Advances in Microbiology
Vol.3 No.4(2013), Article ID:35527,10 pages DOI:10.4236/aim.2013.34050
Synthesis and in Vitro Biological Activities of 4,5-Disubstituted 1,2,4-Triazole-3-Thiols
1Department of Pharmaceutical Chemistry, Riphah International University Islamabad, Islamabad, Pakistan
2Department of Pharmaceutics, Riphah Institute of Pharmaceutical Sciences, Riphah International University Islamabad, Islamabad, Pakistan
Email: *aunmuhammad78@yahoo.com
Copyright © 2013 Humaira Nadeem et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 16 June, 2013; revised 12 July, 2013; accepted 20 July, 2013
Keywords: 4,5-Disubstituted 1,2,4-Triazole-3-Thiols; Antibacterial; Antitumor; Antioxidant; Cytotoxic
ABSTRACT
Purpose: The triazole nucleus is an important part of the therapeutically interesting drug candidate as antimicrobial, analgesic, anticancer, anticonvulsant and anti-inflammatory agents. Methods: Therefore, in this study, twelve 4,5-disubstituted-1,2,4-triazole-3-thiols were synthesized by the reaction of substituted isothiocyanates and hydrazides using the common method of base catalysed intramolecular dehydrative cyclization of substituted thiosemicarbazides 3(a-f) and 4(a-f). The structures of these compounds were characterized by means of FT-IR, 1H-NMR, and elemental analysis data. All these compounds were screened for antibacterial, antioxidant, antitumor and cytotoxic activities. Results: Among these compounds: 5c, 5f and 6f were found active against gram positive cocci, the compounds 5a, 5b, 5d, 6a and 6f showed 85% free radical scavenging effect at 3 ppm when tested for antioxidant activity, 75% tumors inhibition was recorded using 5c, 5d and 6a and brine shrimps lethality assay declared 5a, 5b and 6d was 129.62 µg/ml, 161.577 µg/ml and 81.56 µg/ml respectively. Conclusion: Compounds carrying significant bioactivity can be further studied using animal models to establish their safety profile prior to initiating clinical trials.
1. Introduction
The search for new agent is one of the most challenging tasks to the medicinal chemist. The synthesis of high nitrogen containing heterocyclic systems has been attracting increasing interest over the past decade because of their utility in various applications, such as propellants, explosives, pyrotechnics and especially chemotherapy. In the past few decades, the chemistry of 1,2,4-triazoles and their fused heterocyclic derivatives have received considerable attention owing to their diverse synthetic as well as biological importance. A large number of 1,2,4- triazole-containing ring systems, have been incorporated into a wide variety of therapeutically interesting drug candidates having anti-inflammatory, CNS-stimulant, sedative, antianxiety and antimicrobial agents [1,2] and antimycotic activity such as fluconazole, intraconazole, and voriconazole [3]. Many drugs are known containing 1,2,4-triazole moiety for example triazolam [4], alprazolam [5] etizolam [6] and furacylin [7]. Among these heterocycles, the thione-substituted 1,2,4-triazoles and their derivatives [8] have been found to have a variety of biological activities, for example antibacterial, antifungal, antitubercular, antimycobacterial, anticancer, diuretic, and hypoglycemic properties. Particularly, substituted-1,2, 4-triazoles-3-thiols are among the various heterocycles that have received the most attention during the last two decades as potential antimicrobial agents [9-11]. The literature reveals that substituted1,2,4-triazole has diverse biological potential, and the easy synthetic routes for synthesis have taken attention of the chemists, pharmacologists and researchers [12]. In addition to these important biological applications, mercapto-1,2,4-triazoles are also of great utility in preparative organic chemistry, for example, in the present of various reagents, undergo different types of reactions to yield other heterocyclic compounds, e.g., thiazolotriazoles, triazolothiadiazoles, triazolothiazines, triazolothiazepines and triazolothiadiazines. Recently the most common and useful procedures for the preparation of the mercaptoand thione-substituted 1,2,4-triazole and their utility for the synthesis of well-known heterocyclic ring systems have been reviewed [8]. In view of the diverse biological activities of mercato triazoles, study was planned to synthesize new 4,5-disubstituted-1,2,4-triazole-3-thiones and evaluate them for their antibacterial, antioxidant, antitumor and cytotoxic activities.
2. Experimental
The melting points were recorded on Gallenkamp digital melting point apparatus MFB-595-010M and are uncorkrected. FTIR spectra were recorded on Perkin Elmer Spectrum BX spectrophotometer as KBr pellets. 1HNMR spectra were determined in DMSO-d6 at 300 MHz using a Bruker spectrophotometer AM-300 with chemical shift values reported in δ units (ppm) relative to an internal standard (tetramethylsilane). The elemental analyses were conducted using a LECO-183 CHNS analyzer. Antimicrobial, antioxidant and cytotoxic activity was carried out at the Riphah Institute of Pharmaceutical Sciences, Riphah International University Islamabad, Pakistan. All chemicals were purchased from Merck and Aldrich, and were used without further purification.
2.1. Synthesis of Carboxylic Acid Hydrazides (1a-f)
Respective ester of carboxylic acids (0.041 mol) was dissolved in absolute ethanol (50 - 100 mL). Hydrazine hydrate (80%, 13 mL) was added to this solution and the reaction mixture was refluxed for 7 - 8 hours. The reflux time was monitored by TLC and after the completion of the reaction; the excess hydrazine was distilled off under reduced pressure. The crude solid was collected, washed with water and recrystallized from aqueous ethanol.
2.2. Synthesis of 1, 4-Disubstituted Thiosemicarbazides (3a-f), 4(a-f)
Respective carboxylic acid hydrazide (1a-f, 0.03 moles) was dissolved in ethanol (100 - 200 mL) and a solution of isothiocyanate (2, 0.03 moles) in minimum amount of ethanol was added to it with constant stirring. The reaction mixture was refluxed for 4 - 5 h. The progress of reaction was monitored by TLC. After completion, the reaction mixture was cooled to yield a solid product. The crude product was filtered and recrystallized from 70% ethanol.
