American Journal of Analytical Chemistry
Vol.4 No.1(2013), Article ID:26969,5 pages DOI:10.4236/ajac.2013.41007
Separation of Enantiomers of Clopidogrel on Chiral Stationary Phases by Packed Column Supercritical Fluid Chromatography
1Center for Pharmaceutical Sciences Department, Jawaharlal Nehru Technological University, Hyderabad, India
2Agilent Technologies India Pvt. Ltd., Hyderabad, India
3Drug Control, Hyderabad, India
4Sultan Ul-Uloom College of Pharmacy, Hyderabad, India
Email: *BHAVYA.KHAGGA@gmail.com
Received October 21, 2012; revised November 24, 2012; accepted December 3, 2012
Keywords: Chiral Stationary Phases; SFC; Enantiomer Separation; Supercritical Fluid Chromatography; Clopidogrel
ABSTRACT
A packed column supercritical fluid chromatography (SFC) method for the separation of clopidogrel enantiomers on a chiral stationary phase and CO2 with modifier as mobile phase has been developed at an analytical scale. Among 11 different 2 stationary phases the Chiral cel OD-H column showed by far the best separation properties. The influence of different modifiers, injection solvents, temperature, and pressure, and density of the fluid, respectively, on the separation behaviour has been studied. It was found that the separation behaviour strongly depends on the type of modifier and the modifier content. Temperature and pressure are of less influence.
1. Introduction
Clopidogrel hydrogen sulfate, methyl (+)-(S)-α-(o-chlorophenyl)-6, 7-dihydrothieno [3,2-c] pyridin-5 (4H)-acetate hydrogensulfate, is a thienopyridine derivative that irreversibly blocks ADP receptor. Clopidogrel is chemically related to ticlopidine with superior side effects profile and dosing requirements. The drug which reduces platelet aggregation is extensively used for prevention of thrombosis in patients undergoing placement of a coronary stent [1]. The drug is rapidly, but incompletely, absorbed after oral administration and extensively metabolized to an active metabolite. The parent drug or its active metabolite remains undetectable in plasma. The major circulating compound, however, is an inactive carboxylic derivative, which its blood concentration is used to document the pharmacokinetic profile of clopidogrel [2]. A few analytical methods including gas chromatographymass spectrometry (GC-MS) [3], liquid chromatographymass spectrometry (LC-MS) [4,5] and high-performance liquid chromatography (HPLC) with UV detection [6,7] have been published for determination of the inactive metabolite of clopidogrel in the biological fluids.
Today preparative Chromatography of enantiomers is mostly carried out by liquid chromatography (LC). The use of supercritical fluid chromatography (SFC) may result in higher production rates as the resolution per time in general is better in SFC other advantage of preparative SFC over preparative HPLC include substantial waste reduction. Facilitate product recovery and feasibility of solvent recycling.
Several direct and indirect liquid chromatographic analytical methods involving a variety of chiral and achiral phases for resolution of ibuprofen enantiomers have been reviewed.
A good separation factor between the Clopidogrel isomer was found in SFC with methanol in Carbon dioxide as mobile phase and it was even better in HPLC with ethanol in hexane (Scheme 1).
The aim of this project was to develop as SFC method with binary mobile phase for the separation of clopidogrel enantiomers at an analytical scale which is suitable for an analytical scaling to preparative chromatography like simulated moving bed (SMB) SFC [8].
2. Experimental
2.1. Analytical SFC System
A commercial Thar SFC system with an SFC pump, a modifiee pump and a HP 1050 high pressure diode array detector was used for analytical chromatography. The

Scheme 1. Chemical structure of clopidogrel.
SFC system was equipped with a HP 7673 auto sampler connected to a Rheodyne valve with a fixed 10 µL volume external loop. This system operated in downstream mode, allowed independent pressure and flow control. The column was kept at a constant temperature in the instrument column oven. Data were collected and processed on HP chemStation.
The SFC method can be optimized by variation of the stationary phase, the modifier the temperature and the pressure, respectively density of the fluid. For our investigation we used 11 different analytical chiral columns as stationary phases. The mobile phase consisted of a variety of modifiers entrained in carbon dioxide at 0% - 20% (V/V) and column outlet Pressure ranged from 100 to 190 bar. Column temperature varied from 30˚C to 40˚C. Modifiers included methanol. The total flow of the mobile phase was kept constant at 2 ml/min (pump setting).
