Journal of Sustainable Bioenergy Systems
Vol.3 No.1(2013), Article ID:29087,7 pages DOI:10.4236/jsbs.2013.31007
Utilization of Soluble Starch by Oleaginous Red Yeast Rhodotorula glutinis
1Department of Crop Science, University of Hohenheim, Stuttgart, Germany
2Dave C. Swalm School of Chemical Engineering, Mississippi State University, Starkville, USA
Email: *teresa.schneider@uni-hohenheim.de
Received January 26, 2013; revised February 28, 2013; accepted March 11, 2013
Keywords: Rhodotorula glutinis; Microbial Lipid Production; Starch
ABSTRACT
Starch containing wastewaters from the food and feed industry have been identified as potential cheap carbon sources for the production of microbial lipids. Due to its high potential lipid content the oleaginous yeast Rhodotorula glutinis is often used for fermentations in this field. Moreover it is investigated in the context of microbial carotenoid production, which also requires a cheap source of carbon. Thus, the ability of R. glutinis (ATCC 15125TM) to degrade and utilize soluble starch for the production of lipids has been assessed in this study. While glucose and fructose were readily consumed from the medium, starch was only slightly reduced in one treatment. The yield of fatty acid methyl esters (FAME) was graduated corresponding to the initial sugar contents, with the highest FAME yield (1.5 g∙L−1) at the highest initial sugar content. In the treatment that contained starch as single carbon source, no FAME production was realized. Accordingly, if starchy wastewaters should be used for microbial cultivation with R. glutinis, an enzymatic or chemical pretreatment for starch hydrolysis should be applied, to increase the availability of this carbon source.
1. Introduction
Worldwide, renewable biofuels such as bioethanol and biodiesel contribute a significant share to the overall fuel consumption [1]. Against the background of rising crude oil prices and environmental concerns related with the exploitation of fossil oil resources, politics set strong incentives to further increase the production of biofuels—in Europe mainly biodiesel. So-called 1st generation biodiesel is produced by the transesterification of plant oils like rapeseed and soybean oil with an alcohol— mostly methanol—into fatty acid alkyl (methyl) esters (FAMEs) [2], whereas the terms “FAME” and “biodiesel” are often used synonymously. However, the 1st generation biodiesel derived from agricultural oil crops has been facing increasing criticism in terms of its impact on food prices, climate and ecosystems, so that the question was raised if 1st generation biofuels should actually be further promoted as sustainable energy alternative in the future. Accordingly, research and industry strived to find alternative oil sources for the transesterification into biodiesel. In this context the utilization of oils produced by oleaginous microorganisms has been identified and investigated as one possible approach. These organisms are able to accumulate significant amounts of lipids inside their cells, some as high as 80% of their respective cell dry weight, with similar characteristics as plant oil, when cultivated under nutrient limited and carbon excess conditions [3,4]. In terms of microbial lipid production, the red yeast Rhodotorula glutinis has been studied due to its high potential oil content of up to 72% [4]. It has also been investigated for its ability to produce high value carotenoids, namely β-carotene, torulene and torularhodin, which can be utilized as natural colorants or as ingredients of pharmaceutical products due to their antioxidant and pro-vitamin A properties. For both production pathways the cost of the carbon source has been identified as one of the main cost factors. Thus, the utilization of a cheap carbon source could significantly enhance the economic features of both approaches. Hence, a broad range of potential substrates have been tested for 1) lipid production, ranging from industrial by-products like glycerol [5] and molasses [6] over hydrolyzates of agricultural residues [7] to original waste products like municipal wastewater [8] and 2) carotenoid production, including flour extracts, grape must [9], radish brine [10] and whey [11]. Also wastewaters from the food industry with supposedly high organic contents have gained attention [12,13]. A lot of these substrates contain starch as a principal carbon source. These starch containing wastewaters bear a large potential as cheap carbon sources for the microbial lipid production, if the respective microorganisms are able to utilize starch as rather complex polysaccharide in comparison to easily available monosaccharides. Some molds have shown good results when cultivated on starch as main carbon source [14,15], whereas it has to our knowledge not yet clearly been stated if R. glutinis is actually capable of degrading and utilizing starch as carbon source. Rubio et al. [16] found that strains of R. glutinis did not grow on starch and also its poor performance on starchy wastewaters from potato processing [17] suggest, that starch should be hydrolyzed prior to cultivation [18]. In contrast to these findings, Bhosale and Gadre [19] observed cell growth and carotenoid production by R. glutinis on starch as carbon source. Elsewhere [20,21] it is stated that starch assimilation of R. glutinis is strain specific. Thus, for the present study R. glutinis (ATCC 15125TM) was cultivated on starch and glucose as carbon sources and the production of biomass and microbial lipids was determined in course of the cultivation to evaluate the suitability of starch as carbon source for this specific yeast strain.