2.3. Synthesis of 2, 4-Dihydro-4,5-Disubstituted- 3H-1,2,4-Triazole-3-Thiones 5(a-f), 6(a-f)
General Procedure
The thiosemicarbazides (3a-f) and 4(a-f) were dissolved in aqueous 4N sodium hydroxide solution and refluxed for 4 - 5 h with constant stirring. Progress and completion of reaction was checked by TLC. The reaction mixture was cooled, filtered and the filtrate was acidified to pH of 4 - 5 with 4N hydrochloric acid. The solid product obtained was filtered off, washed thoroughly with water and recrystallized from 70% ethanol.
Synthesis of 4-Hexyl-2,4-dihydro-5-phenyl-3H-1,2,4- triazole-3-thione (5a) Yield 76%, Powder, Mp 110˚C, IR nmax cm−1 (KBr,) 3260 (N-H), 1585 (C = N), 1270 (C = S); 1H-NMR (DMSO-d6, d ppm) 0.79 (t, 3H, J = 6.0Hz, CH3), 1.19 (m, 6H, 3 × CH2), 1.67 (m, 2H, CH2), 4.06 (t, 2H, J = 7.8Hz, CH2-N), 7.47 - 7.54 (m, 5H, Aromatic ring), 12.42 (s, 1H, NH/SH); Elemental analysis: C14H19SN3; Calculated: C 64.36%, N 16.09%, H 7.27%, Found: C 64.15%, N 15.98%, H 7.13%.
Synthesis of 4-Hexyl-2,4-dihydro-5-(2-pyridyl)-3H-1, 2,4-triazole-3-thione (5b) Yield 75%, Powder, Mp 106˚C - 108˚C, IR nmax cm-1 (KBr) 3200 (N-H), 1600 (C = N), 1468 (C = C), 1278 (C = S); 1H-NMR (DMSO-d6, d ppm) 0.83 (t, 3H, J = 6.7 Hz, CH3), 1.28 (m, 6H, 3 × CH2), 1.72 (m, 2H, CH2), 4.13 (t, 2H, J = 6.8 Hz, CH2-N), 7.36 (dd, 1H, 7.0, H-5’ pyridyl ring), 7.80 (t, 1H, J = 7.8 Hz, H-4’ pyridyl ring), 8.01 (d, 1H, J = 7.9 Hz, H-6’ pyridyl ring), 8.60 (d, 1H, J = 4.6 Hz, H-3’ pyridyl ring), 12.65 (s, 1H, SH/NH); Elemental analysis: C13H18SN4; Calculated: C 59.54%, N 21.37%, H 6.87%, Found: C 59.27%, N 21.32%, H 6.56%.
Synthesis of 4-Hexyl-2,4-dihydro-5-(3-pyridyl)-3H-1, 2,4-triazole-3-thione (5c) Yield 72%, Powder, Mp 115˚C, IR nmax cm-1 (KBr) 3240 (N-H), 1595 (C = N), 1495 (C = C), 1278 (C = S); 1H-NMR (DMSO-d6, d ppm) 0.80 (t, 3H, J = 6.9 Hz, CH3), 1.24 (m, 6H, 3 × CH2), 1.76 (m, 2H, CH2), 4.08 (t, 2H, J = 6.8 Hz, CH2-N), 7.46 (dd, 1H, J = 7.8 Hz, J = 5.01, Hz, H-5’ pyridyl ring), 8.13 (d, 1H, J = 7.7 Hz, H-4’ pyridyl ring), 8.59 (d, 1H, J = 4.2 Hz, H-6’ pyridyl ring), 8.89 (d, 1 H, J = 1.8 Hz, H-2’ pyridyl ring); Elemental analysis: C13H18SN4; Calculated: C 59.54%, N 21.37%, H 6.87%, Found: C 59.36%, N 21.29%, H 6.48%.
Synthesis of 4-Hexyl-2,4-dihydro-5-(4-pyridyl)-3H-1, 2,4-triazole-3-thione (5d) Yield 75%, Powder, Mp 143˚C, IR nmax cm-1 (KBr) 3252 (N-H), 1590 (C = N), 1272 (C = S); 1H-NMR (DMSO-d6, d ppm) 0.89 (t, 3H, J = 6.2 Hz, CH3), 1.30 (m, 6H, 3 × CH2), 1.65 (m, 2H, CH2), 4.09 (t, 2H, J = 6.9 Hz, CH2-N), 7.65 (d, 2H, J = 8.1 Hz, H-2’, 6’ pyridyl ring), 8.05 (m, 2H, J = 8.2 Hz, H-3’, 5’ pyridyl ring); Elemental analysis: C13H18SN4; Calculated: C 59.54%, N 21.37%, H 6.87%, Found: C 59.45%, N 21.35%, H 6.80%.
Synthesis of 4-Hexyl-2,4-dihydro-5-(p-methylphenyl)-3H-1,2,4-tiazole-3-thione (5e) Yield 67%, Powder, Mp 90˚C, IR nmax cm-1 (KBr) 3242 (N-H), 1590 (C = N), 1270 (C = S); 1H-NMR (DMSO-d6, d ppm) 0.85 (t, 3H, J = 5.8 Hz, CH3), 1.52 (m, 6H, 3 × CH2), 1.70 (m, 2H, CH2), 2.4 (s, 3H, CH3-Ar), 4.41 (t, 2H, J = 6.8 Hz, CH2-N), 7.38 - 7.42 (m, 4H, Aromatic); Elemental analysis: C15H21SN3; Calculated: C 65.45%, N 15.27%, H 7.63%, Found: C 65.31%, N 15.25%, H 7.56%.
Synthesis of 5-Benzyl-4-hexyl-2,4-dihydro-3H-1,2,4- triazole-3-thione (5f) Yield 69%, Powder, Mp 101˚C, IR nmax cm-1 (KBr) 3226 (N-H), 1600 (C = N), 1380 (C = C), 1280 (C = S). 1H-NMR (DMSO-d6, d ppm) 0.83 (t, 3H, J = 6.0 Hz, CH3), 1.25 - 1.75 (m, 8H, 4CH2), 4.15 (t, 2H, CH2-N), 3.72 (s, 2H, CH2-Ph), 7.29-7.42 (m, 5H, Aromatic); Elemental analysis: C15H21SN3; Calculated: C 65.45%, N 15.27%, H 7.63%, Found: C 65.37%, N 15.19%, H 7.60%.
