American Journal of Analytical Chemistry
Vol.3 No.12A(2012), Article ID:26147,11 pages DOI:10.4236/ajac.2012.312A120
Extraction of the Main Ingredients from Chuanxiong by CO2 Supercritical Fluid Extraction
1School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
2State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macau, China
3Department of Pharmaceutical Analysis and Metabolomics, Jiangsu Province Academy of Traditional Chinese Medicine, Nanjing, China
Email: *linge@cuhk.edu.hk
Received October 31, 2012; revised December 3, 2012; accepted December 14, 2012
Keywords: Chuanxiong Rhizoma; Supercritical Fluid Extraction; Selective Enrichment
ABSTRACT
As one of the most frequently used medicinal herbs in China, Chuanxiong Rhizoma (Chuanxiong) is notable for its beneficial effects in alleviation of cardioand cerebro-vascular disorders. Results from previous phytochemical, pharmacological and pharmacokinetic studies supported the contributions of 10 main components, namely ferulic acid (3), senkyunolide I (4), senkyunolide H (5), senkyunolide A (6), coniferylferulate (7), Z-ligustilide (8), sedanolide (9), 3-butylidenephthalide (10), riligustilide (11) and levistolide A (12), for therapeutic outcomes of the herb. To prepare a Chuanxiong extract, which is selectively enriched with these main components, the supercritical fluid extraction (SCFE) technique using CO2 was chosen in the present study due to its superiority in extraction of lipophilic components, especially thermo-labile components from natural products. Eight Chuanxiong samples were extracted under different SCFE conditions. Contents of 3-12 in SCFE extracts and remained in herbal residues were determined using HPLC-UV and compared with those in 95% ethanol extracts of the respective herbal samples. The results showed that contents of 3-12 were generally enriched by SCFE with higher recoveries achieved for lipophilic constituents, including the three most abundant constituents 6, 7 and 8. Moreover, extraction yield of the less lipophilic 3, 4 and 5 was improved by adding ethanol as entrainer. Higher flow rate of CO2 (10 - 13 L/h vs 9 - 12 L/h) enhanced the overall extraction, while lower temperature (32˚C vs 40˚C) reduced degradation of thermo-labile compounds, in particular 7, 8 and 10. Our established SCFE condition yielded high extraction recoveries for both total (75.6%) and three major chemical ingredients (6: 83.5%, 7: 77%, 8: 78.3%) from Chuanxiong with an adequate overall reproducibility, demonstrating a successful application of SCFE in selective enrichment of certain ingredients and efficient extraction of thermo-labile components from medicinal herbs using specifically designed SCFE conditions.
1. Introduction
Chuanxiong Rhizoma (Chuanxiong) originated from Ligusticum chuanxiong Hort. is one of the most frequently prescribed medicinal herbs in traditional medicinal practice in China. It has attracted extensive research interests due to its notable cardioand cerebro-protective effects.
Previously, our research laboratory has undertaken series studies to characterize the chemical profile, pharmacodynamic properties and pharmacokinetic profile of Chuanxiong and its main constituents with the aim of developing modern Chuanxiong product with known active ingredients and verified bioavailability. As found in our previous studies, ferulic acid (3), senkyunolide I (4), senkyunolide H (5), senkyunolide A (6), coniferyl ferulate (7), Z-ligustilide (8), sedanolide (9), 3-butylidenephthalide (10), riligustilide (11) and levistolide A (12) were the main components in Chuanxiong [1,2] and their contributions to pharmacological activities of Chuanxiong have been verified by our own and other research teams’ studies [3-14]. Moreover, our pharmacokinetic study of Chuanxiong extract also revealed these compounds, especially 6 and 8, as the main in vivo available ingredients of Chuanxiong [15,16]. In addition, it is very interesting to note that although presenting as the relatively minor phthalide in the herb, after ingestion of the herbal extract 3-butylidenephthalide (BuPh, 10) showed comparable systemic exposure to those of the abundant phthalides such as 6 and 8 due to a significant biotransformation from the co-existing 8 [17]. All these results suggested that multiple-components with certain proportions might be the need for the beneficial therapeutic effects of Chuanxiong.
As a result, an extract highly enriched with the aforementioned 10 main ingredients should be prepared for research and development of Chuanxiong-based product. Chuanxiong is usually prepared as decoctions with boiling water in traditional use or extracted using aqueous ethanol in pharmaceutical industry. As the classical method, steam distillation is also commonly used to extract essential oil, which contains the aforementioned 10 main ingredients including active phthalides, from Chuanxiong. The main constituents of Chuanxiong, including coniferyl ferulate, ligustilide and senkyunolide A, were found to be thermoand/or light-labile, and underwent extensive degradation due to exposure to light and/or high temperature during these preparation processes [18]. Thus, the method, that can obtain a high yield extraction of these components from Chuanxiong without thermal degradation, should be taken into consideration.