2. Materials and Methods
Three treatments were designed which differed in the type and composition of the carbon source used. Apart from this, all treatments shared the same basal media, which contained (per liter) 0.5 g yeast extract; 1.0 g Na2HPO4 × 12 H2O; 1.0 g KH2PO4; 0.4 g MgSO4 × 7 H2O; 1.0 g (NH4)2SO4; 6 ml FeSO4 solution (4 g∙L−1 FeSO4 × 7 H2O), and 10 ml trace mineral solution. The trace mineral solution consisted of (per liter) 3.6 g Ca2Cl2 × 2 H2O; 0.75 g ZnSO4 × 7 H2O; 0.13 g CuSO4 × 5 H2O; 0.5 g MnSO4 × H2O; 0.13 g CoCl2 × 6 H2O, and 0.17 g Na2MoO4 × 2 H2O. Table 1 shows the type and amount of carbon source used for the respective treatment. “G100” was set as a control treatment and contained only glucose. In the other treatments fructose was used as easily available monosaccharide in order to be able to distinguish between the initially added carbon source and the glucose resulting out of starch degradation. Generally, the suitability of glucose and fructose as carbon source for R. glutinis was assumed to be equal.
Table 1. Type and composition of carbon sources for the experimental treatments.
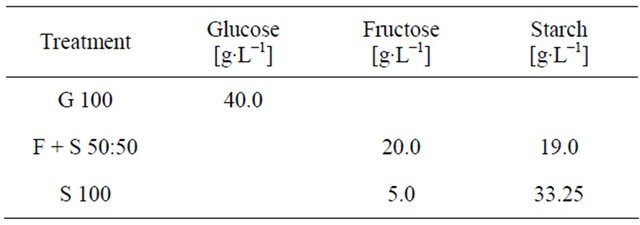
In “S 100” 5 g∙L−1 of fructose were added in order to facilitate the initial adaption of the yeast to the utilization of starch as carbon source. All media were autoclaved and afterwards inoculated with 20 mL of R. glutinis seed culture, which has been cultivated on yeast malt broth (3 g∙L−1 yeast extract, 3 g∙L−1 malt extract, 10 g∙L−1 glucose, 5 g∙L−1 vegetable peptone) for 48 h at 25˚C on a rotary shaker at 115 rpm. Rhodotorula glutinis ((Fresenius) Harrison, anamorph) was obtained from the American Type Culture Collection (ATCC 15125TM). Fermentation was carried out in 1000 mL Erlenmeyer flasks, containing 700 mL of the respective medium, on a rotary shaker at 115 rpm and 25˚C for 240 h. The experiment was carried out in triplicate. 50 mL samples were taken after 0, 48, 120, 168, 216, and 240 h of cultivation and centrifuged at 3000 rpm for 10 min. The supernatant was used for the determination of pH, 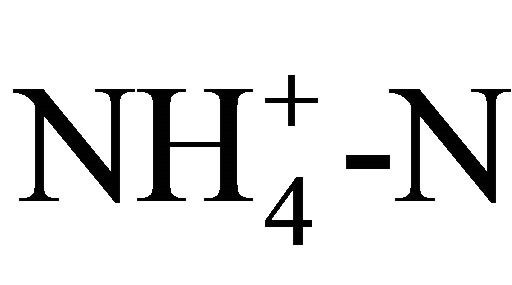 and sugar content.
and sugar content. 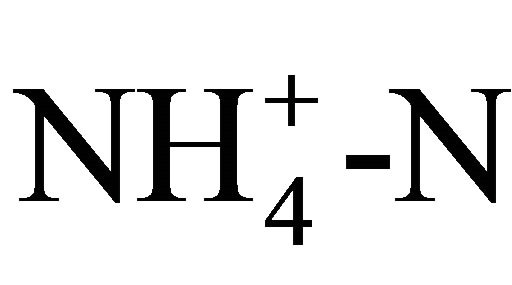 was measured photometrically using reagent vials from Macherey-Nagel (Düren, Germany). 200 µL of the sample is added to the test tube. Ammonium reacts with hypochlorite and salicylate in the presence of sodium nitroprussiate as catalyst to form a blue indophenol. The color strength is then measured with a photometer (NANOCOLOR 400 D, Macherey-Nagel, Düren, Germany) at a wavelength of 690 nm. The sugar content was analyzed via HPLC (Merck-Hitachi HPLC system with DAD detector) using a Phenomenex RezexTM RPMMonosaccharide Pb + 2 (8%) column (300 × 7.8 mm) with water as mobile phase at a flow rate of 0.6 ml∙min−1. The sugar content was determined by comparison to known standards (Figure 1). The remaining cell pellet was freezedried and weighted to determine the cell dry mass. It was observed that the pellet also contained a fraction of undissolved starch.