Synthesis of 4-Cyclohexyl-2,4-dihydro-5-phenyl-3H- 1,2,4-triazole-3-thione (6a) Yield = 68%, Powder, Mp 198˚C, IR nmax cm-1 (KBr) 3215 (N-H), 1585 (C = N), 1415 (C = C), 1282 (C = S). 1H-NMR (DMSO-d6, d ppm) 1.25 - 1.28 (m, 4H, 2 × CH2 cyclohexyl), 1.70 - 1.81 (m, 6H, 3 × CH2 cyclohexyl), 4.38 (m, 1H, CH - N cyclohexyl), 7.32 - 7.38 (m, 5H, Aromatic), 9.0 (bs, 1H, NH/SH); Elemental analysis: C14H17SN3; Calculated: C 64.86%, N 16.21%, H 6.56%, Found: C 64.35%, N 16.08%, H 6.28%.
Synthesis of 4-Cyclohexyl-2,4-dihydro-5-(2-pyridyl)- 3H-1,2,4-triazole-3-thione (6b) Yield 75.4%, Powder, Mp 200˚C, IR nmax cm-1 (KBr) 3210 (N-H), 1585 (C = N), 1410 (C = C); 1285 (C = S). 1H-NMR (DMSO-d6, d ppm) 1.23 - 1.28 (m, 4 H, 2 × CH2 cyclohexyl), 1.67 - 1.79 (m, 6H, 3 × CH2 cyclohexyl), 4.28 (m, 1H, CH-N cyclohexyl), 7.29 (dd, 1H, 7.2, H-5’ pyridyl), 7.78 (t, 1 H, J = 7.5 Hz, H-4’ pyridyl), 8.06 (d, 1H, J = 7.8 Hz, H-6’, 8.47 (d, 1H, J = 5.7 Hz, H-3’ pyridyl), 12.65 (s, 1H, SH/NH); Elemental analysis: C13H16SN4; Calculated: C 60.0%, N 21.53%, H 6.15%, Found: C 59.76%, N 21.43%, H 6.06%.
Synthesis of 4-Cyclohexyl-2,4-dihydro-5-(3-pyridyl)- 3H-1,2,4-triazole-3-thione (6c) Yield 72%, Powder, Mp 210˚C, IR nmax cm-1 (KBr) 3208 (N-H), 1580 (C = N), 1413 (C = C), 1282 (C = S); 1H-NMR (DMSO-d6, d ppm) 1.20 - 1.31 (m, 4H, 2 × CH2 cyclohexyl), 1.60 - 1.75 (m, 6H, 3 × CH2 cyclohexyl), 4.68 (m, 1H, CH-N cyclohexyl), 7.40 (m, 2H, H-6’, 5’ Pyridyl), 7.70 (s, 1H, H-2’ Pyridyl), 8.35 (d, 1H, J = 4.2 Hz, H-4’ Pyridyl), 12.65 (s, 1H, SH/NH); Elemental analysis: C13H16SN4; Calculated: C 60.0%, N 21.53%, H 6.15%, Found: C 59.83%, N 21.50%, H 6.10%.
Synthesis of 4-Cyclohexyl-2,4-dihydro-5-(4-pyridyl)- 3H-1,2,4-triazole-3-thione (6d) Yield 78%, Powder, Mp 206˚C, IR nmax cm-1 (KBr) 3211 (N-H), 1582 (C = N), 1410 (C = C), 1285 (C = S); 1H-NMR (CDCl3, d ppm) 1.23 - 1.32 (m, 4H, 2 × CH2 cyclohexyl), 1.59 - 1.78 (m, 6H, 3 × CH2 cyclohexyl), 4.65 (m, 1H, CH-N cyclohexyl), 7.72 (d, 2H, J = 5.6 Hz, H-2’,6’ Pyridyl), 8.65 (d, 2H, J = 5.6 Hz, H-3’,5’ Pyridyl); Elemental analysis: C13H16SN4; Calculated: C 60.0%, N 21.53%, H 6.15%, Found: C 59.91%, N 21.39%, H 6.08%.
Synthesis of 4-Cyclohexyl-2,4-dihydro-5-(p-methylphenyl)-3H-1,2,4-triazole-3-thione (6e) Yield 65%, powder, Mp 178˚C - 179˚C, IR nmax cm-1 (KBr) 3210 (N-H), 1580 (C = N), 1411 (C = C), 1280 (C = S); 1H-NMR (DMSO-d6, d ppm) 1.28 - 1.75 (m, 10H, 5 × CH2 cyclohexyl), 2.31 (s, 3 H, CH3-Ar), 4.45 (m, 1H, CH-N cyclohexyl), 7.21 - 7.35 (m, 4H, Aromatic); Elemental analysis: C15H19SN3; Calculated: C 65.93%, N 15.38%, H 6.95%, Found: C 65.82%, N 15.31%, H 6.89%.
Synthesis of 4-Cyclohexyl-5-benzyl-2,4-dihydro-3H-1, 2,4-triazole-3-thione (6f) Yield 68%, Powder, Mp 156˚C, IR nmax cm-1 (KBr) 3200 (N-H), 1600 (C = N), 1420 (C = C), 1285 (C = S); 1H-NMR (DMSO-d6, d ppm) 1.25 - 1.79 (m, 10H, 5 × CH2 cyclohexyl), 3.36 (s, 2H, CH2-Ph), 4.50 (m, 1H, CH-N ), 7.25 - 7.43 (m, 5H, aromatic); Elemental analysis: C15H19SN3; Calculated: C 65.93%, N 15.38%, H 6.95%, Found: C 65.91%, N 15.35%, H 6.93%.
2.4. Screening of Compounds by Biological Assays
2.4.1. Antibacterial Activity
Agar well diffusion assay [13,14] was applied to evaluate the laboratory synthesized organic compounds.
2.4.2. Sample Preparation
0.1% solution of organic compounds by using dimethylsulfoxide (DMSO) as solvent was prepared. Ciprofloxacin was used as standard antibiotic in this assay and was prepared in the same way. All test tubes were identified and labeled accordingly.
2.4.3. Test Organism and Inoculums
10 bacterial strains were included and their susceptibility was tested against these compounds. 3 Gram-positive strains including Bacillus subtilis, Staphylococcus aureus, and Micrococcus luteus and 7 Gram-negative strains including Proteus mirabilis, Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, Shigella flexineri, and Klebsiella pneumonia were used.