2.2. Materials and Chemicals
Clopidogrel USP was kindly donated from Dr. Redddy’s laboratories Hyderabad. For method development solutions of about 1% Clopidogrel either in methanol or Propanol have prepared. Carbon dioxide with more than 99.99% was obtained from Ramesh gas agencies Hyderabad. Methanol, Propanol solvents of HPLC grade were purchased from Merck. Analytical (25 × 0.46 cm) Chiral columns Chiral OB-H, OJ-H, Chiral pak AD-H and AS, IC, IA were from Chiral technologies. Kromosil CHI-DMB and CHI-TBB were kindly donated from final chemicals.
3. Preparation of Standard Solutions
Clopidogrel reference standard (50 mg) was accurately weighed transferred to 50 ml volumetric flask, and dissolved in methanol final concentration 1 mg/ml. The resulting solution was sonicated for 10 min and dilute to volume.
4. Determination of Clopidogrel Enantiomers
For construction of the calibration graph, take aliquots (0.2 - 1.3 ml) of 1 mg = ml clopidogrel isomers standard solution into a series of 25 ml measuring flasks. Inject 20 µL of the each solution from each flask and record the chromatograms, maintain the flow rate at 2 ml min and at wave length 215 nm. Measure the ratio of peak area corresponding to concentration of each. Construct a calibration graph representing the relation between concentration and ratio of peak area.
Concentration of unknown samples could be derived from the calibration graph of calculate the following regression equation:
y = 33599x + 1352, R2 = 0.994 (Scheme 2)
y = 83253x + 539.5, R2 = 0.999 (Scheme 3)
where y is peak area of sample, x is concentration of Clopidogrel in mg = ml, and R is correlation coefficient.
Determinations of Clopidogrel Enantiomers in Bricanyl Tablets Weigh 30 tablets accurately and pulverize in a small mortar. Transfer a weighed quantity powder equivalent to 50 mg of clopidogrel into a beaker and extract with methanol using magnetic stirrer. Filter into a 50 ml measuring flask and complete to volume with methanol. Determine clopidogrel concentration by taking (0.2 - 1.3 ml) into 25 ml measuring flask. Complete to volume with methanol and proceed as previously described.
4.1. Method Validation
The methods were validated according to the International Conference on Harmonization guidelines for validation of analytical procedures (ICH-Q2B 1996). Analysis of variance (ANOVA) was used to verify the validity of the methods.
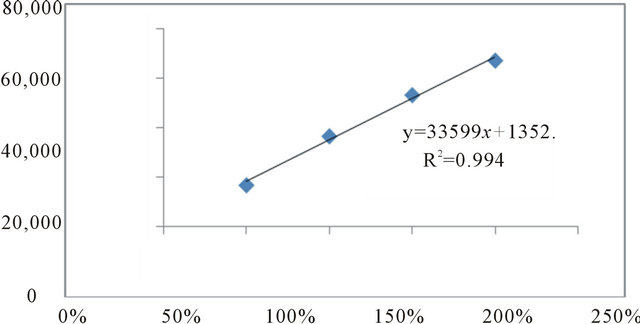
Scheme 2. Linearity graph of S-enantiomer of clopidogrel.

Scheme 3. Linearity graph of R-enantiomer of clopidogrel.
4.2. Linearity
The calibration curve was obtained with seven concentrations of the standard solution, 8 - 52 mg = ml. The solutions were prepared in triplicate. The linearity was evaluated by linear regression analysis, which was calculated by the least square regression method.
4.3. Precision
The precision of the assay was determined by repeatability (intraday) and intermediate precision (interday). Intraday precision was evaluated by assaying the sample, at the same concentration and during the same day. Six sample solutions of 20 mg = ml were prepared and assayed. The intermediate precision (interday) was studied by comparing the assays on different days (3 days).