was measured photometrically using reagent vials from Macherey-Nagel (Düren, Germany). 200 µL of the sample is added to the test tube. Ammonium reacts with hypochlorite and salicylate in the presence of sodium nitroprussiate as catalyst to form a blue indophenol. The color strength is then measured with a photometer (NANOCOLOR 400 D, Macherey-Nagel, Düren, Germany) at a wavelength of 690 nm. The sugar content was analyzed via HPLC (Merck-Hitachi HPLC system with DAD detector) using a Phenomenex RezexTM RPMMonosaccharide Pb + 2 (8%) column (300 × 7.8 mm) with water as mobile phase at a flow rate of 0.6 ml∙min−1. The sugar content was determined by comparison to known standards (Figure 1). The remaining cell pellet was freezedried and weighted to determine the cell dry mass. It was observed that the pellet also contained a fraction of undissolved starch.
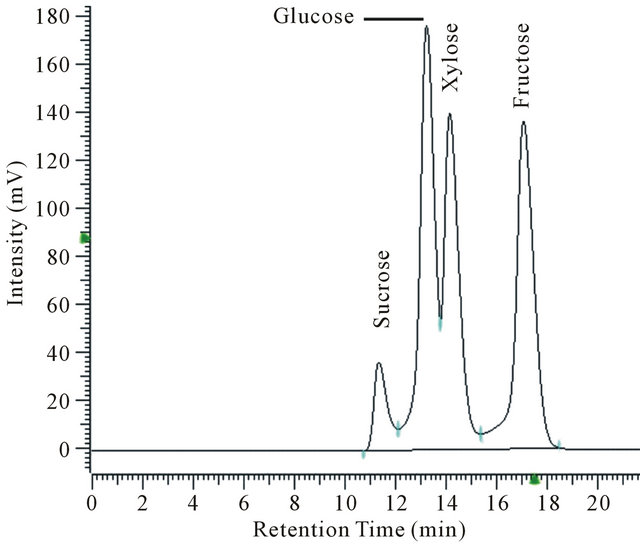
Figure 1. HPLC Chromatogram of standard for sugar analysis containing 10 g∙L−1 sucrose, glucose, xylose, and fructose each.
Thus, the actual biomass production at the respective sampling times was calculated by subtracting the initially measured weight after 0 h, assuming that the fraction of undissolved starch will remain constant over the cultivation time. The lipid content of the dried cell pellet was determined using a modification [22] of the classical Bligh and Dyer [23] extraction with methanol and chloroform as solvents. The final lipid extract was weighted after the solvents were evaporated under nitrogen blow at 50˚C. This extract from the Bligh and Dyer procedure is later referred to as total extractable lipids. Afterward the extracts from sampling times 48 h and 240 h were transesterified with methanol with 2% H2SO4 as catalyst in a waterbath at 60˚C for 2 h. The reaction was quenched with NaHCO3 solution and the resulting FAMEs were extracted with toluene and analyzed using an Agilent 6890 gas chromatograph with flame ionization detector (GC-FID) equipped with an Agilent J&W GC capillary column (30 m × 0.252 mm ID and 0.25 µm film thickness). The different fatty acids were identified by comparison to responses from a known FAME standard (Supelco® 37 component FAME Mix, Sigma Aldrich, München, Germany) (Figure 2). For quantitative determination of FAME production, the peak areas of fatty acids in the samples were compared with the peak areas of fatty acids in a known amount of FAME standard.
For starch analysis samples of the cultivation media were heated in a waterbath to completely dissolve all starch, then diluted with distilled water, mixed with amyloglucosidase (AMG) solution and incubated at 60˚C for 1.5 h in order to degrade starch into glucose. The amyloglucosidase solution was prepared daily by (per 12 samples) dissolving 10.6 mg of AMG (Amyloglucosidase from Aspergillus niger, ~70 U∙mg−1, Sigma Aldrich, München, Germany) in 12 ml buffer solution (pH 4.6). The samples were then filtered and analyzed for their glucose content using HPLC according to the method previously described for the sugar analysis. From the measured glucose contents, the starch content was calculated based on a 90% conversion rate from starch to glucose.
