Open Journal of Medical Microbiology
Vol.3 No.1(2013), Article ID:28576,5 pages DOI:10.4236/ojmm.2013.31007
Field Evaluation of a Transport Medium and Enrichment Broth for Isolation of Campylobacter Species from Human Diarrheal Stool Samples
1Department of Enteric Diseases, Armed Forces Research Institute of Medical Sciences (AFRIMS), US Army Medical Component, Bangkok, Thailand
2Regional Medical Sciences Center, Department of Medical Sciences, Ministry of Public Health, Trang, Thailand
3Regional Medical Sciences Center, Department of Medical Sciences, Ministry of Public Health, Ubon Ratchathani, Thailand
4Queen Sirikit National Institute of Child Health, Bangkok, Thailand
Email: apichais@afrims.org
Received January 12, 2013; revised February 23, 2013; accepted February 28, 2013
Keywords: Humans; Diarrhea microbiology; Stool specimen preservation transportation; Culture media; Campylobacter isolation enrichment
ABSTRACT
Campylobacter continues to be a major cause of bacteria-mediated diarrheal diseases, both for Thai citizens and travelers to Thailand. For field epidemiological studies, appropriate methods for storage, intra-laboratory transport of patients specimens and use of enrichment culture to isolate this organism is critical. Study A, represents patient stool specimens collected in Bangkok and processed for Campylobacter culture within three hours after collection. Study B, represents stool specimens collected from patients in northeast and Southern regions of Thailand in modified Cary-Blair transport medium. These specimens were transported and processed for Campylobacter in Bangkok at varying intervals ranging from 1 to 7 days. Of 900 diarrheal samples examined in study A, a total of 158 were Campylobacter positive through culture. Of these, 145 and 141 isolates were cultured by direct plating and enrichment plating respectively (P = 0.5839). From 1,168 diarrheal stool samples examined in study B, 184 were positive for Campylobacter. Direct and enrichment plating resulted in 139 and 168 culture isolates; respectively (P = 0.0003). Samples from study B delayed in processing for 1 to 3 days, resulted in 46 and 50 isolated by direct and enrichment plating; respectively (P = 0.4545). However, among samples delayed in processing for 4 to 7 days, a total of 128 Campylobacter isolates were cultured, having cultured 93 and 118 isolates through direct and enrichment plating; respectively (P = 0.0003). At present these studies demonstrate that enrichment culture has no benefit when stool specimen collection and immediate processing occur and when there is a processing delay period of 1 - 3 days. However, enrichment culture was beneficial in instances where transport and processing was delayed 4 - 7 days.
1. Introduction
Campylobacter enteritis continues to be a leading cause of diarrheal disease throughout the world; young children being the most susceptible population [1]. Campylobacter is also an important cause of traveler’s diarrhea in Thailand [2]. Campylobacter jejuni (C. jejuni) and Campylobacter coli (C. coli) are the most common etiologies of human Campylobacter enteritis. Increasing fluoroquinolone resistance among these organisms in Thailand is resulting in a significant public health concern [3,4]. For epidemiological studies in diarrheal diseases, immediate culturing for the isolation of Campylobacter from fecal samples is not always possible. Thus, an appropriate method to maintain viability of this organism during storage and intra-laboratory transport is important. Modified Cary-Blair (MCB) medium containing 0.16% agar is considered to be a suitable transport medium for stool samples suspected of harboring Campylobacter species, as well as, other enteric bacterial pathogens. Stool preserved in MCB has demonstrated efficacy at prolonging survival of C. jejuni more effectively at 4˚C than at room temperature more than media containing higher agar concentrations at 4˚C [5]. Routine culture for Campylobacter species is performed using the membrane filtration technique or other selective agar media (e.g. modified Charcoal-Cefoperazone Deoxycholate agar/mCCDA). Enrichment media has been used to enhance recovery of Campylobacter species in the clinical setting, particularly for C. jejuni and C. coli. However, use of enrichment medium to promote recovery of Campylobacter spp. in epidemiological field studies has not been evaluated adequately.
Objectives of the present study were to evaluate use of MCB as transport medium in epidemiological field studies for recovering Campylobacter species from human diarrheal specimens, as well as, evaluation of Preston enrichment broth to improve recovery of this organism from human diarrheal samples processed at variable time points.
