Open Journal of Medical Microbiology
Vol.3 No.2(2013), Article ID:33304,4 pages DOI:10.4236/ojmm.2013.32020
Biopharmaceutical Assessment of Active Components of Deadaleopsis confragosa and Ganoderma lucidum
1Department of Biological Sciences, Ondo State University of Science and Technology, Okitipupa, Nigeria
2Department of Microbiology, Adekunle Ajasin University, Akungba Akoko, Nigeria
3Department of Biological Sciences, Joseph Ayo Babalola University, Ikeji-Arakeji, Nigeria
Email: *fakxoj@yahoo.com
Copyright © 2013 Soji Fakoya et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 21, 2013; revised April 22, 2013; accepted April 30, 2013
Keywords: Ganoderma lucidum; Deadaleopsis confragosa; Antimicrobials; Inhibition Zones; Phytochemicals
ABSTRACT
The spread of multidrug-resistant strains of bacteria makes it necessary to discover new classes of antibacterial and compounds that inhibit these resistant mechanisms. Hence, this study investigated the antimicrobial activities of Ganoderma lucidum and Deadaleopsis confragosa extracts against some bacterial isolates of medical importance. Using agar well diffusion assay, aqueous, ethanolic and petroleum ether extracts were obtained from Ganoderma lucidum and Daedaleopsis confragosa and assayed for antimicrobial on five bacterial species, viz: Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Proteus mirabilis and Klebsiella pneumoniae. In vitro bioassay revealed that the aqueous extract of G. lucidum inhibited P. aeruginosa S. aureus, E. coli and K. Pneumoniae with inhibition zones of 11.0 ± 0.02 mm, 10.0 ± 0.02 mm, 13.0 ± 0.03 mm and 14.0 ± 0.0 mm respectively. The ethanolic extract of G. lucidum also inhibited P. aeruginosa, S. aureus and E. coli with inhibition zones 12.0 ± 0.01 mm, 11.0 ± 0.02 mm and 16.0 ± 0.01 mm. Petroleum ether extract of G. lucidum inhibited P. aeruginosa, S. aureus and E. coli with inhibition zones of 12.0 ± 0.01 mm, 11.0 ± 0.03 mm and 12.0 ± 0.02 mm. For Daedaleopsis confragosa, the aqeous extract inhibited P. aeruginosa and E. coli with inhibition zones of 12.0 ± 0.01 and 12.0 ± 0.02 mm respectively while the petroleum ether extract inhibited S. aureus and E. coli with inhibition zones of 19.0 ± 0.02 mm and 13.0 ± 0.01 mm respectively. All these inhibitions on clinical isolates are therefore attributed to the presence of some bioactive compound as shown by the phytochemical screening of the mushrooms which include tannins, phenolics, flavonoids and saponin.
1. Introduction
Mushroom describes a variety of gilled fungi, with or without stems and the term is used more generally to describe fleshing fruiting bodies of some basidiomycota [1]. Medicinal mushrooms are mushrooms used in the practice of medicine and many species of mushrooms have been used in folk medicines for thousands of years. [2] has also reported the importance of mushrooms as sources of food nutrients as well as for medicinal purposes in the orients. [3] also reported that some mushrooms inhibit tumor growth and enhance aspect of the immune system and thus, have been subjects of research for approximately 50 years. However, studied mushroom extracts have consistently shown to be safe and well tolerated, also, the medicinal value of these mushrooms lied in some chemical substances that produce a definite physiological action on human body [3]. It is recognized in some developing countries that mushrooms are the main medicinal source to treat infectious diseases. In these countries living conditions are crowded by poor hygiene, diarrhea and dysentery and some other deadly diseases caused by bacteria, fungi and other microorganisms resulting to high morbidity and mortality.
Research conducted with mushroom has led to the discovery of drugs such as penicillin, endosporin, griseofulvin, cephalosporin and so on. Examples of mushroom species with medicinal potentials of importance include Reishi (Ganoderma lucidum), shiitake (Lentinula edodes), Grifola fondosa, G. unbellaus and so on. Reishi is also known to contain various chemical substances, including more than 119 different types of triterpenes and several types of polysaccharides [4]. Presence of immune polysacchardes also known as beta-glucan makes these medicinal mushrooms of high medicinal value [5].