All data was statistically analyzed using Sigma Stat
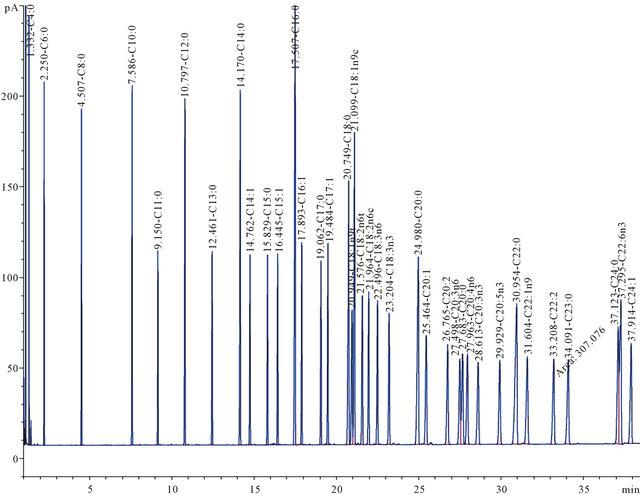
Figure 2. GC chromatogram of FAME standard solution.
3.5 (Jandel Scientific, USA). The statistical significance of differences in mean values was calculated using single factor variance analysis with the Tukey-Test at the 5% level of probability.
3. Results and Discussion
3.1. Sugar and Starch Consumption
As Figure 3 shows, the monosaccharides glucose and fructose were steadily consumed in course of cultivation. Thus, based on the different initial sugar contents, the easily available carbon sources were exhausted after 48 h in S 100 and after 168 h in F + S 50:50. In treatment G 100 glucose was available throughout the cultivation with 75 % of total glucose consumed after 240 h.
The initially measured starch content of 18.1 g∙L−1 in F + S 50:50 did not vary significantly over the duration of the fermentation. In treatment S 100 the starch content decreased significantly from 33.2 g∙L−1 at 120 h to 27.5 g∙L−1 at 168 h. This indicates that some type of mechanism for starch degradation must be available in R. glutinis. Verstraete et al. [24] reported the presence of an amylolytic Rhodotorula strain in activated sludge. However, at the same time it is rather incomprehensible that after this point no further decrease in starch was detected. If different carbon sources are offered for microbial growth, the easily available sugars are preferentially utilized. If this source is exhausted, cells switch to the second source, whereas at that time often a certain lag period can be observed, since it needs some time until the
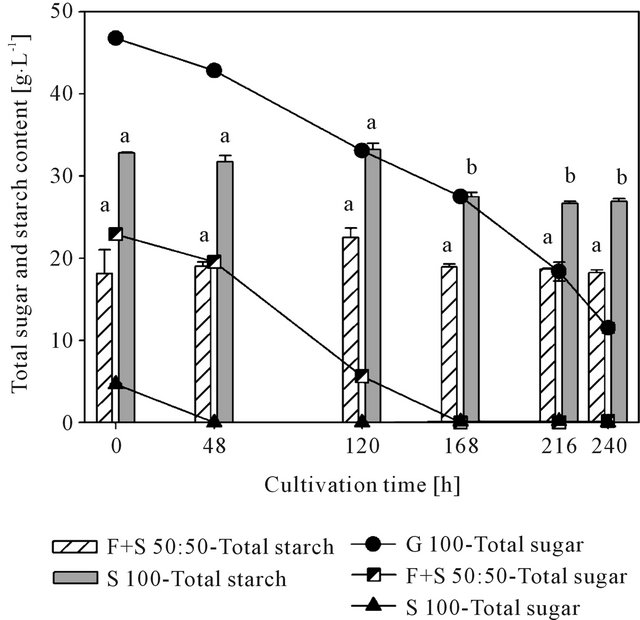
Figure 3. Utilization of monosaccarides and starch by Rhodotorula glutinis cultivated on glucose (G 100), a fructose/starch mixture (F + S 50:50), and starch (S 100). Different lower case letters within a treatment indicate significant differences at 5% level of probability.
required enzymes for the degradation of the respective carbon source are produced. In the present study this lag phase was quite long and could be observed in S 100 between 48 h (fructose exhaustion) and 168 h (starch utilization). In F + S 50:50 fructose was available until 168 h. Thus, it can be assumed that in this treatment after a lag phase a certain degree of starch utilization might occur. To verify this assumption, the cultivation time should be increased in further studies.