2. Materials and Methods
2.1. Source of Specimens
All samples tested were obtained from the Multi-Center Study of Prevalence and Drug Resistant Pattern of Campylobacter and Other Enteric Pathogens in Thailand. Samples were approved by the Ethical Review Committee of Research in Human Subjects, Ministry of Public Health, Nonthaburi, Thailand and the Human Subjects Research Review Board, US Army Medical Research and Material Command, Fort Detrick, MD. After obtaining informed consent from a parent or guardian, a stool sample was collected from children aged 3 months to 5 years who presented to the hospital with diarrhea. All stools were collected in a clean wide mouth container by a nurse or a health care provider.
2.1.1. Study A
Fresh stool samples were collected from January 2003 to October 2006 from children who presented to the OPD ward of the Queen Sirikit National Institute of Child Health, Bangkok, Thailand. All samples were transported to the Armed Forces Research Institute of Medical Sciences (AFRIMS) laboratory, Bangkok, Thailand and processed for culture within 3 hours after collection. Transport medium was not used in this study.
2.1.2. Study B
Stool samples collected from the northeast region of Thailand during October 2004 to December 2006 originated from children who presented to the OPD of the Suppasitprasong Hospital. Stool samples collected within the southern region of Thailand from October 2004 to October 2006 originated from children who presented to the OPDs of the Trung Hospital and Patalung Hospital. In all cases, three polyester tipped swabs were used to harvest fecal material from each stool sample with subsequent inoculation in MCB transport medium. All inoculated MCB transport medium were refrigerated at 2˚C - 8˚C until transported in Styrofoam boxes with ice packs to the AFRIMS laboratory. Specimen processing occurred within 7 days of collection.
2.2. Media
Semi-solid, 0.16% (w/v), Modified Cary-Blair (MCB) transport medium [6] was used in this study. Brucella agar plates (Becton, Dickinson and Company, MD, USA) supplemented with 5% sheep blood (BAP), and mCCDA (Oxoid Ltd., Hampshire, England), supplemented with 32 mg/L cefoperazone and 10 mg/L Amphotericin B (Sigma-Aldrich.Inc, St. Louis, USA) were prepared according to the manufacturer’s instructions.
2.3. Specimen Processing
Upon receipt at the laboratory, approximately 0.5 gram of each fresh stool sample or 3 fecal swabs were diluted 1:10 with an appropriate volume of sterile normal saline in sterile test tubes. Each fecal suspension was cultured as follows;
2.3.1. Direct Plating
Filtration technique as described by Steele and McDermott [7] was modified as follows; five to six drops of fecal suspension were separately applied on top of a 47-mm, 0.65 µm sterile cellulose acetate membrane filter (Millipore, Bedford, USA) centrally placed on the surface of a BAP, followed by membrane removal after 30 minutes of filtration under ambient conditions. Selective technique was performed by streaking a single drop of fecal suspension onto the mCCDA.
2.3.2. Enrichment and Plating after Enrichment
Approximately 0.5 mL of each fecal suspension was inoculated into 7 mL of Preston broth (Oxoid, UK), keeping caps loose during incubation. Every 18 - 24 hours a new set of BA filtration plates and mCCDA plates were inoculated.
2.3.3. Incubation
Inoculated BAP and mCCDA plates were incubated at 37˚C under microaerobic conditions (5% O2, 10% CO2, 85% N2), generated by a trigas-incubator (Nuaire, Plymouth, USA) for up to 72 hours and 18 - 24 hours for Preston enrichment broths.
2.4. Plate Reading
BAP and mCCDA plates were examined after 48 hours of incubation. Typical colonies resembling those of Campylobacter were picked to perform identification. Specimens were determined to be Campylobacter culture negative for both direct and enrichment plating after 72 hours of no growth.
2.5. Identification of Campylobacter
Initial identification of Campylobacter was determined by colony morphology, Gram stain, and oxidase test. Confirmatory identification was determined through phenotypic tests [8], including catalase, hippurate hydrolysis, nitrate reduction, indoxyl acetate hydrolysis, H2S production in TSI, growth at 37˚C aerobically, ability to grow at 25˚C and 42˚C microaerobically, and susceptibility to Nalidixic Acid (30 µg) and cephalothin (30 µg) disks.
2.6. Data Analysis
In each study, improvement of Campylobacter isolation by Preston broth were measured by comparing the numbers of Campylobacter-positive samples from enrichment culture to the number from direct plating. Non-parametric McNemar test was applied to each study’s results to assess efficacy of enrichment. A cricital level (α) of 0.05 was selected to denote statistical significance.