Mushrooms are also known to contain antioxidants such as tocopherols, phenolic compounds and carotenoids. Research has shown that some medicinal mushrooms may be able to lower elevated blood sugar levels. Mushrooms noted for this ability include Reishi, Agaricus campestris, A. blazei, Agrocybe aegerita, and Cordyceps [6]. Explanation for this effect is limited, with the exception of the maitake mushroom which has ability to lower blood sugar levels [7]. It has been explained by the fact that the mushroom naturally contains a compound known as an alpha-glycosidase inhibitor [8].
Antimicrobial agents vary in their selective toxicity. Some act in a non-selective manner and have similar effect on all types of cells. Antimicrobial agents with selective toxicity are especially useful as chemotherapeutic agents in such treating infectious diseases and may be a function of specific receptor requirement for drug attachment [9]. Therefore, the aim of this work is to determine the antimicrobial activities and bioactive ingredients of Ganoderma lucidum and Daedaleopsis confragosa and compare the efficacy of the mushroom extracts with some standard antibiotics.
2. Materials and Methods
2.1. Collection of Mushroom Samples
Mushroom samples used for this research work were obtained on the 16th April, 2010 from a cocoa farm very close to Joseph Ayo Babalola University, Ikeji-Arakeji, Osun State, Nigeria. They were identified in the Department of Biological Science, Joseph Ayo Babalola University and authenticated at the Department of Microbiology, Federal University of Technology, Akure. The mushrooms were washed with distilled water and sundried for four days. It was later oven dried for 24 hr at 45˚C. The dried samples were pulverized using industrial blender (exceler).
2.2. Collection of Isolates
Clinical isolates of Staphylococcus aureus, Escherichia coli, Proteus vulgaris, Klebsiella pneumonia and Pseudomonas aeruginosa were collected from Obafemi Awolowo University teaching hospital, Ile-Ife, Osun State, Nigeria. The isolates were resuscitated in nutrient agar and maintained at 4˚C for further use.
2.3. Phytochemical Analysis
The following bioactive compounds were assayed for: tannis, phenolics, saponins, flavoniods, steroid and phlobatannins using the method described by Sofowora et al. [10].
2.4. Determination of the Antimicrobial Activity of the Mushroom Samples
Antimicrobial potentials of extracts from G. lucidum, D. confragosa and some standard antibiotics were assayed for using the method of [11].
2.5. Statistical Analysis
Quantitative data were expressed as mean ± standard deviation. Statistical evaluation of the data was performed using one-way analysis of variance followed by Duncan’s multiple range test at 5% level of significance i.e. P ≤ 0.05 [12].
3. Results
3.1. Bioactive Components of G. lucidum and D. confragosa
Table 1 shows the phytochemical components present in the aqeous, ethanolic and petroleum ether extracts of G. lucidum and D. confragosa.
3.2. Antimicrobial Activities of G. lucidum and D. confragosa
Table 2 shows the antimicrobial activities of the mushroom samples. Highest zone of inhibition of 16.0 ± 0.01 mm was observed against E. coli on ethanolic extract of G. lucidum while P. vulgaris and K. pneumonia were resistant. Petroleum ether extract of G. lucidum inhibited P. aeruginosa, S. aureus and E. coli with 12.0 ± 0.01 mm, 11.0 ± 0.03 mm and 12.0 ± 0.02 mm respectively. However, P. vulgaris, and K. pneumoniae were found to be resistant. Also, aqueous extract of G. lucidum against P. aeruginosa, S. aureus, E. coli and K. pneumoniae was pronounced with inhibitory zones of 11.0 ± 0.02 mm, 10.0 ± 0.02 mm, 13.0 ± 0.03 mm and 14.0 ± 0.00 mm respectively while P. mirabilis was resistant. The ethanolic extract of D. confragosa against P. aeruginosa, S. aureus, P. vulgaris, E. coli and K. pneumoniae were not noticeable while S. aureus and E. coli had 19.0 ± 0.02 mm and 13.0 ± 0.01 mm for the petroleum ether extract of D. confragosa with P. aeruginosa, P. vulgaris and K. pneumoniae being resistant. For the antibacterial activities of the aqueous extract of D. confragosa, S. aureus and E. coli had zones of inhibition of 12.0 ± 0.01 mm and 12.0 ± 0.01 mm respectively with P. aeruginosa, P. vulgaris and K. pneumoniae being resistant. The standard antibiotics assayed for showed the Amoxil against P. aeruginosa, S. aureus and E. coli with inhibition zones of 11.0 ± 0.02 mm, 14.0 ± 0.01 mm and 8.0 ± 0.01 mm respectively, while Ampicillin inhibited S. aureus, P. vulgaris, E. coli and K. pneumoniae with inbihition zones of 11.0 ± 0.02 mm, 14.0 ± 0.03 mm, 11.0 ± 0.02 mm and 18.0 ± 0.01 mm respectively. Ampiclox also inhibited S.