2.4.4. Media Preparation and Sterilization
Nutrient agar medium (28 g) was accurately weighed and suspended in 1000 ml of distilled water in a conical flask. It was heated on a water bath to dissolve the medium completely. Direct heating was avoided as it may lead to charring of the medium components and render it useless for the purpose. The conical flask containing the nutrient agar medium was plugged with the help of non-absorbent cotton bung and then cotton bung were properly covered with aluminum foil. The medium was then sterilized by autoclaving at 15-lbs per square inch pressure for 18 minutes.
2.4.5. Procedure
The already prepared solutions were tested for antibacterial activity using agar well-diffusion assay. The strains of microorganisms obtained were inoculated in conical flask containing 100 ml of nutrient broth. These conical flasks were incubated at 37˚C for 24 hours and were referred to as seeded broth. Prepared media was poured on Petri dishes and inoculated with the test organisms from the seeded broth using sterile cotton swabs. Sterile stainless borer of 4-millimeter width was used for well formation. Each well was introduced with 40 μl of sample. The plates were incubated overnight at 37˚C. Antibacterial activity was assigned by measuring the inhibition zone formed around the wells. Ciprofloxacin was used as standards. All data on antibacterial activity were average of triplicate.
2.5. In Vitro Antioxidant Activity
2.5.1. Sample Preparation
1, 1-Diphenyl-2-picryl-hydrazyl (DPPH) was purchased from Sigma-Aldrich (USA). In this assay, ascorbic acid was used as “standard”; methanol was taken as “blank”, and DPPH was used as “control”. The solvents and other chemicals were of analytical grade. The reaction tubes, in triplicates, were wrapped in aluminum foil and kept at 30˚C for 30 min in dark. All measurements were done under dim light. Spectrophotometric measurements were done at 517 nm using UV-visible Spectrophotometer.
2.5.2. DPPH Radical Scavenging Activity
The antioxidant activity of the samples, on the basis of the scavenging activity of the stable DPPH free radical, was determined by the method described by [15]. One milliliter of the sample (0.75, 1.5, and 3 µg/ml) was added to 3 ml of a 0.1 mmol/l methanol solution of DPPH. Absorbance at 517 nm was determined after 30 min, and the percent inhibition activity was calculated using this formula: % DPPH radical scavenging activity = [(Absorbance of control-Absorbance of test sample)/ (Absorbance of control)] × 100.
2.5.3. Crown Gall Tumors on Potato Discs: Antitumor Activity
Crown gall assay was performed by using the potato discs according to Mclaughlin and Rogers, 1998 [16]. Fresh potato were used in this assay and was placed in bacto-agar, sample was poured onto the discs and incubated for three weeks. Each petri plate contains 5 discs and results were recorded in triplicates. Positive and negative controls were included in the assay. The percent inhibition of crown gall tumors on potato discs were calculated using the following the formula: % inhibition
= 100 – (average number tumors of sample/average number tumors of control) × 100.
2.6. Brine Shrimps Lethality Assay
2.6.1. Hatching of Shrimp Eggs
Brine shrimp lethality bioassay was carried out according to a method described elsewhere [17] to investigate the cytotoxicity of 4,5-disubstituted 1,2,4-triazole-3-thiols.
In brief, a rectangular dish (22 × 32 cm) was divided into two unequal compartments with plastic divider of 2 mm with several holes and filled with artificial seawater (prepared using 38 g sea salt per liter of water and adjusted to pH 8.5 using 1 N NaOH, filter). Approximately 25 mg eggs (Artemia salina Sera, Heidelberg, Germany) were sprinkled in the larger compartment, which was darkened, while the smaller compartment was illuminated to attract the hatched shrimps. After 48 hours, phototropic nauplii (brine shrimp larvae) were collected by pipette from the lightened side.
2.6.2. Preparation of Vials for Testing
The stock solution was prepared by dissolving 0.02 g test compound in 2 ml dimethyl sulphoxide (DMSO). 1.8 ml of the brine was added to 0.2 ml of the stock to give 1000 ppm solution. Subsequent concentrations of 100 and 10 ppm were obtained from this. Ten nauplii were drawn through a glass capillary and placed in test tube containing 4.0 ml of brine solution and 0.5 ml of test compound concentration and made up to 5 ml with brine solution. Tests for each concentration were done in triplicate. A control experiment containing 5 ml of brine solution with two drops of DMSO and ten nauplii was set alongside. The experiments were maintained at room temperature for 24 h under light and the surviving larvae counted. The lethality of the extracts was calculated from the mean survival larvae of compounds treated and control using Finney’s probit analysis was used to determine the LD50 of each compound [18].
3. Results and Discussion
4,5-disubstituted-3H-1,2,4-triazole-3-thiones 5(a-f), 6 (a-f), were obtained in good yields by the dehydrative cyclization of 1,4-disubstituted thiosemicarbazides in the presence of sodium hydroxide following Scheme 1. The reaction, in general, was quite successful; however, the yields of 3-pyridyl group bearing 1, 2, 4-triazole-3-thiones were comparatively less than those of 2- and 4-pyridyl substituted triazoles. Purity of synthesized triazoles was ascertained by TLC and a single spot was observed in each case. The physical data of the synthesized compounds is given in Table 1.
The characterization of the isolated triazoles was based on IR spectral data, 1H-NMR and elemental analysis
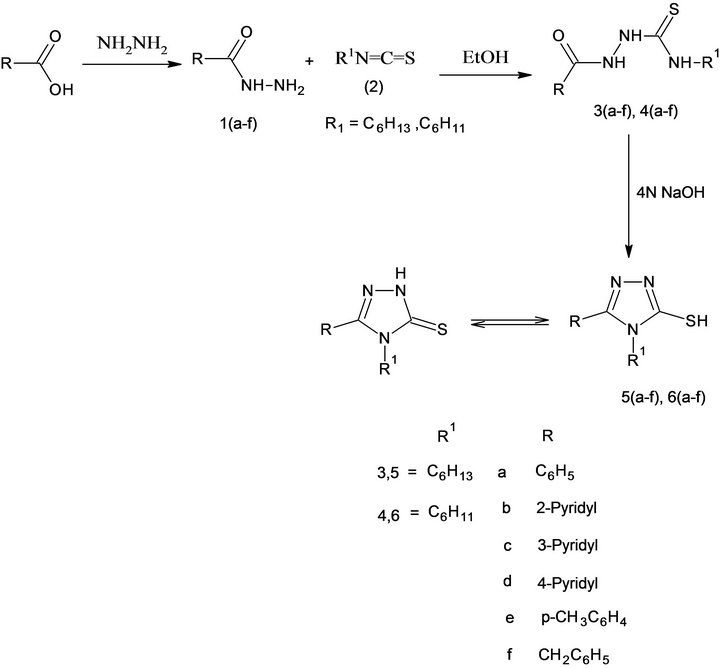
Scheme 1.. Synthesis of 2, 4-Dihydro-4, 5-disubstituteD-3 H-1, 2, 4-triazole-3-thiones 5(a-f), and 6(a-f).
