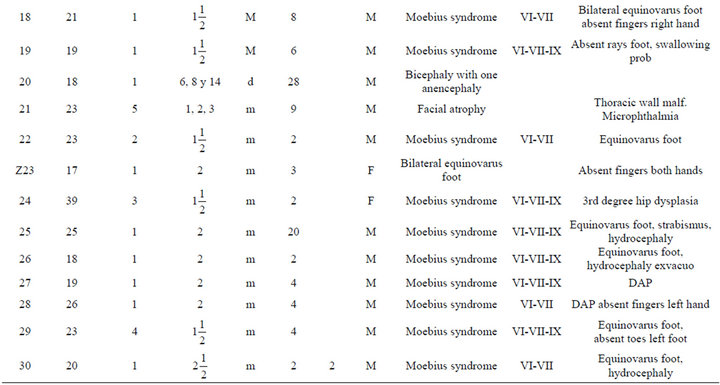
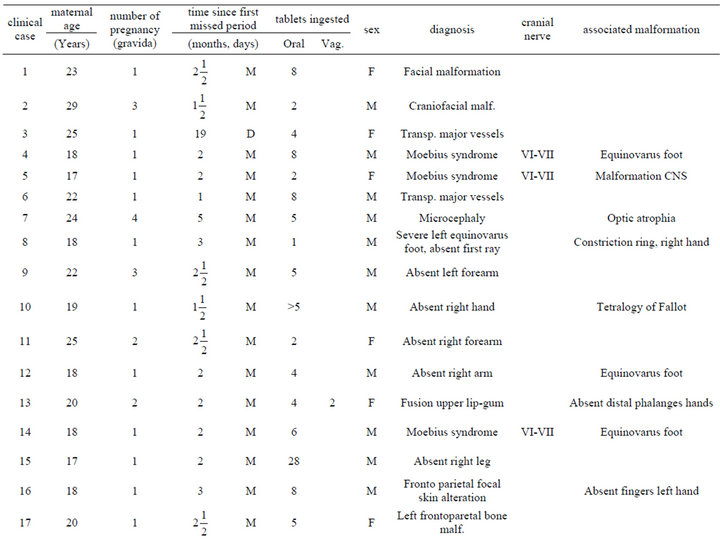
Table 1. Description of infant malformations cases associated with the use of misoprostol during early pregnancy. Hospital del niño de panama (panama’s children’s hospital). Years 1995-2003.
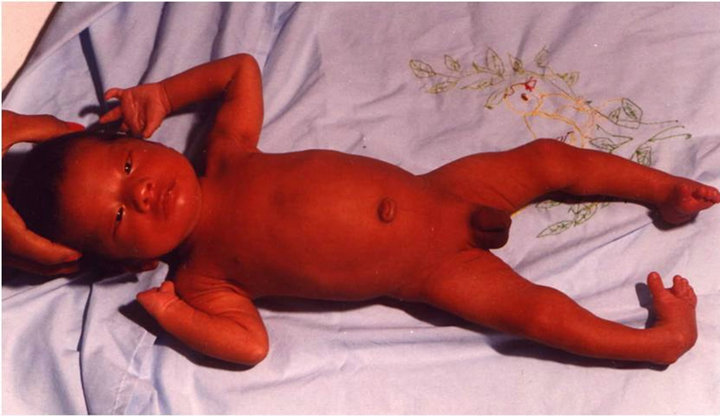
Figure 1. Case # 18. Moebius syndrome, bilateral equinovarus foot and absent finger at right hand.
Moebius syndrome patients had no other associated anomalies.
Among the patients with craniofacial anomalies, there were also 12 who had central nervous system abnormalities. In this group, there were 2 cases of anencephaly (one with constriction rings of the lower limbs and bilateral equinovarus deformity of foot, (see Figure 2) and the other with bicephalous (conjoined twin) with only one of the heads having anencephaly (see Figure 3). Six patients hadhydrocephaly; of these, 3 only had hydrocephaly and 3 had hydrocephally seen together with Moebius syndrome.
Limb anomalies were the second most common abnormality, present in 43 individuals (68%). 25 associated to Moebius Syndrome, 7 were associated with another significant malformation and 11 were the principal abnormality. Thirteen had unilateral or bilateral equinovarus foot deformity. Five had limb abnormalities together with other abnormalities (see Figure 4). And six cases with secondary malformations probably secondary to amniotic bands (one case with anencephaly and constriction ring at extremities, one facial malformation, eye and lips, and four with constriction rings at fingers. with Interestingly, the first cases of misoprostol teratogenicity in Panama were associated predominantly with limb anomalies however, more recently Moebius syndrome appears to be more common than limb anomalies. This may reflect a change in the agent taken, the dosage, or the timing when taken by themselves.