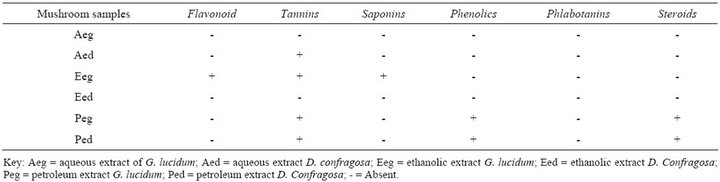
Table 1. Phytochemical components of D. confragosa and G. lucidum.
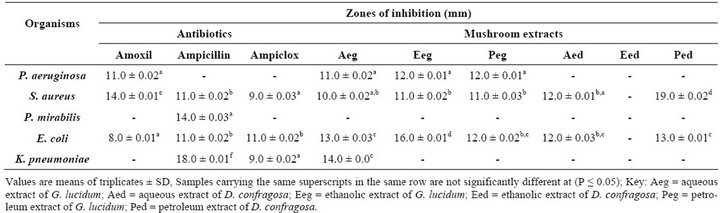
Table 2. Antimicrobial activities or potentials of mushroom extracts D. confragosa and G. lucidum.
aureus, E. coli and K. pneumoniae with inhibition zones of 9.0 ± 0.03 mm, 11.0 ± 0.02 mm and 9.0 ± 0.02 mm respectively as shown in Table 2.
4. Discussion
The result obtained in this study revealed that the aqueous extract of G. lucidum contain tannins which has an anti-inflammatory potential that helps to control gastritis, esophagitis and enteritis [5]. It can also heal burns, stops bleeding, prevents kidney damage and also of great importance in the curing of sore throats, diarrhea and dysentery in human, while the ethanolic extract of G. lucidum contains saponins, tannins and flavonoids. Saponins, tannins eand phenolics were present in petroleum ether extract of G. lucidum. However, this presence of saponin in particular indicates it potential to be used as protective against coronary heart disease, stroke and cancer [5], while flavonoids are active against nausea and gastro-enteric infections [13]. The aqueous and petroleum ether extract of D. confragosa contains tannins and phenolics, respectively except for the ethanolic extract of D. confragosa which has no phytochemical group.
The inhibitory potentials of the mushroom extracts could be attributed to the presence of phytochemicals also known as bioactive agents [14] because without these bioactive agents, the clinical organisms tend to show resistant. The ethanolic extract of G. lucidum shows the highest antibacterial potential with an inhibition zone of 16 mm while the petroleum ether extract of D. confragosa equally shows a high zone of inhibition of 19.0 mm. In comparison, the aqeous extracts of Ganoderma lucidum showed a greater antibacterial potential with their inhibittion zones greater than that of the commercial antibiotics assay for except for ampicillin which had greater inhibittory potentials. This however is in agreement with the report of [15] that Ganoderma lucidum had great antibacterial potential. Also, the aqueous, ethanol and petroleum ether extract of G. lucidum had a broad spectrum of antimicrobial activities due to the presence of more phytochemical groups compared to the aqeous and petroleum ether extracts of D. confragosa. This therefore confirms the report of [13] that the extracts of G. lucidum is more of medicinal importance in the treatment of diarrhea, kidney infection, sore throat enteric infections and so on. In conclusion, results obtained from this study show that some of the solvents (aqueous, ethanol and petroleum ether) used for the extraction had a broad spectrum of activity which makes it very effective against infection causing organisms except for the ethanolic extract of D. confragosa. However, if the active ingredients of these extracts are characterized and purified through further research, it is possible that crystallized therapeutic antibiotics could be produced from them. Further research could also be carried out on these mushrooms’ extracts
(G. lucidum and D. confragosa) in order to ascertain their selective toxicity.