4.4. Accuracy
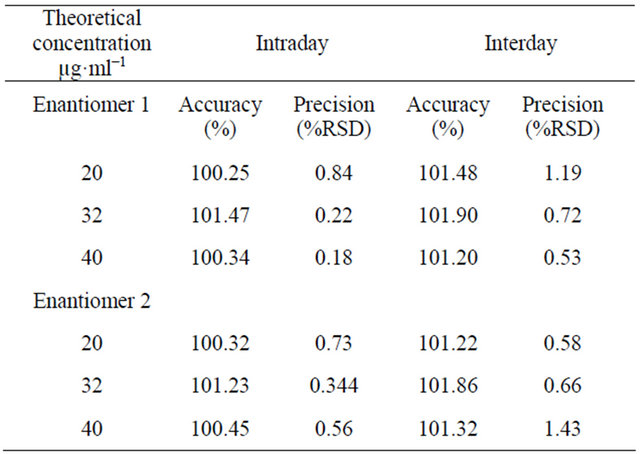
The accuracy of an analytical method is determined by how close the test results obtained by that method come to the true value. It can be determined by application of the analytical procedure to an analyte of known purity (for the drug substance) or by recovery studies, where a known amount of standard is spiked in the placebo (for drug product). In the present study, a number of different solutions were prepared with a known added amount of drug substance and injected in triplicate. Percent recoveries of response factor (area and concentration) were calculated as shown in Table 1, which indicated the accuracy of the proposed method.
5. Robustness
The robustness of the SFC method was determined by analysis of samples under a variety of conditions by making small changes in the mobile-phase composition, in the flow rate (1.8 - 2.2 ml/min), in the temperature of
Table 1. Intraday and interday accuracy and precision data of HPLC method for clopidogrel.
the column (20˚C - 300˚C), and by changing the wavelength (220 - 225 nm).
6. LOD and LOQ
Limit of detection (LOD) is defined as the lowest concentration of an analyte in a sample that can be detected, but not necessarily quantified, and the limit of quantification (LOQ) is defined as the lowest.
7. Separation and Determination of Clopidogrel Enantiomers
Concentration of analyte in sample that can be determined with acceptable precision and accuracy.
8. Results and Discussion
The development of stereoselective SFC method for the determination of enantiomeric drugs has received considerable attention in recent years because of its importance in analysis of quality control of pharmaceutical formulations. We developed an SFC method for the separation and quantitation of Clopidogrel enantiomers in its pharmaceutical formulations. The chromatographic conditions were adjusted to provide a reliable assay performance. Mobile-phase selection was based on peak parameters, runtime, ease of preparation, and cost. A typical chromatogram is shown in Figure 1 for the analysis and separation of a sample solution of Clopidogrel enantiomers. The retention time was observed at 9.77 min for enantiomer 1, at 13.46 min for enantiomer 2.
The LOD and LOQ obtained using the slope and standard deviation of the intercept from three curves and determined by the linear regression line, were 0.4 and 0.7 mg/ml–1, respectively. These values were also used in an experimental assay confirming the calculation. The calibration curves for Clopidogrel enantiomers were constructed by plotting concentration versus peak area and showed good linearity in the 12 - 60 mg/ml range as shown in Figures 2 and 3. The representative linear equations were y = 0.0239 x + 0.0267 for enantiomer 1 and y = 0.0238 x – 0.0201 for enantiomer 2 with high correlation coefficients (r = 0.9995 and r = 0.9995, respectively).
Accuracy and precision of the proposed method were assessed by performing triplicate analyses of the standard solutions. Three different concentrations, diluted with the mobile phase, were prepared in the linear range of the calibration curve and analyzed to determine intraday variability and accuracy. The interand intraday precision were calculated as the RSD%. The results and the mean values are shown in Table 1, demonstrating good precision and accuracy. When chromatographic conditions were intentionally altered, no significant effect was
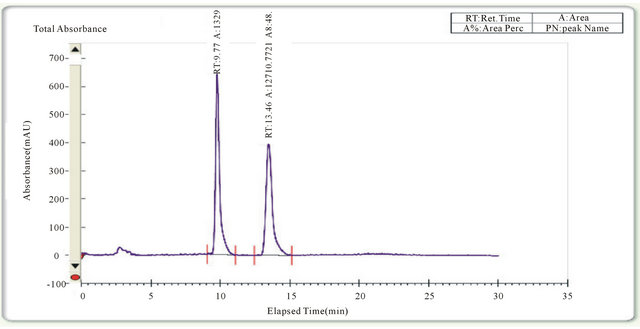
Figure 1. Chromatogram of Clopidogrel (40 mg = ml) on a Chiral cel OD-H column (250 × 4.6 nm) by advanced separation technologies Inc. (ASTEC) using a mixture super critical fluid liquid and 2-propanol as a mobile phase and flow rate of 2 ml/min at 215 nm.

Figure 2. Linearity of concentration of Clopidogrel enantiomer 1 to peak area of Clopidogrel enantiomer 1 to peak area of internal reference standard (ratio of peak area) using SFC.