Over the past decades, supercritical fluid extraction (SFE) has been widely applied in many areas with the most common use in decaffeination of coffee and tea, extraction of hop components for beer flavouring and extraction of essential oils, oleoresins, flavors and aromas from spices and botanicals [19,20]. As an alternative to the traditional non-supercritical liquid extraction of plant materials, SFE has gained an increased attention with a growing concern of the quality and safety of herbal medicines and foods worldwide [21]. The princeple of this advanced extraction technology is using a gas or liquid which takes on many properties of both gas and liquid after being compressed and heated to a “supercritical” phase to penetrate and extract targeted molecules from source materials [22]. This “fluid” offers very attractive extraction characteristics, such as rapid mass transfer, faster completion of extraction, and stronger penetration into the material to be extracted than the conventional liquid solvent, owing to its favorable diffusivity, viscosity, surface tension, etc. Solvents commonly used in supercritical fluid include carbon dioxide (CO2), ethane, ethylene, propane, etc. Because of the special properties of CO2, such as inert, non-toxic, inexpensive, easily available and environmentally-friendly, carbon dioxide (CO2) is the most desirable SFE solvent for extraction of natural products for foods and medicines, and supercritical fluid extraction using CO2 (SCFE) is the today’s most popular technique for rapid and contamination-free extraction in herbal pharmaceutical industries [23]. Moreover, its near-ambient critical temperature (31.1˚C) and low critical pressure (7.38 MPa) and selectivity for lipophilic constituents make it ideally suitable for extraction of essential oils and thermo-labile constituents from natural products. To increase the solubility of polar compounds, small amounts of polar or non-polar co-solvents called entrainers or modifiers, like ethanol, ethyl acetate, and water, is usually added into the supercritical CO2 to selectively extract and enrich these polar compounds [24]. Ethanol is the modifier of choice in pharmaceutical and food industries because it is nontoxic and generally recognized as safe.
To date, there are only a few reports on the SCFE of Chuanxiong [25-30]. All these studies were operated at 40˚C or a higher temperature. Apparently, due to the instability of major components in Chuanxiong, this high temperature should not be suitable for the extraction of some of the thermo-labile components, in particular the original main component ligustilide and coniferyl ferulate. In most previous reports, the SCFE extracts of Chuanxiong were prepared under a fixed condition with aims to obtain the lipophilic components. Thus, entrainer was not added to improve extraction of less lipophilic constituents with exception of the studies from Yuan’s and Wu’s groups [28,29], which used ethanol and CHCl3 as the entrainer, respectively. The extraction yields of total essential oils were generally very low (<5%) [25,26], when compared with the extracts obtained by steamdistilled extraction. Moreover, the constituents in the SCFE extracts were analyzed using GC-MS technique by comparison with data reported in the literature owing to unavailability of authentic standards [25,26,28,29] or the extraction efficiency of the SCFE was evaluated with only one component, such as ligustilide [27,30] or the overall UV absorbance of all ingredients in the extracts [28]. Again, gas chromatography is not appropriate for the analysis of thermo-labile components in Chuanxiong. The UV absorption is also not suitable for monitoring extraction of desired components.
Therefore, the aim of the present study was to develop a suitable SCFE method for the extraction and enrichment of the main naturally occurring ingredients of Chuanxiong. The main components 3-12 in the extracts were simultaneously determined using HPLC-UV for the assessment of suitable SCFE conditions. The developed SCFE method provided an extract that is enriched in components 3-12 for future development of Chuanxiong-based proprietary products.
2. Materials and Methods
2.1. Reagents and Materials
HPLC grade methanol (E. Merck, Darmstadt, Germany) was used for the HPLC analysis. Ninety-five percent ethanol (AR) was used for the extraction of Chuanxiong samples. Hexane (AR), ethyl acetate (AR) and chloroform (AR) from BDH (VWR Int. Ltd., Dorset, UK) were utilized in isolation and purification of authentic compounds.
Eight dried Chuanxiong samples (CX-R 1-6, 9-10) were extracted by SCFE in the present study. Their sources and sample codes are listed in Table 1. All dried Chuanxiong samples were only subjected to sun drying without any further processing. The voucher specimens of these samples were deposited at the Department of Pharmacology, the Chinese University of Hong Kong, and the identities of all non-processed Chuanxiong samples were authenticated according to the monograph documented in China Pharmacopoeia [31].
Ferulic acid (3, 99% purity) from Acros Organics (Geel, Belgium), Z-ligustilide (8) of 1 mg/ml in acetonitrile from ChromaDex (Santa Ana, CA, USA), sedanolide (9, 97% purity) and a-naphthaflavone (utilized as internal standard, IS) from Sigma Chemical Company (St. Louis, Mo, USA), and 3-butylidenephthalide (10, 96% purity) from Aldrich Chemical Company (St. Louis, Mo, USA) were purchased and used as received. Senkyunolide I (4), senkyunolide H (5), senkyunolide A (6), coniferylferulate (7), riligustilide (11) and levistolide A (12) were isolated from commercial Chuanxiong SCFE extracts (Masson Pharmaceutical Co. Ltd. Guangzhou, China) as described in our previous study [1]. The purity of each isolated compound was determined by HPLC (pure compound: 6: 95%, 4: 99.7%, 5: 94%, 7: 94%, 11: 97%, 12: 98%.
2.2. SCFE Procedure
Briefly, dried Chuanxiong samples (CX-R 1-6, 9-10) were cut into small pieces (about 1 mm) and then loaded (420 grams) into the extractor (1 liter). CO2 supercritical fluid continuously flowed through the extractor via a high-pressure pump. After extraction under conditions described in Tables 2(a)-(c), the extract-laden CO2 then flowed to the separator via a pressure reduction valve and the extracts precipitated in the separator after reduction of pressure, while CO2 free of extracts was recycled and flowed back to the extractor.