REFERENCES
- R. Griffiths, W. Richards, M. Johnson, U. McCann and R. Jesse, “Mystical-Type Experiences Occasioned by Psilocybin Mediate the Attribution of Personal Meaning and Spiritual Significance 14 Months Later,” Journal of Psychopharmacology, Vol. 22, No. 6, 2008, pp. 621-632. doi:10.1177/0269881108094300
- U. Lindequist, T. H. J. Niedermeyer and W. D. Julich, “The Pharmacological Potential of Mushrooms—Review,” Evidence-Based Complementary and Alternative Medicine, Vol. 2, No. 3, 2005, pp. 285-299. doi:10.1093/ecam/neh107
- J. W. Bennett and M. Klich, “Mycotoxins,” Clinical Microbiology Reviews, Vol. 16, No. 3, 2003, pp. 497-516. doi:10.1128/CMR.16.3.497-516.2003
- C. Hsieh and F. Yang, “Reusing Soy Residue for the Solid-State Fermentation of Ganoderma lucidum,” Bioresource Technology, Vol. 91, No. 1, 2004, pp. 105-109. doi:10.1016/S0960-8524(03)00157-3
- J. L. Mau, H. C. Lin and C. C. Chen, “Non-Volatile Components of Several Medicinal Mushrooms,” Food Research International, Vol. 34, No. 6, 2001, pp. 521-526. doi:10.1016/S0963-9969(01)00067-9
- Y. Masuda, Y. Murata, M. Hayashi and H. Nanba, “Inhibitory Effect of MD-Fraction on Tumor Metastasis: Involvement of NK Cell Activation and Suppression of Intercellular Adhesion Molecule (ICAM)-1 Expression in Lung Vascular Endothelial Cells,” Biological & Pharmaceutical Bulletin, Vol. 31, No. 6, 2008, pp. 1104-1108. doi:10.1248/bpb.31.1104
- A. T. Borchers, A. Krishnamurthy, C. L. Keen, F. J. Meyers and M. E. Gershwin, “The Immunobiology of Mushrooms,” Experimental Biology and Medicine, Vol. 233, No. 3, 2008, pp. 259-276. doi:10.3181/0708-MR-227
- T. Pillai, C. Nair and K. Janardhanan, “Polysaccharides Isolated from Ganoderma Lucidum Occurring in Southern Parts of India, Protects Radiation Induced Damages Both in Vitro and in Vivo,” Environmental Toxicology and Pharmacology, Vol. 26, No. 1, 2008, pp. 80-85.
- C. N. Harold and D. G. Thomas, “Antimicrobial Chemotherapy,” Medical Microbiology, 4th Edition, University of Texas Medical Branch, Galveston, 1996.
- C. Perez, M. Pauli and P. Bazerque, “An Antimicrobial Assay by the Agar Well Diffusion Method,” Acta Biologae et Medicine Experimentalis, Vol. 15, 1990, pp. 113- 115.
- A. E. Sofowora and A. Odebiyi, “Medicinal Plants and Traditional Medicinal in Africa,” 2nd Edition, Spectrum Books Ltd., Ibadan, 1993, pp. 134-156.
- J. H. Zar, “Biostatistical Analysis,” Prentice-Hall, Upper Saddle River, 1984.
- B. Boh, M. Berovic, J. Zhang and L. Zhi-Bin, “Ganoderma lucidum and Its Pharmaceutically Active Compounds,” Biotechnology Annual Review, Vol. 13, 2007, pp. 265-301. doi:10.1016/S1387-2656(07)13010-6
- B. U. Shamaki, U. K. Sandabe, I. A. Fanna, O. O. Adamu, Y. A. Geidam, I. I. Umar, M. S. Adamu, “Proximate Composition, Phytochemical and Elemental Analysis of Some Organic Solvent Extract of The Wild Mushroom Ganoderma lucidum,” Journal of Natural Sciences Research, Vol. 2, No. 4, 2012.
- S. Sekaran, S. Elumalai, B. Ramalingam and K. Devendiran, “Evaluation of Antibacterial and Antifungal Activiity of Ganoderma lucidum (curtis) p. Karst Fruit Bodies Extracts,” World Journal of Science and Technology, Vol. 1, No. 6, 2011, pp. 8-11.
NOTES
*Corresponding author.