3. Results and Discussion
Table 1 shows the results of Campylobacter species isolated from direct and enrichment plating in studies A and B. Of the 900 fresh stool specimens collected from Study A, without using MCB transport medium, 158 were Campylobacter-positive, 145 isolates by direct plating, and 141 isolates through enrichment plating (P = 0.5839). From 1168 preserved stool samples in MCB from Study B, 184 were positive for Campylobacter spp. Of these, 139 were cultured by direct plating, while 168 isolates were cultured through enrichment plating (P = 0.0003). Numbers of species-specific Campylobacter isolates from the two studies are shown in Table 1. In both studies C. jejuni comprised the majority of isolates, while the remaining isolates comprised C. coli. There was no marked improvement, Study A, using enrichment broth for the recovery of Campylobacter species from fresh stool specimens (P = 0.5839). However, Study B methods demonstrated significant improvement in C. jejuni isolation from archived stool specimens utilizing enrichment medium (P = 0.0006), but not for other Campylobacter species (most likely due in part to low numbers of cells from other Campylobacter spp.).
A total of 1168 fecal swab samples were received in MCB transport medium after an average of 6 days in transit prior to being processed for Campylobacter isolation; Table 2. An overall isolation rate by direct and enrichment plating was 15.8% (184/1168). Utilizing Preston enrichment medium resulted in a 2.5% (14.4% versus 11.9%) higher isolation rate of Campylobacter spp. in contrast to direct inoculation. Of 477 stool specimens examined within 3 days, 56 Campylobacter-positive cultures were assayed; 6 isolates by direct plating only, 10 isolates through enrichment plating only, and 40 isolates through both direct and enrichment plating; Table 2. Only 1% (4/477) more Campylobacter-positive specimens were detected from preserved human stool in MCB by enrichment plating than by direct plating (P = 0.4545). This data suggests that enrichment broth did not significantly enhance recovery of any Campylobacter species, though these specimens had been held in MCB at 2˚C - 8˚C for 1 to 3 days. Of 691 fecal swabs held in MCB transport medium for 4 - 7 days, 128 Campylobacter
Table 1. Campylobacter isolates from stool specimens using direct and enrichment plating, Study A and Study B.
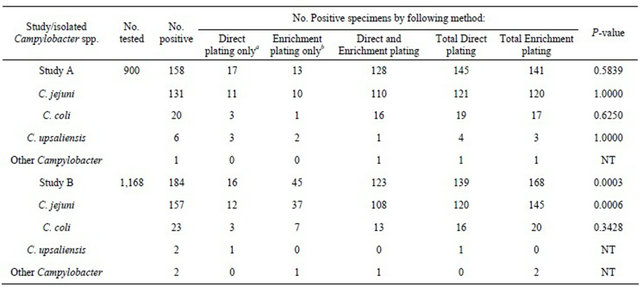
a.Specimens positive by direct plating but negative by enrichment plating. bSpecimens positive by enrichment plating but negative by direct plating. NT, not tested.
Table 2. Campylobacter isolates using and duration of delay in transport before processing, Study B.
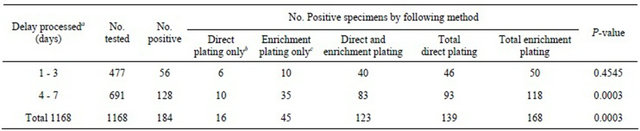
aMedian = 6 days, bSpecimens positive by direct plating but negative by enrichment plating, cSpecimens positive by enrichment plating but negative by direct plating.
positive cultures were assayed; 10 isolates by direct plating only, 35 isolates through enrichment plating only, and 83 isolates through both direct and enrichment plating; Table 2. These results suggest an increase in Campylobacter positive cultures by enrichment plating in comparison to direct plating. Suggesting Preston enrichment broth did significantly, (P = 0.0003), improve recovery of Campylobacter species from fecal specimens with delayed processing times from 4 to 7 days.