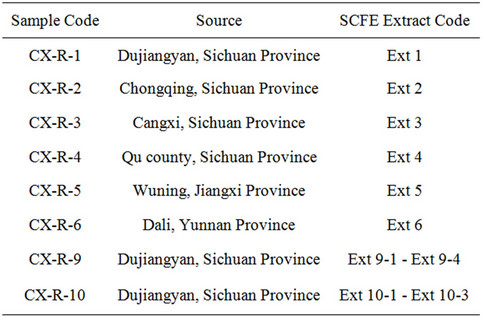
Table 1. Sources and respective extract of Chuanxiong samples.
Among ten main components in Chuanxiong samples, compounds 6-12 were lipophilic and could be easily extracted by SCFE. Compounds 3-5, which either contained one carboxylic and one hydroxyl group (3) or two hydroxyl groups (4 and 5), were relatively less lipophilic. To improve the extraction of compounds 3-5, ethanol was used as an entrainer in the present study.
Firstly, six dried Chuanxiong samples (CX-R 1-6) were extracted under the same SCFE condition (Table 2(a)) to yield six extracts (Ext 1 - Ext 6) for the evaluation of extraction variations in different herbal samples. Secondly, modification of various parameters as listed in Table 2(b) was carried out with sample CX-R 9 to obtained 4 extracts (Ext 9-1 - Ext 9-4) for the improvement of extraction efficiency. Finally, reproducibility of the selected SCFE condition (Table 2(c)) for Chuanxiong was assessed with sample CX-R 10 and its 3 extracts (Ext 10-1 - Ext 10-3).
2.3. Preparation of Different Chuanxiong Extracts
Extraction of dried Chuanxiong samples (CX-R 1-6 and 9-10) using SCFE were conducted by Masson Pharmaceutical Co. Ltd under the conditions indicated in Tables 2(a)-(c) to give Chuanxiong SCFE extracts (Ext 1-6, 9-10). SCFE extract of each herb was analyzed under the HPLC chromatographic conditions reported previously by our group [2]. The contents of compounds 3-12 were calculated from the calibration curves constructed as described below and compared with their contents in the respective 95% ethanol extract of each herbal sample that determined according to our previous report [2]. The extraction efficiency of the SCFE method was evaluated using the following equation:

In addition, after SCFE, the residues (residue 1 - 6 and 9) were further extracted with 95% ethanol in the same manner as described previously for preparation of the 95% ethanol extract of the herb [2] and contents of compounds 3-12 in residual extracts were determined and calculated from the corresponding calibration curves [2]. The percentages of the ingredients remaining in the residues were calculated with the following equation:
2.4. Quantification of Main Components 3-12 in Chuanxiong SCFE Extracts
The HPLC-UV analytical conditions were the same as reported previously by our group [2]. The calibration curves of components 3-12 were constructed as follows:
 (a)
(a)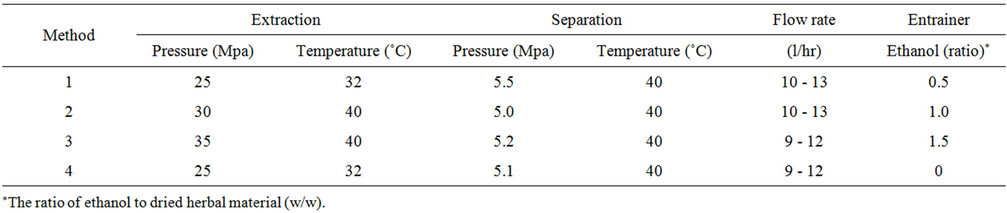 (b)
(b) (c)
(c)
Table 2. (a) SCFE conditions for the extraction of different Chuanxiong samples; (b) Modification of SCFE conditions; (c) Reproducibility of the selected SCFE condition.
A methanol stock solution containing all ten authentic compounds 3-12 was prepared and diluted to appropriate concentrations to construct calibration curves. Each calibration curve contained six different concentrations and was performed in triplicate. a-Naphthaflavone was used as an internal standard with the concentration of 1 g/ml for all analyzes. An aliquot (20 μl) of each solution was subjected to HPLC-UV analysis. Four wavelengths at 254, 274, 284 and 294 nm were chosen as the monitoring wavelengths for different analytes, respectively [2]. Calibration curves were constructed by plotting the concentration of each analyte as a function of peak area ratio of spiked analyte to the internal standard.
Two concentrations at low and high levels of the corresponding calibration curve of each analyte were chosen to test intraand inter-day variability. Known quantities of compounds 3-12 and the internal standard were spiked into methanol to achieve the desired concentrations. The resultant solutions were then subjected to HPLC-UV analysis and quantity of each analyte was calculated from corresponding calibration curves. Each sample was analyzed in triplicate to determine the intra-day variability. The inter-day reproducibility was obtained by analyzing the samples on three separate days. The relative standard deviation (RSD) was taken as a measure of precision and the percentage difference between amounts determined and spiked was considered as a measure of accuracy. To determine detection limitations, aliquots of analytes were added into methanol to provide concentrations of ten analytes ranging from 2 to 100 ng/ml in the final solutions. The detection limit of each analyte was determined when the signal-to-noise (S/N) ratio of the tested peak area was greater than five. All data were presented as mean ± standard deviation (S.D).