data. The infrared spectra of all the triazoles showed absorption due to C = S in the region 1270 - 1290 cm−1, in addition to an N-H absorption in the region 3170 - 3260 cm−1. In each case the absence of carbonyl absorption (1675 - 1684 cm−1) in the IR spectra of the triazoles as compared to corresponding thiosemicarbazides indicated that the dehydrative cyclization reaction had occurred. Absence of SH and presence of NH and C = S absorptions established that the triazoles obtained are present in their thione form rather than the thiol form. This is in agreement to the previous observations.
In the 1H-NMR spectra of 5(a-f) having 4-hexyl group attached to nitrogen atom, methyl protons were found to resonate at 0.79 - 0.83 ppm as triplet and a multiplet at 1.19 - 1.25 ppm integrating to six protons was observed due to three methylene groups of hexyl moiety. Another multiplet due to two protons of methylene group appeared at 1.62 - 1.72 ppm. The methylene protons attached to nitrogen atom resonated downfield as triplet in the region of 4.06 - 4.13 ppm. 1H-NMR spectra of compounds 6(a-f) exhibits a downfield multiplet at 4.39 - 5.01 ppm due to CH attached with nitrogen. The other methylene protons of the cyclohexyl group exhibit two mutiplets each at 1.09 - 1.35 ppm and 1.60 - 1.83 ppm
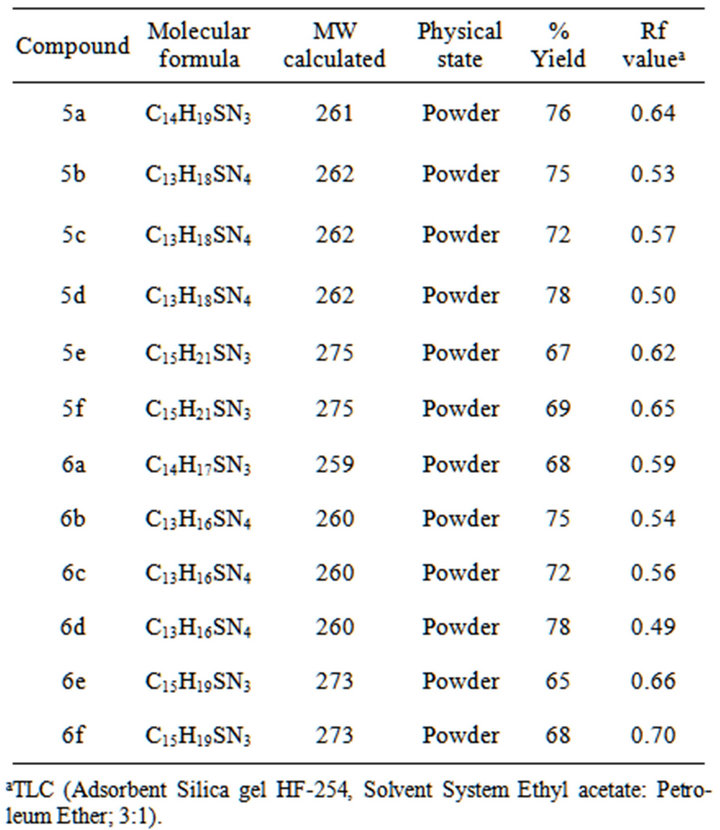
Table 1. Physical data of triazoles 5(a-f) and 6(a-f).
aTLC (Adsorbent Silica gel HF-254, Solvent System Ethyl acetate: Petroleum Ether; 3:1).
respectively in all the 4-cyclohexyl substituted triazoles. The labile proton which is attached to nitrogen or sulfur due to tautomerization appeared downfield in the range 12.40 - 12.65 ppm. In case of 5a and 6a, the phenyl protons exhibited a multiplet at 7.47 - 7.54 ppm. In triazoles 5, 6(b-d) bearing isomeric pyridyl groups, the pyridyl protons resonated in the expected region of 7.40 - 8.80 ppm.
In case of compound 5e and 6e, the singlet for p-methyl protons appeared at 2.3 ppm, whereas in 5f and 6f, the methylene protons of benzyl group resonated as a singlet at 3.72 ppm. The aromatic protons resonated in the region 7.29 - 7.42 ppm.
3.1. Antibacterial Activity
The antibacterial activity of synthesized compounds against the selected bacterial strains was checked by agar well diffusion assay. The well diffusion assay for antibacterial activity showed significant reduction in bacterial growth (Table 2).
It has been observed that among all the tested bacterial organisms, the Gram positive bacterial strain, Staphylococcus aureus followed by the Micrococcus luteus were found to be more susceptible to the organic samples by giving maximum inhibition zone of 16 mm and 14 mm respectively and the Gram negative strains were least susceptible comparatively. Pyridyl substituted triazoles 5b, 6b, 5c and 6c showed moderate activities against certain bacterial strains. 5c and 6f showed broad spectrum activity against most of the selected bacterial strains including Gram positive and negative strains (Figure 1).
3.2. DPPH Radical Scavenging Activity
DPPH radical scavenging antioxidant activity is due to their hydrogen donating ability. DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule. The reduction capability of DPPH radicals was determined by the decrease in its absorbance at 517 nm induced by antioxidants. It is visually obvious as a dis-coloration from purple to yellow. Hence, DPPH is usually used as a substrate to evaluate anti-oxidative activity of antioxidants. The antioxidant activities of the synthesized compounds at three different concentrations are given in Table 3. 5c (92.5%) and 5f (92.3%) have shown a significant decrease in the concentration of DPPH radical due to the scavenging ability of these compounds. Ascorbic acid was used as standard which showed 95.7% scavenging effect at concentration of 3 µg/ml. These results showed that these organic compounds have a noticeable effect on scavenging free radicals. Free radical scavenging effect was also increased with an increasing concentration.