Isolation of Campylobacter is hampered by its fastidious nature and may be overgrown by normal flora present in stool specimens. Although isolation techniques have been improved, isolation of this organism is still difficult due to delays in transit to reference laboratories. Appropriate methods for transportation, storage of clinical samples, and enrichment are critical for recoverability of pathogens in field epidemiological studies. Particularly important when immediate processing of fecal specimens is not always possible. Tanner and Bullin [9] compared storage of 7 aliquots of positive human fecal specimens kept at +4˚C and at room temperature, after 2 days only one of seven samples stored at room temperature was positive, whereas all seven samples stored at +4˚C were still positive. Blaser et al. [10] reported that C. fetus subsp. jejuni was recoverable from 5 positive human stools from 9 to 22 days when refrigerated at 4˚C, but for only 2 to 8 days when left at room temperature. Wang et al. [5] reported 90% survival of C. jejuni in human fecal specimens stored in MCB after 5 - 8 days, (with decreased agar concentration, 1.6 g/liter), at 4˚C without enrichment and 50% survival after 2 weeks. Enrichment media has been used for many years to improve isolation of Campylobacter spp. from human, animal, food and environmental samples. Martin and others [11] reported a significant increase of C. jejuni after enrichment in Campylobacter enrichment broth. SjÖgren and coworkers [12] used semisolid motility test medium as enrichment for Campylobacter spp. from in/out patients whose samples were processed within 24 hours. Differences in cultured isolates were only seen in outpatients and sample qualities were suggested to be the variable factors. Bolton and Robertson [13] observed that Preston enrichment broth was suitable for recovering Campylobacter species from human stool specimen and other biological sources (animal, avian and environmental). They also found that enrichment was not necessary for laboratory diagnosis of acute infections in human when great numbers of Campylobacter were present in stool, but was necessary when the number of C.jejuni was lower than 104 organisms/gram of stool.
In the present study, it is probable that most specimens contained viable Campylobacter organisms in sufficient numbers to be easily detected by one or both agar media in the primary plating step. Of 158 positive Campylobacter isolates, 13 were recovered only after enrichment (Table 1). This suggests that even though the diarrheal stool specimens were processed for culture within 3 hours of collection, some of them may have contained insufficient numbers of Campylobacter to be detected by direct plating. However, no significant difference in the total number of Campylobacter isolated from direct plating and after enrichment in Preston broth were detected (P = 0.5839) (Table 1). Additionally, 11 C. jejuni, 3 C. coli and 3 C. upsaliensis isolates were recovered when plated directly, but were negative after enrichment (Table 1). This suggests that these Campylobacter species might be susceptible to at least one antimicrobial in the enrichment broth, and therefore could not be recovered in secondary plating. Though all specimens were received and processed, on average, 6 days after collection, 11.9% (139/1,168) of these specimens yielded Campylobacter in the direct plating step (Table 1). When Preston enrichment broth was incorporated, the isolation rate increased from 11.9% to 14.4% and the overall isolation rate by combining direct plating and enrichment plating was 15.8% (Table 2).
Successful isolation is dependent upon the quality of collection, storage and processing of the clinical specimens. For decades, Cary-Blair (with 0.5% agar) cottontipped applicators have been routinely used for transport/ storage and collection of human stool samples collected at AFRIMS field-sites. These preserved stools were processed for culture both at the field-site laboratory and at AFRIMS’s laboratory. Commonly, it is observed that fastidious enteric bacterial pathogens (Shigella and Campylobacter species) were routinely isolated at field-site laboratories. However, after extended storage of 1 week there was a decrease in colony forming units (CFU). One possible factor are the fatty acids present in cotton which, are known growth inhibitors of Shigella [14] and may also inhibit Campylobacter species. Implementation of MCB and polyester-tipped swabs resulted in comparable isolation rates of these pathogens in our laboratory to those of field-site laboratories. However, culture dependent variables, still need to be ascertained.
4. Conclusion
Presently this study demonstrates that modified CaryBlair medium with polyester-tipped swabs is a suitable transport system for increasing survival rates of Campylobacter in human stool samples when stored and transported at 2˚C - 8˚C. Preston enrichment broth was not necessary for the enrichment of Campylobacter spp. from human diarrheal stool samples, if the specimens were collected and processed immediately or within 3 days. However, Preston enrichment broth improved the recoverability of Campylobacter species if the specimens were delayed for processing up to 7 days, particularly with respect to C. jejuni which was consistently detected in this study.
5. Acknowledgements
We are grateful for the funding received from the Military Infectious Diseases Research Program (MIDRP), Silver Spring, MD, USA. We thank Siriporn Sornsakrin, Umaporn Suksawad and Ovath Thonglee for specimen collection; Wilawan Oransathid, Paksathorn Puripanyakom for specimen processing and identification of Campylobacter species; Dr. Trent Peacock and Pavinee Yongpatinat for their assistance on manuscript preparation.