3.2. Biomass Production
The results of sugar and starch consumption were in line with the data obtained from biomass and lipid production (Figure 4). Until 168 h the treatments G 100 and F + S 50:50 showed almost an identical increase in cell mass production along with a simultaneous decrease of the ammonium content, with ammonium as nitrogen source being completely exhausted after 120 h. The further increase in biomass was probably facilitated by the utilization of the yeast extract, which was initially added to the medium as secondary nitrogen source. In G 100 the cell mass continued to increase until 216 h, reaching a biomass yield of 12.3 g∙L−1. Since glucose was still available at this point it can be assumed that nitrogen was the limiting factor for further biomass production. In F + S 50:50 biomass production ceased after 168 h at 9.1 g∙L−1. This can be explained by the limitation of carbon, since the entire fructose was consumed from the medium and
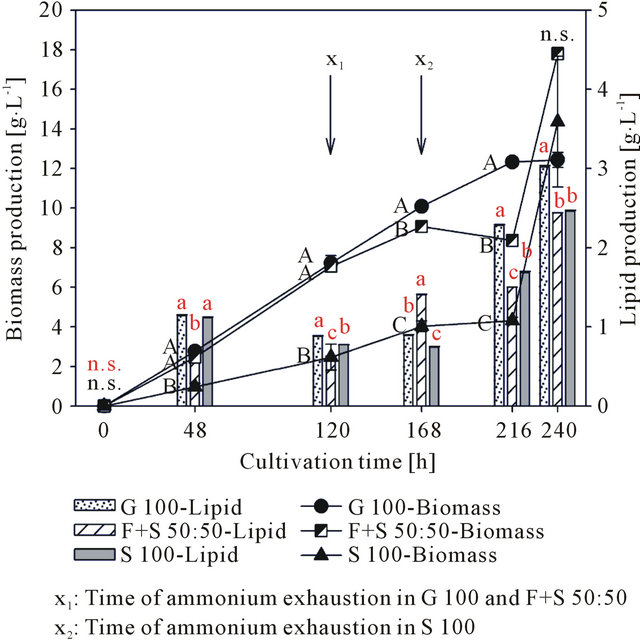
Figure 4. Biomass and lipid production of Rhodotorula glutinis cultivated with glucose (G 100), a fructose/starch mixture (F + S 50:50), and starch (S 100) as carbon source. Different lower case and capital letters indicate significant differences between the treatments for lipid and biomass production, respectively, at 5% level of probability.
starch as secondary carbon source was not utilized. In treatment S 100, fructose was exhausted after 48 h of cultivation, while no starch utilization could be observed until 120 h. Despite this limited availability of carbon the cell mass increased more or less steadily until 168 h.
However, compared to the other treatments biomass production was much lower, yielding less than half (4.0 g∙L−1) of the cell mass compared to G 100 and F + S 50:50 after 168 h. Due to the fact, that nitrogen was utilized for the formation of biomass, ammonium was consumed from the media more slowly in S 100, being exhausted after 168 h of cultivation. The increase of biomass production after 216 h in F + S 50:50 and S 100 cannot be coherently explained. However, it is noticeable that the increase was almost identical for both treatments containing starch and it was also confirmed by photometrical cell density determination. Thus, it is unlikely that these increases are a matter of coincidence or flawed measurements. A production of actual cell mass is unlikely since nitrogen was exhausted at this point. Since the cell pellet after freeze drying was contaminated with residues of undissolved starch, the biomass production was calculated by subtracting the initially measured pellet weight at 0 h. For this, a stable proportion if residual starch was assumed. Thus, a decrease in the fraction of undissolved starch could appear as an increase in biomass production. However, this assumption is not supported by the fact, that no decrease in the starch content was detected.
3.3. Lipid and FAME Production
Regarding lipid production, it needs to be differentiated between the gravimetrically determined extractable lipid fraction as obtained by the Bligh and Dyer extraction (Figure 4) and the amount of saponifiable lipids represented by the amount of FAMEs as detected using GC analysis (Figure 5), because next to this saponifiable fraction of lipids the Bligh and Dyer extracts contain many other compounds that will dissolve in chloroform, e.g. certain carotenoids.
Still the gravimetrical results for lipid production are valuable indicators regarding the cycle of lipid production during microbial cultivation. Furthermore it needs to be considered, that not all extracted lipids are necessarily derived from the process of De novo lipid synthesis, which is defined as the production of storage lipids from sugar as carbon source. Since lipids are also found in cell membranes, an increasing lipid yield can be to some extend associated to a rise in biomass production. The gravimetrical lipid production (Figure 4) can roughly be divided into three phases, which are similar for all treatments: 1) an increase in lipid production during the first 48 h of growth, 2) a stagnation of lipid production in the
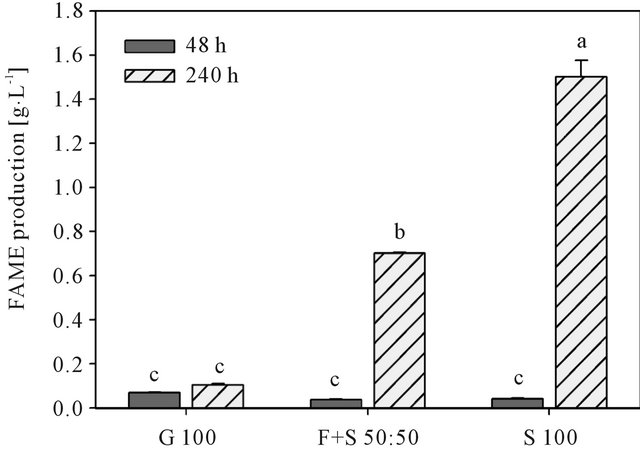
Figure 5. FAME production by Rhodotorula glutinis cultivated on glucose (G 100), starch (S 100) and a fructose/ starch mixture (F + S 50:50) after 48 and 240 h of cultivation. Different lower case letters indicate significant differences at 5% level of probability.