3. Results and Discussions
3.1. Calibration Curves for Determination of Compounds 3-12 in Chuanxiong SCFE Extracts
Under the reported chromatographic conditions [2], the intercepts, slopes, concentration ranges and detection limits for compounds 3-12 in SCFE extracts were summarized in Table 3. All 10 analytes showed good linearity over concentration ranges investigated. The limits of detection (LOD) for all analytes were the same as previous reported [2], and the limits of quantitation (LOQ) calculated as per gram extract were 0.8 μg for 3, 0.4 μg for compounds 4, 6, 9, 10 and 11, 0.2 μg for 5 and 8, 0.6μg for 7, and 2.0 μg for 12, respectively. Furthermore, using our previously well-developed quantitative HPLCUV method [2], the results obtained from the present method validation were similar [2] and also revealed good precisions with overall intraand inter-day variations of less than 9% and 12%, respectively, and accuracies higher than 90% for all analytes.
3.2. SCFE of Different Chuanxiong Samples under the Same Condition
Contents of compounds 3-12 in 95% ethanol extracts of six dried Chuanxiong samples (CX-R 1-6) have been determined and reported [2]. Great variations were found in both individual and total contents of compounds 3-12 and three major compounds 6, 7 and 8 (18.52 - 34.93 mg/g) accounted for about 86% - 95% of the total content of dried Chuanxiong samples.
After extraction under the conditions summarized in Table 2(a), all the main ingredients in herbs were extracted into SCFE extracts (Figures 1-3), and three major ingredients 6, 7 and 8 contributed to about 89% - 96% of the total content in SCFE extracts. Furthermore, contents of all compounds investigated in the extracts were significantly higher than those in the corresponding herbs. For example, a total content and the sum of contents of three major compounds 6, 7 and 8 in six SCFE extracts were about 7 - 15 times and 5 - 15, 6 - 15 and 8 - 18 times higher than those in original herbs, indicating successful enrichments of all main ingredients in the extracts.
However, in some samples, particularly samples 4 and 5, composition ratios of different ingredients in SCFE extracts were different from that in original herbs (Figure 3), suggesting the existence of selective extractions towards certain Chuanxiong ingredients by using SCFE.
The extraction recovery of SCFE for both individual and total ingredients were calculated based on extraction yield, which was 5.0% (Ext 1), 6.1% (Ext 2), 5.6% (Ext 3), 6.0% (Ext 4), 7.5% (Ext 5) and 6.0% (Ext 6), respectively. As shown in Figure 3, based on an average value of six extracts, extraction recovery of each individual ingredient varied greatly from sample to sample ranging
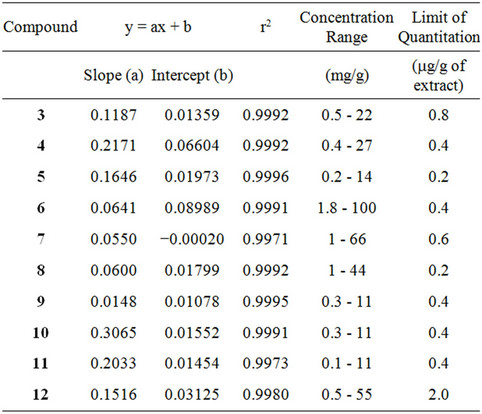
Table 3. Calibration curves for determination of compounds 3-12 in SCFE extracts.
from 20% - 50%, indicating that herbal sample itself could influence the outcome of SCFE. In general, higher recoveries were obtained for the lipophilic constituents, such as three major ingredients 6, 7, and 8, whereas recoveries for relatively less lipophilic compounds 3, 4 and 5 were poor in the absence of ethanol as an entrainer (Figure 3). Except Ext 3, the overall recoveries of the 10 main components in SCFE extracts were about 50% - 60%, while the recovery of Ext 3 reached 83%.
In addition, contents of ten main ingredients remaining in the residual materials after SCFE were also determined. The results (Table 4(a)) showed that less than 17% of total components remained in the residual materials. Taking into account a sum of the extraction recovery and the percentage remaining in the residual material for all ingredients determined, there was no significant loss for sample CX-R-3, while about 28% - 45% loss of the investigated compounds were found for all other Chuanxiong samples during SCFE.
3.3. Modifications of SCFE Condition
To improve the extraction recovery and reduce the loss of main ingredients during SCFE, dried Chuanxiong sample (CX-R-9) was extracted by using four different SCFE conditions as indicated in Table 2(b) to give SCFE extracts Ext 9-1, 9-2, 9-3 and 9-4, respectively. Different amounts of ethanol entrainer were added to improve the extraction of relatively less lipophilic compounds 3-5.
With modified SCFE conditions, the extraction yields for these four SCFE extracts were improved to 7.9%, 7.9%, 8.5% and 7.2%, respectively. Chemical profile and contents of the three major ingredients in all four extracts were highly similar, while the total contents in samples Ext 9-1 and Ext 9-2 were significantly higher than those in other two samples (Figure 4), indicating that higher flow rate of CO2 improved the overall extraction. In the presence of ethanol (Ext 9-1, 9-2 and 9-3), con-
 (a)
(a) (b)
(b)
Table 4. (a) Percentage of total 10 components remaining in the residues of six different Chuanxiong samples extracted under the same conditions; (b) Percentage of total 10 components remaining in the residues of Chuanxiong sample CX-R-9 extracted under four different conditions.