Analysis of maternal profiles indicates that the age range of mothers was from 13 to 39 years, with an average age of 22.7 at the time of misoprostol consumption.
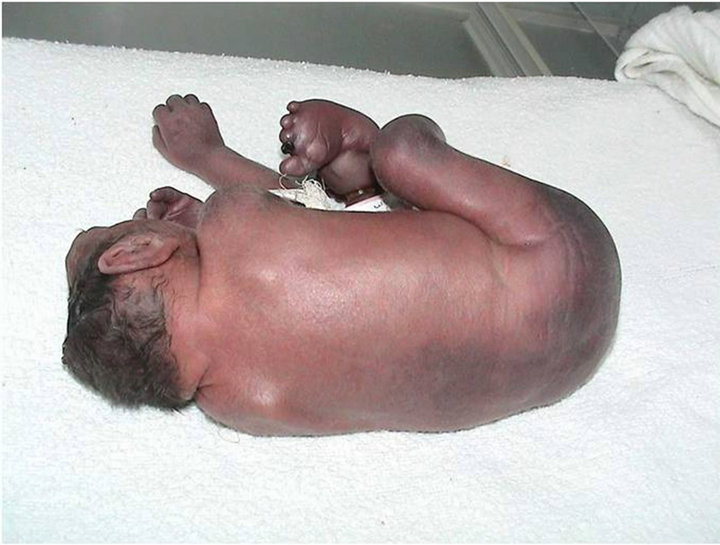
Figure 2. Anencephaly newborn girl case # 60.
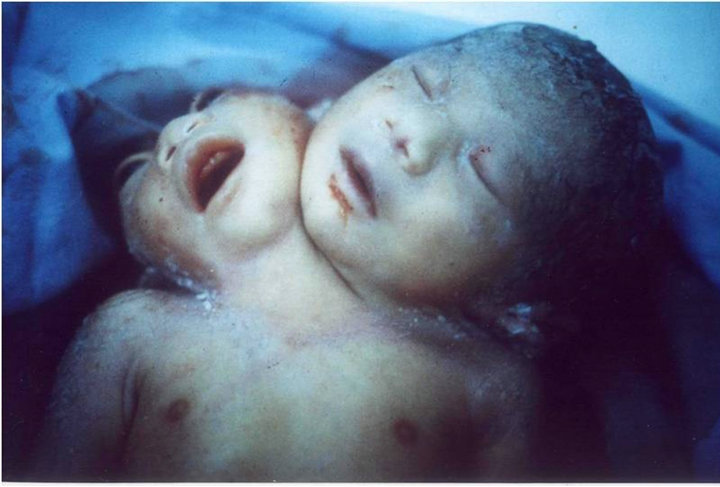
Figure 3. Case # 20. Newborn bicephaly with one anencephaly.
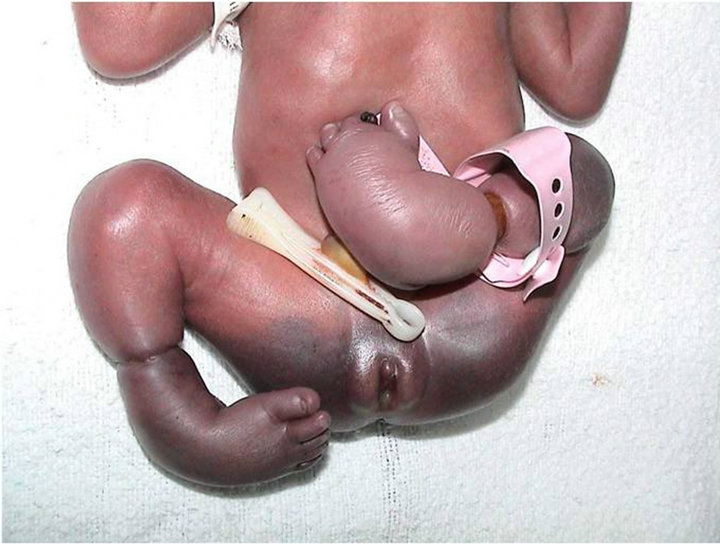
Figure 4. See constriction rings at lower limbs and bilateral equinovarus foot case # 60.
The largest age group was 17 to 19 years with 37.9% of the mothers in this age group. For 57% of women, this was their first pregnancy.