middle of cultivation until 168 h, and 3) an increase in lipid production at the end of cultivation after 168 h (G 100 and S 100) and 216 h (F + S 50:50), respectively. The initial increase is probably related to the simultaneous onset of biomass production as described previously and thus cell growth induced. The second phase of lipid production 3) just starts after ammonium is exhausted from the medium (Figure 4). This is characteristically for De novo lipid synthesis, since carbon is only channelled into the production of storage lipids, when a limitation of a growth required nutrient (mostly nitrogen) restricts cell growth processes [4]. With 3.0 g∙L−1 after 240 h, G 100 showed the highest lipid production. Other reports where shake flask cultivations of R. glutinis were applied observed similar yields [25-27]. However, treatments F + S 50:50 (2.4 g∙L−1) and S 100 (2.5 g∙L−1) had only slightly lower values for lipid production. This is rather surprising, since at that time in both treatments fructose was exhausted and starch was not or only slightly degraded, thus a carbon limitation could be assumed, which would usually hamper the production of biomass and lipids. The effect of the differences in the initial amounts of easily available sugars in the different treatments becomes obvious with the chromatographically determined FAME production (Figure 5). Since only glucose and fructose have been consumed as carbon sources, the FAME production follows the pattern of initial sugar content, whereas G 100 with the highest sugar content yields the highest amount of FAMEs, followed by F + S 50:50 with around half the initial sugar content and FAME production. S 100 had the lowest sugar content and only small amounts of starch were utilized. Thus, carbon was limited and no significant FAME production could be observed.
The fatty acid composition (Table 2) showed a pre-
Table 2. Fatty acid composition [%] of microbial lipids from Rhodotorula glutinis after 240 h of cultivation on different carbon sources.
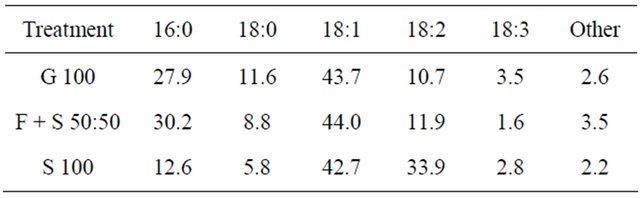
dominance of palmitic (C 16:0) and oleic (C 18:1) acid, which is characteristic for R. glutinis [26,28]. Generally in all treatments the long-chain C 16 and C 18 fatty acid account for the vast majority with over 97% of total fatty acids. Thus, the microbial oils can be transesterified into biodiesel just like conventional plant oil. Moreover, the high share of oleic acid has beneficial effects on biodiesel fuel quality [29]. The comparably high content of linoleic acid (C 18:2) in S 100 and the fact that linoleic acid is often found in the lipids of cell membranes indicate that in this treatment biomass associated lipids derived from cell membranes prevailed.
The obtained results generally suggest that the mechanisms of starch utilization are poorly developed in R. glutinis. However, since in treatment S 100 a significant reduction of starch was measured, it cannot be ruled out, that this strain of R. glutinis over time develops the required enzymatic systems for starch utilization. For further experiments the cultivation time should be increased to observe if starch is further utilized. However, in the context of starch containing wastewaters as potential carbon source for microbial lipid and biodiesel production, it can be stated that a suitable enzymatic or thermochemical pretreatment for starch hydrolysis would increase the amount of available carbon and thus potentially increase the lipid yields. Other possible measures for increased starch utilization could be the co-cultivation with amylolytic yeast strains (e.g. [18]) or the use of specific strains of R. glutinis, which are adapted to the utilization of starch.