Figure 1. Representative HPLC-UV chromatograms of Chuanxiong samples and the respective SCFE extracts monitored at 294 nm (Note: Compound 9 could not be determined at 294 nm, but was measured at 254 nm, data not shown in this figure).
tents of compounds 3, 4 and 5 were significantly higher than that in Ext 9-4 extracted in the absence of ethanol, demonstrating that ethanol as an entrainer improved the extraction of less lipophilic ingredients. However, increase in amount of ethanol added from a ratio of 0.5 (ethanol: dried herbal material, w/w) (Ext 9-1) to 1.5 (Ext 9-3) did not significantly further enhance extraction capacity towards these three less lipophilic components, suggesting that the addition of ethanol with the ratio of 0.5 was adequate. Further comparing Ext 9-1 with Ext 9-2, quantities of some thermo-labile compounds, in particular 7, 8 and 10, were markedly lower in Ext 9-2, indicating that extraction at relatively higher temperature (40˚C versus 32˚C) might led to degradation of some thermolabile components.
As shown in Figure 5, with improved extraction yields, the extraction recoveries for most ingredients in Ext 9-1, 9-2 and 9-3 increased significantly and the total re-
 (a) (b)
(a) (b)
Figure 2. Contents of ten main ingredients 3-12 in six different Chuanxiong samples (a) and in the respective SCFE extracts obtained under the same conditions (b).
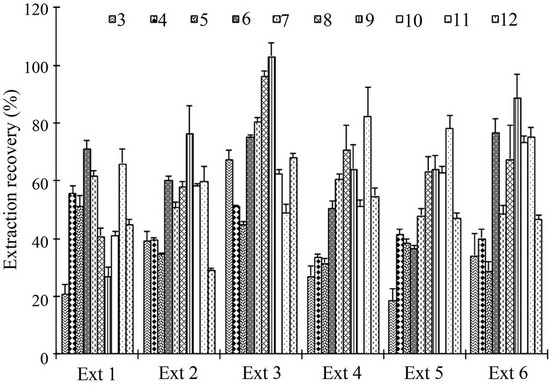
Figure 3. Extraction recovery of ten main ingredients 3-12 from six different Chuanxiong samples under the same SCFE conditions.
 (a) (b)
(a) (b)
Figure 4. Contents of ten main ingredients 3-12 in Chuanxiong sample CX-R-9 (a) and its SCFE extracts obtained under different conditions (b).
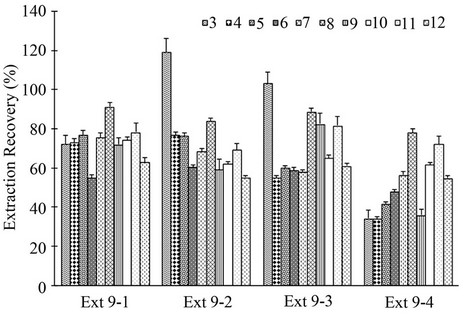
Figure 5. Extraction recovery of ten main ingredients 3-12 from Chuanxiong sample CX-R-9 under different conditions.
covery reached about 72% - 75%. While, in the absence of entrainer the extraction recovery for less lipophilic compounds in Ext 9-4 was only about 30% - 40% and subsequently the total recovery in this extract was also lower (about 60%), further confirming that the addition of ethanol as an entrainer was helpful for the extraction of less lipophilic compounds. In Ext 9-2 and Ext 9-3, extraction recovery for compound 3 was even higher than 100% (119% and 103%, respectively). According to previous reports and the results obtained by our group [18], thermal instability of 7 leading to the formation of 3 via hydrolysis pathway may explain this high recovery. The present results further demonstrated that temperature could be one of important factors affecting extraction, and hydrolysis of 7 to 3 might occur at relatively higher extraction temperature (40˚C for Ext 9-2 and 9-3 versus 32˚C for Ext 9-1 and 9-4) during the extraction.
Furthermore, a total amount of ten main ingredients 3-12 remaining in the residual materials after SCFE reduced to 4% - 8% under these four modified extraction conditions (Table 4(b)). There were about 17% - 24% loss of the investigated compounds from the Chuanxiong sample during SCFE.
Based on the above data, the SCFE condition for the extraction of Ext 9-1 provided an overall high extraction recovery for all main ingredients with different lipophilicities. Furthermore, the extraction temperature used in this condition was appropriate for the extraction of thermo-labile compounds, in particular 7, 8 and 10. Therefore, the SCFE condition for Ext 9-1 was considered to be the best among four conditions examined and selected for the further reproducibility test.
3.4. Reproducibility of the Selected SCFE Condition
Dried Chuanxiong sample (CX-R-10) was extracted under the above selected SCFE condition (Table 2(c)) on three separate days to test the extraction reproducibility. The extraction yields were 7.0 (Ext 10-1), 7.2 (Ext 10-2) and 7.8% (Ext 10-3), respectively, and the average extraction yield was 7.3% with variations less than 6%. Contents of the ten main components investigated in both herbal sample and SCFE extracts were also determined and shown in Figure 6. The results demonstrated that chemical profiles of all three SCFE extracts were similar and not different from that of the original herb.