REFERENCES
- M. Zilbauer, N. Dorrell, B. W. Wren and M. Bajaj-Elliott, “Campylobacter jejuni-Mediated Disease Pathogenesis: An Update,” Transactions of the Royal Society of Tropical Medicine and Hygiene, Vol. 102, No. 2, 2008, pp. 123-129. doi:10.1016/j.trstmh.2007.09.019
- J. W. Sanders, D. W. Isenbarger, S. E. Walz, L. W. Pang, D. A. Scott, C. Tamminga, et al., “An Observational Clinic-Based Study of Diarrheal Illness in Deployed United States Military Personnel in Thailand: Presentation and Outcome of Campylobacter Infection,” The American Journal of Tropical Medicine and Hygiene, Vol. 67, No. 5, 2002, pp. 533-538.
- D. W. Isenbarger, C. W. Hoge, A. Srijan, C. Pitarangsi, N. Vithayasai, L. Bodhidatta, et al., “Comparative Antibiotic Resistance of Diarrheal Pathogens from Vietnam and Thailand, 1996-1999,” Emerging Infectious Diseases, Vol. 8, No. 2, 2002, pp. 175-180. doi:10.3201/eid0802.010145
- C. W. Hoge, J. M. Gambel, A. Srijan, C. Pitrangsi and P. Echeverria, “Trends in Antibiotic Resistance among Diarrheal Pathogens Isolated in Thailand over 15 Years,” Clinical Infectious Diseases, Vol. 26, No. 2, 1998, pp. 341-345. doi:10.1086/516303
- W. L. Wang, L. B. Reller, B. Smallwood, N. W. Luechtefeld and M. J. Blaser, “Evaluation of Transport Media for Campylobacter jejuni in Human Fecal Specimens,” Journal of Clinical Microbiology, Vol. 18, No. 4, 1983, pp. 803-807.
- N. W. Luechtefeld, W. L. Wang, M. J. Blaser and L. B. Reller, “Evaluation of Transport and Storage Techniques for Isolation of Campylobacter fetus subsp. jejuni from Turkey Cecal Specimens,” Journal of Clinical Microbiology, Vol. 13, No. 3, 1981, pp. 438-443.
- T. W. Steele and S. N. McDermott, “The Use of Membrane Filters Applied Directly to the Surface of Agar Plates for the Isolation of Campylobacter jejuni from Feces,” Pathology, Vol. 16, No. 3, 1984, pp. 263-265. doi:10.3109/00313028409068535
- S. L. W. On, B. Holmes and M. Sackin, “A Probability Matrix for the Identification of Campylobacters, Helicobacters, and Allied Taxa,” Journal of Applied Bacteriology, Vol. 81, 1996, pp. 425-432.
- E. I. Tanner and C. H. Bullin, “Campylobacter Enteritis,” British Medical Journal, Vol. 2, No. 6086, 1977, p. 579. doi:10.1136/bmj.2.6086.579-a
- M. J. Blaser, H. L. Hardesty, B. Powers and W. L. Wang, “Survival of Campylobacter fetus subsp. jejuni in Biological Milieus,” Journal of Clinical Microbiology, Vol. 11, No. 4, 1980, pp. 309-313.
- W. T. Martin, C. M. Patton, G. K. Morris, M. E. Potter and N. D. Puhr, “Selective Enrichment Broth Medium for Isolation of Campylobacter jejuni,” Journal of Clinical Microbiology, Vol. 17, No. 5, 1983, pp. 853-855.
- E. Sjogren, G. B. Lindblom and B. Kaijser, “Comparison of Different Procedures, Transport Media, and Enrichment Media for Isolation of Campylobacter Species from Healthy Laying Hens and Humans with Diarrhea,” Journal of Clinical Microbiology, Vol. 25, No. 10, 1987, pp. 1966-1968.
- F. J. Bolton and L. Robertson, “A Selective Medium for Isolating Campylobacter jejuni/coli,” Journal of Clinical Pathology, Vol. 35, No. 4, 1982, pp. 462-467. doi:10.1136/jcp.35.4.462
- M. Pezzlo, “Processing and Interpretation of Bacterial Fecal Cultures,” In: H. D. Isenberg, Ed., Essential Procedures for Clinical Microbiology, ASM Press, Washington DC, 1998, pp. 90-94.