3.3. Antitumor Activity
Percent inhibition results showed that all samples had anti-crown gall activity. 75% inhibition was recorded for sample 5c followed 58% inhibition for 6d (Figure 2). Methotrexate when used as positive control demonstrated 83% anti-crown gall activity on potato discs (Table 4). In general pyridyl substituted triazoles showed greater inhibition as compared to phenyl substituted triazoles. The mean tumors inhibition by these compounds has been shown in Figure 3.
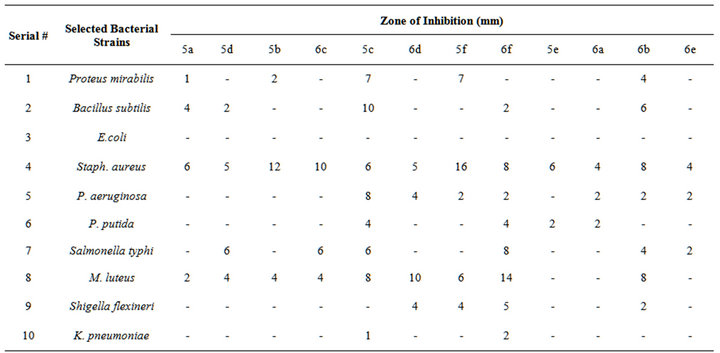
Table 2. Antibacterial activity of synthesized organic compounds against selected bacterial strains.
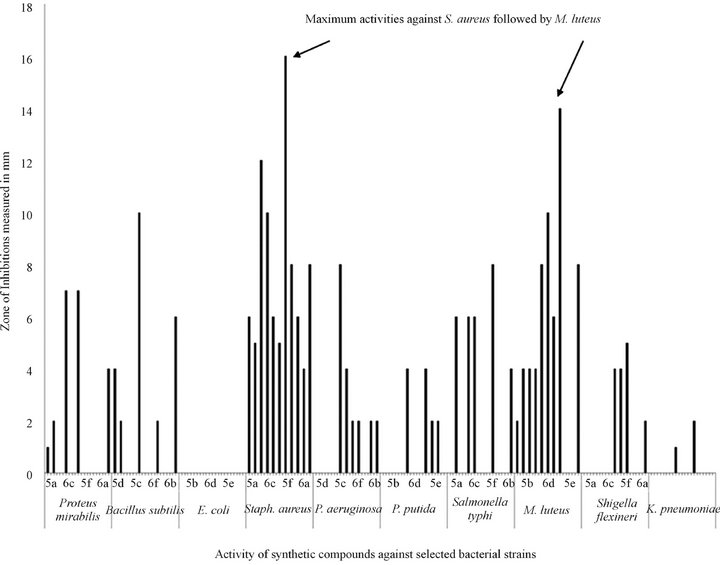
Figure 1. Activity of laboratory synthesized organic compounds against selected bacterial strains. Activity was measured in terms of zone of inhibitions (mm).
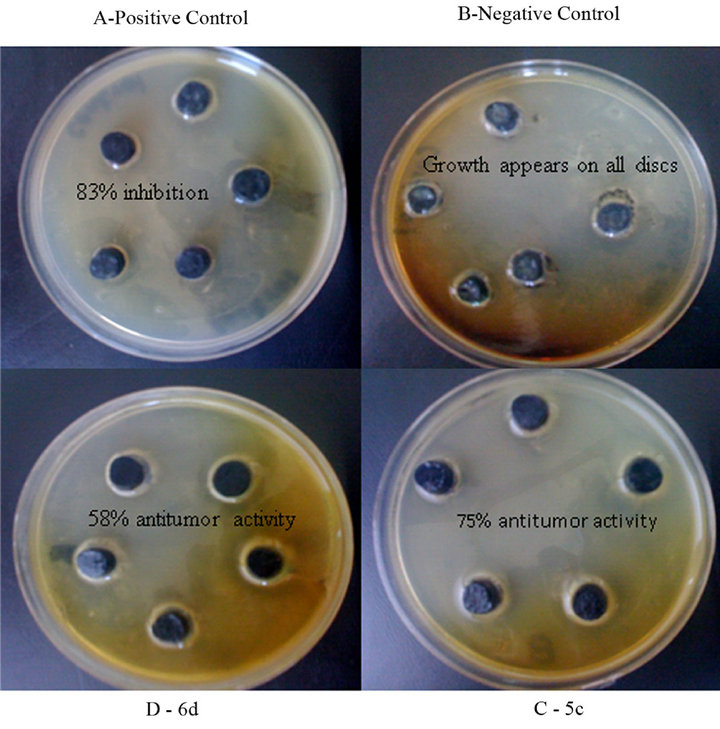
Figure 2. Inhibition of crown gall tumors on potato disc (A) Methotrexate used as Positive Control (B) Agrobacterium tumefaciens as Negative Control (C) Sample compound 5c showed 75 % tumors inhibition (D) Sample compound 6d showed 58 % tumors inhibition.
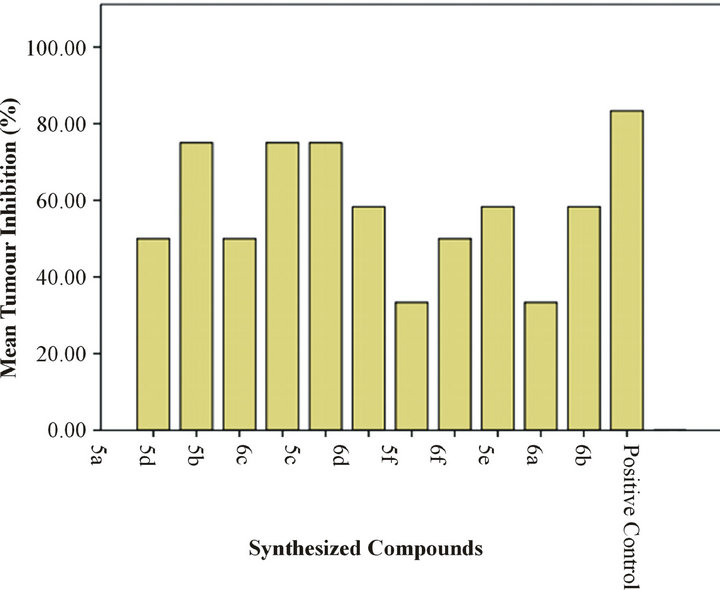
Figure 3. Mean tumors inhibition of laboratory synthesized compounds. Methotrexate was used as positive control which showed more than 80 % inhibition activity.