4. Conclusion
Wastewaters from food industry with high starch contents are a promising cheap carbon source as feedstock for microbial lipid and biodiesel production and also of potential interest for the microbial production of carotenoids. When red yeast R. glutinis was cultivated on soluble starch as carbon source, it yet showed a poor ability of starch degradation and utilization. Thus, before these wastewaters are used as fermentation medium it is advisable to conduct an additional pretreatment step for starch hydrolysis. However, since some literature sources report starch utilization by R. glutinis, the present results might be strain specific.
REFERENCES
- International Energy Agency, “Renewable Energy Outlook,” World Energy Outlook 2012, IEA Publications, Paris, 2012, p. 211.
- G. Knothe, “Introduction: What Is Biodiesel?” In: G. Knothe, J. van Gerpen and J. Krahl, Eds., The Biodiesel Handbook, AOCS Press, Urbana, 2005, pp. 9-11. doi:10.1201/9781439822357.ch1
- C. Ratledge, “Single Cell Oils—Have They a Biotechnological Future?” Trends in Biotechnology, Vol. 11, No. 7, 1993, pp. 278-284. doi:10.1016/0167-7799(93)90015-2
- C. Ratledge and Z. Cohen, “Microbial and Algal Oils: Do They Have a Future for Biodiesel or as Commodity Oils?” Lipid Technology, Vol. 20, No. 7, 2008, pp. 155-160. doi:10.1002/lite.200800044
- E. R. Easterling, W. T. French, R. Hernandez and M. Licha., “The Effect of Glycerol as Sole and Secondary Substrate on Growth and Fatty Acid Composition of Rhodotorula glutinis,” Bioresource Technology, Vol. 100, No. 1, 2009, pp. 356-361. doi:10.1016/j.biortech.2008.05.030
- R. M. Alvarez, B. Rodríguez, J. M. Romano, A. O. Diaz, D. Miró, et al., “Lipid Accumulation in Rhodotorula glutinis on Sugar Cane Molasses in Single-Stage Continuous Culture,” World Journal of Microbiology and Biotechnology, Vol. 8, No. 2, 1992, pp. 214-215. doi:10.1007/BF01195853
- X. Yu, Y. Zheng, K. Dorgan and S. Chen, “Oil Production by Oleaginous Yeasts Using the Hydrolysate from Pretreatment of Wheat Straw with Dilute Sulfuric Acid,” Bioresource Technology, Vol. 102, No. 10, 2011, pp. 6134- 6140. doi:10.1016/j.biortech.2011.02.081
- Z. Chi, Y. Zhang, A. Jiang and Z. Chen, “Lipid Production by Culturing Oleaginous Yeast and Algae with Food Waste and Municipal Wastewater in an Integrated Process,” Applied Biochemistry and Biotechnology, Vol. 165, No. 2, 2011, pp. 442-453. doi:10.1007/s12010-011-9263-6
- P. Buzzini and A. Martini, “Production of Carotenoids by Strains of Rhodotorula glutinis Cultured in Raw Materials of Agro-Industrial Origin,” Bioresource Technology, Vol. 71, No. 1, 2000, pp. 41-44. doi:10.1016/S0960-8524(99)00056-5
- C. Malisorn and W. Suntornsuk, “Optimization of β-Carotene Production by Rhodotorula glutinis DM28 in Fermented Radish Brine,” Bioresource Technology, Vol. 99, No. 7, 2008, pp. 2281-2287. doi:10.1016/j.biortech.2007.05.019
- G. Frengova, E. Simova, K. Pavlova, B. Beshkova and D. Grigorova, “Formation of Carotenoids by Rhodotorula glutinis in Whey Ultrafiltrate,” Biotechnology and Bioengineering, Vol. 44, No. 8, 1994, pp. 888-894. doi:10.1002/bit.260440804
- T. Schneider, S. Graeff-Hönninger, W. T. French, R. Hernandez, W. Claupein and N. Merkt, “Microbial Lipids for Biodiesel Production and Carotenoids as Value Added By-Products—Screening of Industrial Wastewaters as Suitable Feedstock for Oleaginous Red Yeast Rhodotorula glutinis,” Proceedings of the 20th European Biomass Conference and Exhibition, Milan, 18-22 June 2012, pp. 1541-1546.
- R. Wild, S. Patil, M. Popovic, M. Zappi, S. Dufreche and R. Bajpaj, “Lipids from Lipomyces starkeyi,” Food Technology and Biotechnology, Vol. 48, No. 3, 2010, pp. 329- 335.