4. Misoprostol Use and Administration
The minimal amount of misoprostol ingested was 200 μg in one tablet and the maximum was 5600 μg in 28 tablets, with an average of 5 - 6 tablets (1000 to 1200 μg) per pregnant woman. In 98.5% of women, the use of misoprostol was during 1.5 - 2 month of pregnancy. Only one case (1.5%) reported ingesting the prostaglandin (5 tablets) during the second trimester (5th month). This pregnancy resulted in a newborn with microcephaly and atrophy of the optic nerves.
Only one mother did not know the amount of misoprostol she had ingested since her husband had pulverized the tablets and had given them to her in a drink. She only knew that she had amenorrhea and then ingested the medication.
In 56 cases (89%) misoprostol was taken only orally. In 7 cases (11%), the drug was administered both orally and vaginally after the first missed period in 98.5%. In forty-five or 71.4% of the cases, the information about ingestion was verified by the mother. If there was any doubt about the dating of ingestion, the information was verified by calculation in relation to the last menstrual period. In all cases, the mothers reported that they had used the misoprostol believing it would help them terminate the pregnancy in an expedient way without complications.
5. Discussion
Our study is not a case control study, but rather an observational study over an 8-year period. Our hospital is the only level 3 pediatric hospital in Panama and it is likely that this report contains a majority of the cases of malformations associated with the use of misoprostol during pregnancy in Panama during these 8 years. The study concludes the observation that misoprostol, when used early in pregnancy to terminate pregnancy, may instead lead to congenital anomalies when the pregnancies donot terminate. The potential that the treated pregnancies may continue has not been broadly appreciated and the risk for anomalies in the child was not expected by these mothers, further studies examining the relative risk and long term outcome are needed.
Since many of our cases appeared to have rupture of amniotic membrane. We postulate that the strong uterine contractions produced by synthetic prostaglandins also lead to rupture of amniotic membranes and loss of amniotic fluid resulting in amniotic bands, amputations, and deformations.
Reports of congenital anomalies in the children born to mothers who have ingested some type of synthetic prostaglandin analog have been described in the literature for the last 20 years. First report in 1983, Collins and Mahoney [5] published the case of a 34-week newborn with hydrocephaly and digital anomalies. The mother who had requested an abortion had been enrolled in a research protocol in which she was initially given 1 mg of 15 metil F2 alpha orally and after an hour given another 3 mg. In 2002, Wheler et al. [6] reported a boy with hypotonia, growth restriction, and multiple anomalies whose 23 year old mother had received methotrexate followed by misoprostol during the 8th week of pregnancy to terminate the pregnancy; however, she decided to continue the pregnancy.
Moebius syndrome has been associated with the use of E1 synthetic prostaglandin (misoprostol) during the first trimester of pregnancy use. In 1993, Gonzalez et al. [1] reported 7 Brazilian babies with Moebius syndrome, with or without digital anomalies. In 1998, Pastuszak et al. [7] identified 96 infants with Moebius syndrome and matched them with 96 infants with neural-tube defects. The mean age at the time of the diagnosis of Moebius syndrome was 16 months (range 0.5 to 78). And the diagnosis of neural tube defects was made within 1 week of birth in most cases. Among the mothers of the infants with Moebius syndrome 47 (49 percent) had used misoprostol in the first trimester of pregnancy. As compared with only 3 of the mothers of the 96 infants with neural tube defects.
The mechanism(s) by which limb anomalies and Moebius syndrome occur in the offspring of mothers who have used misoprostol in the first trimester is unknown. It is assumed to be a vascular mechanism since misoprostol has a major effect on vasculature. In 1995, Shepard [8] postulated that the possible mechanism for Moebius syndrome was related to vascular constriction at a critical time in neuronal maturation. He noted that the cranial nerves VI and VII were preferentially affected. The nuclei of these nervecells were in the ventral part of the rhombencephalon. He postulated that the uterine contraction might produce cephalocaudal constriction of blood flow, affected the area of the VI and VII cranial nerves more severely and presumably lead to ischemia, necrosis, and cell death of those cranial nerve nuclei. Vargas et al. [4] studied a total of 93 cases and 279 controls which were recruited in eight participating center. Prenatal exposure to misoprostol was identified in 32 infants diagnosed with vascular disruption defects (34.4%) compared with only 12 (4.3%) in the control group (P < 0.0000001).