Furthermore, reproducibility of extraction recovery for each of different ingredients investigated and a total content of all ingredients were also determined (Figure 7). Except for compounds 3 and 4, reproducibility for each individual ingredient and an overall reproducibility for total components were excellent with less than 8.5% variations. Whereas, variations of extraction recovery for compound 3 and 4 were slightly higher (about 17.7% and 12.1%). Nevertheless, these variations were still considered to be adequate, because reproducible extraction of these less lipophilic components by SCFE was recognized difficult in general [24]. Furthermore, compounds 3 and 4 were the minor ingredients in Chuanxiong herb and were reported not as the major components contributing to the therapeutic outcomes of Chuanxiong [32,33]. Therefore, the overall extraction reproducibility for the developed SCFE condition was considered to be adequate for the extraction of all main ingredients in Chuanxiong herb.
In summary, extraction recovery for both total and individual ingredients in Chuanxiong samples varied from sample to sample, and can be improved by the modification of extraction conditions via adjustment of various parameters, such as temperature, pressure, CO2 flow rate and amounts of the entrainer added. SCFE condition used in the extraction of Ext 9-1 was demonstrated as the best one among all conditions tested. This condition yielded high extraction recoveries for the total (75.6%) and the three major chemical ingredients (6: 83.5%, 7: 77%, 8: 78.3%) with an adequate overall reproducibility. The developed SCFE method also provided the advantage of extraction at low temperature (32˚C), which might significantly reduce the degradation of certain thermo-labile major components in Chuanxiong herb.
4. Conclusion
In conclusion, the results obtained from the present study using Chuanxiong as an example herb demonstrated that SCFE can be used as a general method for the extraction of different chemical ingredients from medicinal herbs with advantages including high extraction yield of thermo-labile components and selective extraction of certain ingredients using specifically designed SCFE conditions.
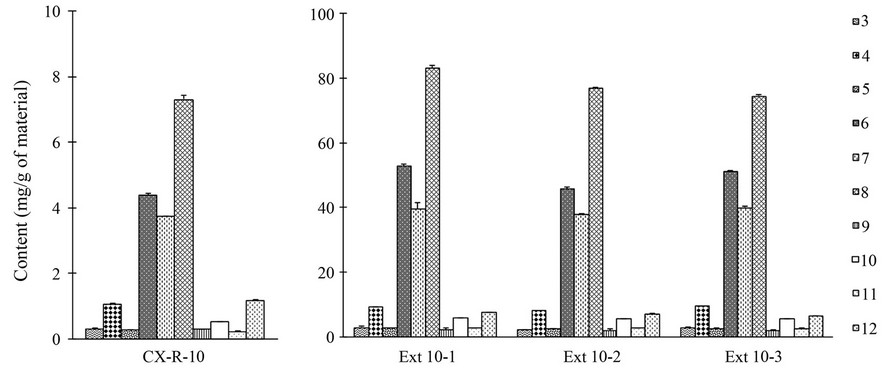
Figure 6. Content of ten main ingredients 3-12 in Chuanxiong herbal sample CX-R-10 (a) and in its SCFE extracts obtained under selected condition on three separate days (b).
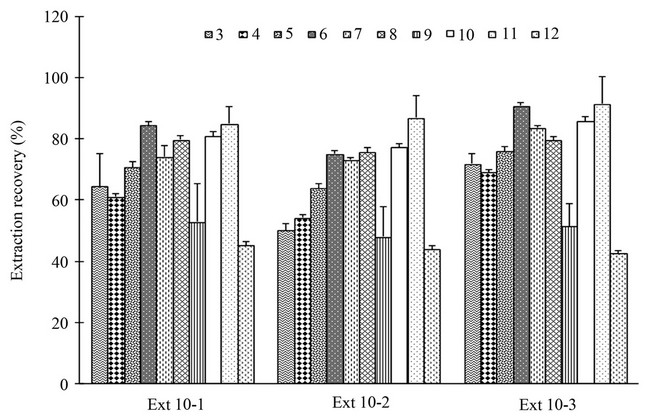
Figure 7. Extraction recovery of ten main ingredients 3-12 from Chuanxiong sample CX-R-10 under selected SCFE conditions on three separate days.
5. Acknowledgements
The authors would like to thank Mr. Bo Jin and Mr. Hancha Liu at Masson Pharmaceutical Co. Ltd., Guang zhou, China, for their technical assistance of SCFE.
REFERENCES
- S. L. Li, S. S.-K. Chan, G. Lin, L. Ling, R. Yan, H. S. Chung and Y. K. Tam, “Simultaneous Analysis of the Main Chemical Ingredients of Ligusticum Chuanxiong by On-Line High Performance Liquid Chromatography-Diode Array Detector-Mass Spectrometry,” Planta Medica, Vol. 69, No. 5, 2003, pp. 445-451. Hdoi:10.1016/j.jpba.2004.09.054
- R. Yan, S. L. Li, H. S. Chung, Y. K. Tam and G. Lin, “Simultaneous Quantification of Twelve Bioactive Components of Ligusticum Chuanxiong Hort. by High-Performance Liquid Chromatography,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 37, No. 1, 2005, pp. 87-95. Hdoi:10.1016/j.jpba.2004.09.054
- W. C. Ko, J. R. Sheu, S. H. Tzeng and C. M. Chen, “The Selective Antianginal Effect without Changing Blood Pressure of Butylidenephthalide in Conscious Rats,” Planta Medica, Vol. 64, No. 3, 1998, pp. 229-232. Hdoi:10.1055/s-2006-957415
- W. C. Ko, C. C. Liao, C. H. Shih, C. B. Lei and C. M. Chen, “Relaxant Effects of Butylidenephthalide in Isolated Dog Blood Vessels,” Planta Medica, Vol. 68, No. 11, 2002, pp. 1004-1009. Hdoi:10.1055/s-2002-35671
- T. Naito, K. Kubota, Y. Shimoda, T. Sato, Y. Ikeya, M. Okada and M. Maruno, “Effects of Constituents in a Chinese Crude Drug, Ligustici Chuanxiong Rhizoma on Vasoconstriction and Blood Viscosity,” Natural Medicine, Vol. 49, 1995, pp. 288-292.