- S. Papanikolaou, M. Galiotou-Panayotou, S. Fakas, M. Komaitis and G. Aggelis, “Lipid Production by Oleaginous Mucorales Cultivated on Renewable Carbon Sources,” European Journal of Lipid Science and Technology, Vol. 109, No. 11, 2007, pp. 1060-1070. doi:10.1002/ejlt.200700169
- S. D. Dyal and S. S. Narine, “Implications for the Use of Mortierella Fungi in the Industrial Production of Essential Fatty Acids,” Food Research International, Vol. 38, No. 4, 2005, pp. 445-467. doi:10.1016/j.foodres.2004.11.002
- M. C. Rubio, R. Runco and A. R. Navarro, “Invertase from a Strain of Rhodotorula glutinis,” Phytochemistry, Vol. 61, No. 6, 2001, pp. 605-609. doi:10.1016/S0031-9422(02)00336-9
- T. Schneider, S. Graeff-Hönninger, W. T. French, R. Hernandez, W. Claupein, W. E. Holmes and N. Merkt, “Screening of Industrial Wastewaters as Feedstock for the Microbial Production of Oils for Biodiesel Production and HighQuality Pigments,” Journal of Combustion, Vol. 2012, 2012, Article ID: 153410. doi:10.1155/2012/153410
- P. Buzzini, “Batch and Fed-Batch Carotenoid Production by Rhodotorula glutinis—Debaryomyces castellii CoCultures in Corn Syrup,” Journal of Applied Microbiology, Vol. 90, No. 5, 2001, pp. 843-847. doi:10.1046/j.1365-2672.2001.01319.x
- P. B. Bhosale and R. V. Gadre, “Production of β-Carotene by a Mutant of Rhodotorula glutinis,” Applied Microbiology and Biotechnology, Vol. 55, No. 4, 2001, pp. 423- 427. doi:10.1007/s002530000570
- CBS-KNAW Fungal Biodiversity Centre Database, 2011. http://www.cbs.knaw.nl/collections/BioloMICS.aspx?Table=Yeasts%20species&Name=Rhodotorula%20glutinis%20var.%20glutinis&Fields=All&ExactMatch=T
- Y. Yeeh, “Rhodotorula,” In: R. Robinson, Ed., Encyclopedia of Food Microbiology, 1999, pp. 1900-1905. doi:10.1006/rwfm.1999.1340
- G. Zhang, W. T. French, R. Hernandez, E. Alley and M. Paraschivescu, “Effects of Furfural and Acetic Acid on Growth and Lipid Production from Glucose and Xylose by Rhodotorula glutinis,” Biomass and Bioenergy, Vol. 35, No. 1, 2011, pp. 734-740. doi:10.1016/j.biombioe.2010.10.009
- E. G. Bligh and W. J. Dyer, “A Rapid Method of Total Lipid Extraction and Purification,” Canadian Journal of Biochemistry and Physiology, Vol. 37, No. 8, 1959, pp. 911-917. doi:10.1139/o59-099
- W. Verstraete, J. P. Voets and J. Mottar, “Competitive Ability of Amylolytic Bacteria in Activated Sludge,” Archives of Microbiology, Vol. 104, No. 1, 1975, pp. 279- 283. doi:10.1007/BF00447337
- B. Cheirslip, W. Suwannarat and R. Niyomdecha, “Mixed Culture of Oleaginous Yeast Rhodotorula glutinis and Microalgae Chlorella vulgaris for Lipid Production from Industrail Wastes and Its Use as Biodiesel Feedstock,” New Biotechnology, Vol. 28, No. 4, 2011, pp. 362-368. doi:10.1016/j.nbt.2011.01.004
- C. Saenge, B. Cheirslip, T. T. Suksaroge and T. Bourtoom, “Efficient Concomitant Production of Lipids and Carotenoids by Oleaginous Red Yeast Rhodotorula glutinis Cultured in Palm Oil Mill Effluent and Application of Lipids for Biodiesel Production,” Biotechnology and Bioprocess Engineering, Vol. 16, No. 1, 2011, pp. 23-33. doi:10.1007/s12257-010-0083-2
- C. Dai, J. Tao, F. Xie, Y.-J. Dai and M. Zhao, “Biodiesel Generation from Oleaginous Yeast Rhodotorula glutinis with Xylose Assimilating Capacity,” African Journal of Biotechnology, Vol. 6, No. 18, 2007, pp. 2130-2134.
- V. Perrier, E. Dubreucq and P. Galzy, “Fatty Acid and Carotenoid Composition of Rhodotorula strains,” Archives of Microbiology, Vol. 164, No. 3, 1995, pp. 173-179. doi:10.1007/BF02529968
- G. Knothe, “Dependence of Biodiesel Fuel Properties on the Structure of Fatty Acid Alkyl Esters,” Fuel Processing Technology, Vol. 86, No. 10, 2005, pp. 1059-1070. doi:10.1016/j.fuproc.2004.11.002
NOTES
*Corresponding author.