6. Findings
Of the 63 affected infants, 36 presented with Moebius syndrome, 13 with malformations of the limbs, and 12 with craniofacial malformations and 2 with complex cardiac malformations; however, many had more than one anomaly. Two of the 63 had neural tube defects.
7. Interpretation
Early prenatal exposure to misoprostol is associated with Moebius syndrome and abnormalities of the limbs that are thought to be due to vascular disruption at the time of maternal ingestion [9,10]. In addition, many of these patients had features suggestive of amniotic membrane rupture. Anomalies secondary to amnion rupture included: loss of amniotic fluid and amniotic bands leading to constriction rings, amputations, and deformities.
REFERENCES
- C. H. Gonzalez, F. Vargas, A. B. Perez, C. A. Kim, D. Brunoni, M. J. Marques-Dias, C. R. Leone, J. Correa Neto, J. C. Llerena Junior and J. C. de Almeida, “Limb Deficiency with or without Moebius Sequence in Seven Brazilian Children Associated with Misoprostol Use in the First Trimester of Pregnancy,” American Journal of Medical Genetics, Vol. 47, No. 1, 1993, pp. 59-64. doi:10.1002/ajmg.1320470113
- K. Blanchard, B. Winikoff and C. Ellertson, “Use of Misoprostol during Pregnancy and Mobius’ Syndrome in Infants,” The New England Journal of Medicine, Vol. 339, No. 21, 1998, pp. 1553-1554. doi:10.1056/NEJM199811193392114
- L. Schüller, A. Pastuszak, M. T. Sanseverino, I. M. Orioli, D. Brunoni and G. Koren, “Pregnancy Outcome after Abortion Attempt with Misoprostol,” Teratology, Vol. 55, No. 36, 1997, pp. 1-28.
- F. R. Vargas, L. Schuler-Faccini, D. Brunoni, C. Kim, V. F. Meloni, S. M. Sugayama, L. Albano, J. C. Llerena, J. C. Almeida, A. Duarte, D. P. Cavalcanti Jr., E. Goloni-Bertollo, A. Conte, G. Koren and A. Addis, “Prenatal Exposure to Misoprostol and Vascular Disruption Defects: A Case-Control Study,” American Journal of Medical Genetics, Vol. 95, No. 4, 2000, pp. 302-306. doi:10.1002/1096-8628(20001211)95:4<302::AID-AJMG2>3.0.CO;2-B
- F. S. Collins and M. Mahoney, “Hydrocephalus and Abnormal Digits after First-Trimester Prostaglandin Abortion Attempt,” Journal of Pediatrics, Vol. 102, No. 4, 1983, pp. 620-621. doi:10.1016/S0022-3476(83)80204-2
- M. Wheeler, P. O’Meara and M. Stanford, “Fetal Methotrexate and Misoprostol Exposure: The Past Revisited,” Teratology, Vol. 66, No. 2, 2002, pp. 73-76. doi:10.1002/tera.10052
- A. L. Pastuszak, L. Schüler, C. E. Speck-Martins, K. E. Coelho, S. M. Cordello, F. Vargas, D. Brunoni, I. V. Schwarz, M. Larrandaburu, H. Safattle, V. F. Meloni and G. Koren, “Use of Misoprostol during Pregnancy and Mobius’ Syndrome in Infants,” The New England Journal of Medicine, Vol. 338, No. 26, 1998, pp. 1881-1885. doi:10.1056/NEJM199806253382604
- T. H. Shepard, “Mobius Syndrome after Misoprostol a Possible Teratogenic Mechanism,” Lancet, Vol. 346, No. 8977, 1995, p. 780. doi:10.1016/S0140-6736(95)91540-0
- K. E. Coelho, M. F. Sarmento, C. M. Veiga, C. E. SpeckMartins, H. P. Safatle, C. V. Castro and N. Niikawa, “Misoprostol Embryotoxicity: Clinical Evaluation of Fifteen Patients with Arthrogryposis,” American Journal of Medical Genetics, Vol. 95, No. 4, 2000, pp. 297-301. doi:10.1002/1096-8628(20001211)95:4<297::AID-AJMG1>3.0.CO;2-K
- C. H. Gonzalez, M. J. Marques-Dias, C. A. Kim, S. M. Sugayama, J. A. Da Paz, S. M. Huson and L. B. Holmes, “Congenital Abnormalities in Brazilian Children Associated with Misoprostol Misuse in First Trimester of Pregnancy,” Lancet, Vol. 351, No. 9116, 1998, pp. 1624- 1627. doi;10.1016/S0140-6736(97)12363-7
NOTES
*Corresponding author.