- C. M. Teng, W. Y. Chen, W. C. Ko and C. Ouyang, “Antiplatelet Effect of Butylidenephthalide,” Biochimica et Biophysica Acta, Vol. 924, No. 3, 1987, pp. 375-382. Hdoi:10.1016/0304-4165(87)90151-6
- S. S.-K. Chan, A. O.-K. Choi, R. L. Jones and G. Lin, “Mechanisms Underlying the Vasorelaxing Effects of Butylidenephthalide, an Active Constituent of Ligusticum Chuanxiong, in Rat Isolated Aorta,” European Journal of Pharmacology, Vol. 537, No. 1-3, 2006, pp. 111-117. Hdoi:10.1016/j.ejphar.2006.03.015
- S. S.-K. Chan, T. Y. Cheng and G. Lin, “Relaxation Effects of Ligustilide and Senkyunolide A, Two Main Constituents of Ligusticum Chuanxiong, in Rat Isolated Aorta,” Journal of Ethnopharmacology, Vol. 111, No. 3, 2007, pp. 677-680. Hdoi:10.1016/j.jep.2006.12.018H
- S. S.-K. Chan, R. L. Jones and G. Lin, “Synergistic Interaction between the Ligusticum Chuanxiong Constituent Butylidenephthalide and the Nitric Oxide Donor Sodium Nitroprusside in Rat Isolated Aorta,” Journal of Ethnopharmacology, Vol. 122, No. 2, 2008, pp. 308-312. Hdoi:10.1016/j.jep.2009.01.002
- W. L.-T. Kan, C. H. Cho, J. A. Rudd and G. Lin, “Study of the anti-Proliferative Effects of Phthalides from Angelica Sinensis on Colon Cancer Cells,” Journal of Ethnopharmacology, Vol. 120, No. 1, 2008, pp. 36-43. Hdoi:10.1016/j.jep.2008.07.027
- L. Liu , Z. Q. Ning, S. Shan, K. Zhang, T. Deng, X. P. Lu and Y. Y. Cheng, “Phthalide Lactones from Ligusticum Chuanxiong Inhibit Lipopolysaccharide-Induced TNFAlpha Production and TNF-Alpha-Mediated NF-KappaB Activation,” Planta Medica, Vol. 71, No. 9, 2005, pp. 808-813. Hdoi:10.1055/s-2005-871231
- J. W. Chung, R. J. Choi, E. K. Seo, J. W. Nam, M. S. Dong, E. M. Shin, L. Y. Guo and Y. S. Kim, “Anti-Inflammatory Effects of (Z)-Ligustilide through Suppression of Mitogen-Activated Protein Kinases and Nuclear Factor-κB Activation Pathways,” Archives of Pharmacal Research, Vol. 35, No. 4, 2012, pp. 723-732. Hdoi:10.1007/s12272-012-0417-z
- Z. Feng, Y. Lu, X. Wu, P. Zhao, J. Li, B. Peng, Z. Qian and L. Zhu, “Ligustilide Alleviates Brain Damage and Improves Cognitive Function in Rats of Chronic Cerebral Hypoperfusion,” Journal of Ethnopharmacology, Vol. 144, No. 2, 2012, pp. 313-321. Hdoi:10.1016/j.jep.2012.09.014
- T. F. Lee, Y. L. Lin and Y. T. Huang, “Studies on antiProliferative Effects of Phthalides from Ligusticum Chuanxiong in Hepatic Stellate Cells,” Planta Medica, Vol. 73, No. 6, 2007, pp. 527-534. Hdoi:10.1097/FTD.0b013e31802c5862H
- R. Yan, G. Lin, N. L. Ko and Y. K. Tam, “Low Oral Bioavailability and Pharmacokinetics of Senkyunolide A, a Major Bioactive Component in Rhizoma Chuanxiong, in the Rat,” Therapeutic Drug Monitoring, Vol. 29, No. 1, 2007, pp. 49-56. Hdoi:10.1097/FTD.0b013e31802c5862
- R. Yan, N. L. Ko, S. L. Li, Y. K. Tam and G. Lin, “Pharmacokinetics and Metabolism of Ligustilide, a Major Bioactive Component in Rhizoma Chuanxiong, in the Rat,” Drug Metabolism and Disposition, Vol. 36, No. 2, 2008, pp. 400-408. Hdoi:10.1124/dmd.107.017707
- R. Yan, N. L. Ko, B. Ma, Y. K. Tam and G. Lin, “Pharmacokinetic Interaction among co-Existing Ingredient Leading to Significant Systemic Exposure of Z-Butylidenephthalide, a Minor Ingredient in Chuanxiong Rhizoma, in Rat,” Current Drug Metabolism, Vol. 13, No. 5, 2012, pp. 524-534. Hdoi:10.2174/1389200211209050524
- S. L. Li, R. Yan, Y. K. Tam and G. Lin, “Post-Harvest Alteration of the Main Chemical Ingredients in Ligusticum Chuanxiong Hort. (Rhizoma Chuanxiong),” Chemical and Pharmaceutical Bulletin, Vol. 55, No. 1, 2007, pp. 140-144. Hdoi:10.1248/cpb.55.140