3.4. Brine Shrimp Lethality Assay
The brine-shrimp bioassay finds the lethality of samples toward brine-shrimp larvae (known as nauplii). The shrimp nauplii have been used for a number of experi-
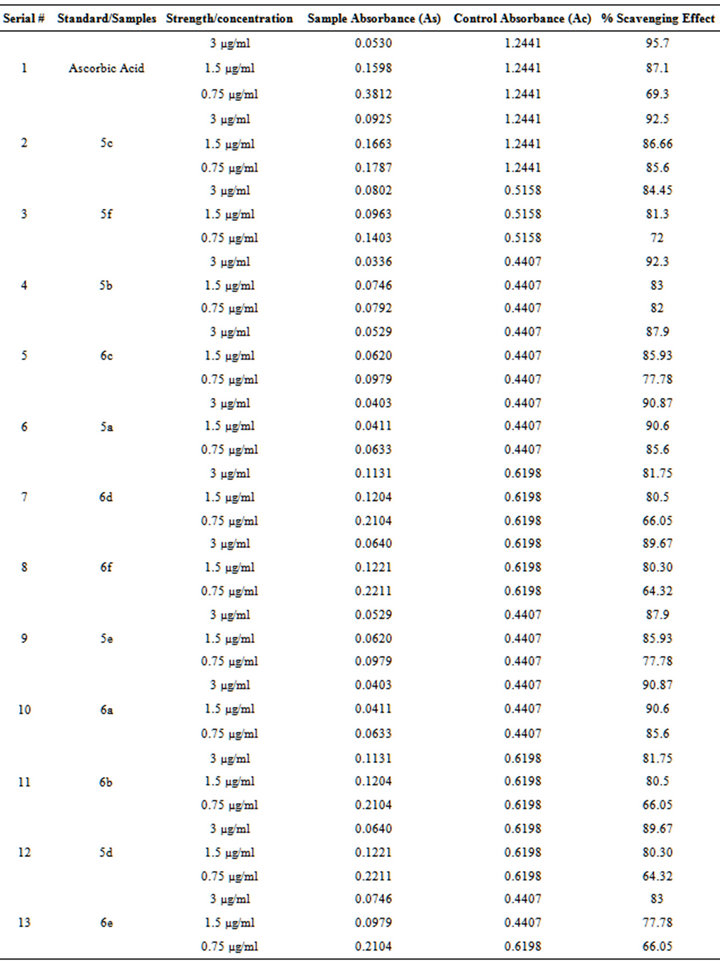
Table 3. DPPH radical scavenging effect and % inhibition of our synthesized compounds.
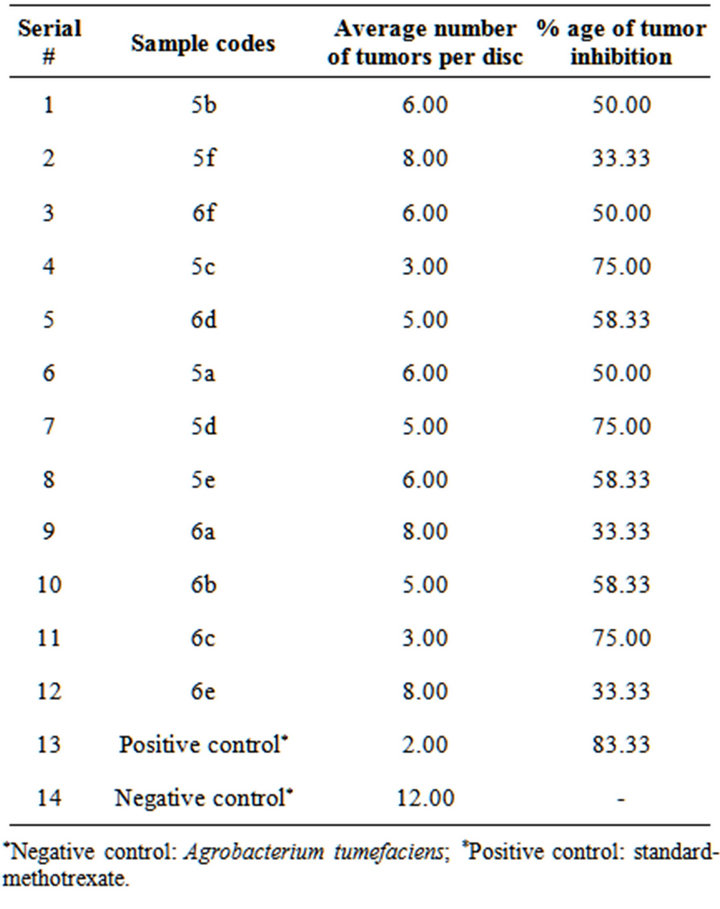
Table 4. Anti-crown gall activities of synthesized compounds.
ments in which various samples are tested at concentration of 1000 μg/ml, 100 μg/ml and 10 μg/ml in vials containing 5 ml of brine and ten nauplii in each of the three replicates [17]. After enumerating the number of shrimps surviving after 24 hours, the values for these compounds were calculated using Finney’s probit analysis. The compounds (5a, 5b & 6d), exhibited significant brine shrimp lethality with ED50 values 129.62 µg/ml, 161.577 µg/ml and 81.56 µg/ml respectively, while compounds (5f, 6a and 6b) showed moderate lethality with LD50 values of 200 µg/ml to 800 µg/ml. The results below 200 µg/ml are considered as significant values [19]. The degree of lethality was found to be directly proportional to the concentration of the extract. Maximum mortalities took place at a concentration of 1000 μg/ml whereas least mortalities were at 10 μg/ml concentration (Figure 4).