- S. Lee, M. K. Park, K. H. Kim and Y.-S. Kim, “Effect of Supercritical Carbon Dioxide Decaffeination on Volatile Components of Green Teas,” Journal of Food Science, Vol. 72, No. 7, 2007, pp. S497-S502. Hdoi:10.1111/j.1750-3841.2007.00446.x
- J. P. Coelho, A. F. Cristino, P. G. Matos, A. P. Rauter, B. P. Nobre, R. L. Mendes, J. G. Barroso, A. Mainar, J. S. Urieta, J. M. Fareleira, H. Sovová and A. F. Palavra, “Extraction of Volatile Oil from Aromatic Plants with Supercritical Carbon Dioxide: Experiments and Modeling,” Molecules, Vol. 17, No. 9, 2012, pp. 10550-10573. Hdoi:10.3390/molecules170910550
- S. M. Pourmortazavi and S. S. Hajimirsadeghi, “Supercritical Fluid Extraction in Plant Essential and Volatile Oil Analysis,” Journal of Chromatography A, Vol. 1163, No. 1-2, 2007, pp. 2-24. Hdoi:10.1016/j.chroma.2007.06.021
- M. A. McHugh and V. J. Krukonis, “Supercritical Fluid Extraction: Principles and Practice (Butterworth-Heinemann Series in Chemical Engineering),” ButterworthHeinemann, Oxford, 1994.
- Q. Lang and C. M. Wai, “Supercritical Fluid Extraction in Herbal and Natural Product Studies—A Practical Review,” Talanta, Vol. 53, No. 4, 2001, pp. 771-782. Hdoi:10.1016/S0039-9140(00)00557-9
- M. Mukhopadhyay, “Natural Extracts Using Supercritical Carbon Dioxide,” CRC Press, Boca Raton, 2000. Hdoi:10.1248/cpb.35.1427
- Z. Y. Hong, X. Z. Wang, J. Le, D. C. Zhang, Y. F. Chai and Z. L. Liu, “Supercritical Fluid Extraction of Essential Oil from Dry Rhizome of Ligusticum Chuanxiong Hort and Their Characterization by GC/MS,” Journal of Chinese Pharmaceutical Sciences, Vol. 11, No. 2, 2002, pp. 31-34. Hdoi:10.1016/j.juro.2006.06.135
- Q. Ruan, Y. Zhang, Y. Y. Hu and X. X. He, “Influences of Different Extraction Mathods on the Chemical Contents of Chuanxiong Essential Oils,” China Journal of Chinese Materia Medica, Vol. 28, No. 6, 2003, pp. 572- 574.
- G. T. Wu, L. F. Shi, J. H. Hu and L. Li, “The Determination of Ligustilide in Ligusticum Chuanxiong Hort. by Supercritical Fluid Extraction,” Acta Pharmaceutica Sinica, Vol. 33, No. 6, 1998, pp. 457-460.
- Y. F. Yuan, J. Zhou, X. M. Zheng and L. Li, “Studies on Volatile Oil in Ligusticum Chuanxiong by Supercritical Fluid Extraction,” Chinese Pharmaceutical Journal, Vol. 35, No. 2, 2000, pp. 84-87.
- X. L. Guo, Y. F. Feng, H. M. Liang, G. F. Chen and Y. Gao, “Analysis of Chemical Constituents in Supercritical CO2 Extract of Ligusticum Chuanxiong Hort. By GCMS,” Chinese Journal of Pharmaceuticals, Vol. 36, No. 8, 2005, pp. 472-474.
- Y. Lan, L. Zheng, Y. Huang, A. Wang, Y. Li, X. He and Y. Wang, “Studies on Extraction of Ligusticum Chuanxiong in Hongye Xintong Soft Capsule by Super Critical Fluid Extraction,” Zhongguo ZhongYao ZaZhi, Vol. 34, No. 2, 2009, pp. 161-164.
- The State Pharmacopoeia Commission of China, “Rhizoma Chuanxiong,” Pharmacopoeia of the People’s Republic of China, Chemical Industry Press, Beijing, 2010, p. 38.
- M. Kobayashi, M. Fujita and H. Mitsuhashi, “Components of Cnidium Officinale Makino: Occurrence of Pregnenolone, Coniferyl Ferulate and Hydroxyphthalide,” Chemical & Pharmaceutical Bulletin, Vol. 32, No. 9, 1984, pp. 3770-3773. Hdoi:10.1248/cpb.32.3770
- M. Kobayashi, M. Fujita and H. Mitsuhashi, “Studies on the Constituents of Umbelliferae Plants. XV. Constituents of Cnidium Officinale: Occurrence of Pregnenolone, Coniferyl Ferulate and Hydroxyphthalide,” Chemical & Pharmaceutical Bulletin, Vol. 35, No. 4, 1987, pp. 1427-1433. Hdoi:10.1248/cpb.35.1427H
NOTES
*Corresponding author.