4. Conclusion
The synthesis and in vitro biological activities of a series of 4,5-disubstituted-1,2,4-triazole-3-thiones 5(a-f) and 6 (a-f) were performed in this study. In general, the compounds having pyridyl groups showed good antibacterial activity. All the compounds showed moderate to good antioxidant activity comparable to that of standard drug ascorbic acid. Free radical scavenging effect was also increased with an increasing concentration. Antitumor
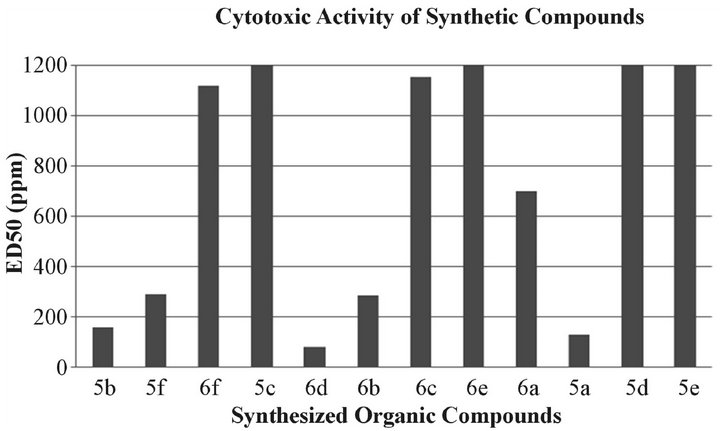
Figure 4. Cytotoxic activity analysis of laboratory synthesized organic compounds using brine shrimps. ED50 of 5a, 5b and 6d was 129.62 µg/ml, 161.577 µg/ml and 81.56 µg/ml respectively.
and cytotoxic activities were moderate to low for all the compounds. Keeping in view the results obtained, it is suggested that further analogs be synthesized and evaluated biologically in search in safe and potent drugs.
REFERENCES
- N. D. Heindel and J. R. Reid, “4-Amino-3-Mercapto-4H- 1,2,4-Triazoles and Propargyl Aldehydes: A New Route to 3-R-8-Aryl-1,2,4-Triazolo[3,4-b]-1,3,4-Thiadiazepines,” Journal of Heterocyclic Chemistry, Vol. 17, No. 5, 1980, p. 1087. doi:10.1002/jhet.5570170547
- B. S. Holla, B. Kalluraya, K. R. Sridhar, E. Drake, L. M. Thomas, K. K. Bhandary and M. S. Levine, “Synthesis, Structural Characterization, Crystallographic Analysis and Antibacterial Properties of Some Nitrofuryl Triazolo [3,4-b]-1,3,4-Thiadiazines,” European Journal of Medicinal Chemistry, Vol. 29, No. 4, 1994, pp. 301-308.
- J. Haber, “Present Status and Perspectives on Antimycoties with Systematic Effects,” Casopis Lekaru Ceskych, Vol. 140, No. 19, 2001, pp. 596-604.
- A. Brucato, A. Coppola, S. Gianguzza and P. M. Provenzan, “Triazolam: Characteristics of Its Depressive Action,” Bollettino della Società italiana di biologia sperimentale, Vol. 54, No. 11, 1978, pp. 1051-1057.
- D. L. Coffen and R. I. Fryer, US Patent (1974) 3(849): 434, Chemical Abstracts, 1975, 82: 730044v.
- M. Shiroki, T. Tahara and K. Araki, Japanese Patent NO. 75100096, 1975, Chemical Abstracts, 84: 59588k.
- F. D. Povelitsa and A. G. Gural, “Antibiotiki,” Moscow, 1973;18,71; Chemical Abstracts, 78, 93044.
- R. M. Shaker. “The Chemistry of Mercaptoand ThioneSubstituted 1,2,4-Triazoles and Their Utility in Heterocyclic Synthesis,” ARKIVOC, No. 9, 2006, pp. 59-112. doi:10.3998/ark.5550190.0007.904
- G. Mazzone, F. Bonina, R. R. Arrigo and G. Blandino “Synthesis of 1-Aroyl-4H(R)-Thiosemicarbazides, the Corresponding 5-Aryl 4H(R)-1,2,4-Triazolin-3-Thiones and Some Derivatives of Pharmaceutical Interest,” Farmaco-Edizione Scientifica, Vol. 36, No. 3, 1981, pp. 181- 196.
- S. Narayanaswami and K. Richardson, European Patent, 1983, 96:569; Chemical Abstracts, 1984. 100: 139122t.
- M. B. Gravestock, European Patent, 1983, 94:146, Chemical Abstracts, 1984, 100: 139118w.
- R. Sharma, D. P. Nagde and G. L. Talesara, ARKIVOC, Vol. 1, 2006, pp. 1-12.
- K. S. Sen, F. S. Haque and C. S. Pal, “Nutrient Optimization for Production of Broad Spectrum Antibiotics by Streptomyces Antibiotics Strain 154,” Acta Microbiologica et Immunologica Hungarica, Vol. 42, No. 2, 1995, pp. 155-162.
- A. W. Bauer, W. M. Kirby, J. C. Sherries and M. Truck, “Antibiotic Susceptibility Testing by a Standardized Single Disk Method,” American Journal of Clinical Pathology, Vol. 45, No. 4, 1996, pp. 426-493.
- A. Braca, N. De Tommasi, L. Di Bari, C. Pizza, M. Politi and I. Morelli, “Antioxidant Principles from Bauhinia Terapotensis,” Journal of Natural Products, Vol. 64, No. 7, 2001, pp. 892-895. doi:10.1021/np0100845
- J. L. Mclaughlin and L. L. Rogers, “The Use of Biological Assays to Evaluate Botanicals,” Drug Information Journal, Vol. 32, No. 2, 1998, pp. 513-524.
- B. N. Meyer, N. R. Ferrigni, J. E. Putnam, L. B. Jacobsen, D. E. Nichols and J. L. McLaughlin, “Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents,” Planta Medica, Vol. 45, No. 5, 1982, pp. 31-34. doi:10.1055/s-2007-971236
- D. J. Finney, “Probit Analysis,” Cambridge University Press, Cambridge, 1971.
- H. O. Oladimeji, R. Nia and E. E. Essien, “In Vitro Anti-Microbial and Brine-Shrimp Lethality Potential of the Leaves and Stem of Calotropis procera (Ait),” African Journal of Biomedical Research, Vol. 9, No. 3, 2006, pp. 205-211.
NOTES
*Corresponding author.