Figure 3. Linearity of concentration of Clopidogrel enantiomer 2 to peak area of Clopidogrel enantiomer 2 to peak area of internal reference standard (ratio of peak area) using SFC.
observed in the chromatogram, confirming the robustness of the method. The intraday precision obtained by the proposed method showed a RSD of 0.41% and 0.54% for both enantiomer 1 and enantiomer 2, respectively. Interday variability was calculated and showed RSD values of 0.81% and 0.89% for both enantiomers, respectively, as shown in Table 1.
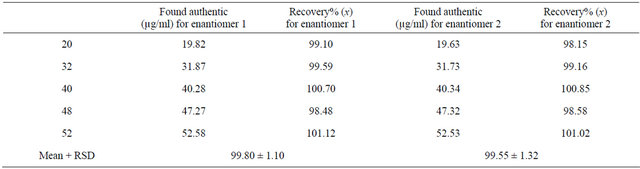
Results of the determination of terbutaline in preva and pharmaceutical tablet formulations are shown in Tables 2 and 3. The accuracies of the HPLC method for enantiomers 1 and 2 were 99.80% and 99.55%, respectively, confirming the accuracy of the proposed methods. The results are expressed in Table 4.
Table 2. Results of the determination of clopidogrel in tablets by SFC.
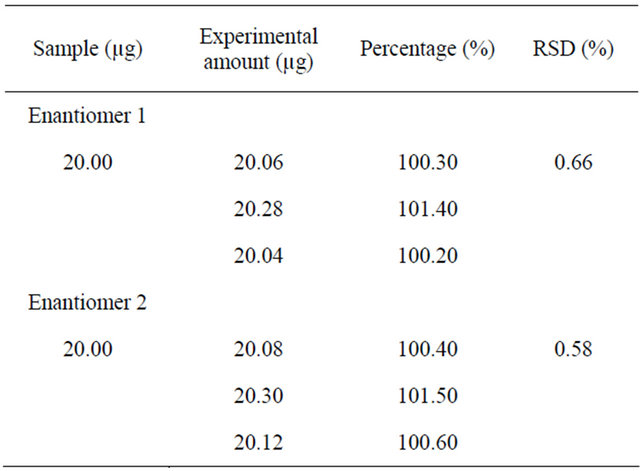
Table 3. Results of standard addition of authentic clopidogrel to preva tablets.
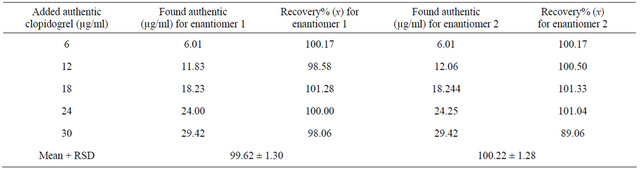
Table 4. Determination of authentic clopidogrel via the suggested SFC method.
9. Conclusion
The proposed SFC method described a quantitative determination and separation of Clopidogrel enantiomers in bulk drug and pharmaceutical tablet formulations. The validated SFCmethod is fast, precise, accurate, and efficient and can be applied in routine analyses in Regular production laboratories.
REFERENCES
- P. W. Majerus and D. M. Tollefsen, “Blood Coagulation and Anticoagulant, Thrombolytic, and Antiplatelet Drugs,” In: L. L. Brunton, Ed., The Pharmacological Basis of Therapeutics, 11th Edition, The McGraw-Hill Companies, New York, 2006, p. 1483.
- J. M. Herbert, D. Frehel, E. Vallee, G. Kieffer, D. Gouy, Y. Berger, G. Defreyn and J. P. Maffrand, “Clopidogrel, a Novel Antiplatelet and Antithrombotic Agent,” Cardiovascular Drug Reviews, Vol. 11, No. 2, 1993, pp. 180-198. doi:10.1111/j.1527-3466.1993.tb00275.x
- P. Lagorce, Y. Perez, J. Ortiz, J. Necciari and F. Bressolle, “Assay Method for the Carboxylic Acid Metabolite of Clopidogrel in Human Plasma by Gas ChromatographyMass Spectrometry,” Journal of Chromatography B: Biomedical Sciences and Applications, Vol. 720, No. 1-2, 1998, pp. 107-117. doi:10.1016/S0378-4347(98)00452-6
- H. Ksycinska, P. Rudzki and M. Bukowska-Kiliszek, “Determination of Clopidogrel Metabolite (SR26334) in Human Plasma by LC-MS,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 41, No. 2, 2006, pp. 533-539. doi:10.1016/j.jpba.2005.11.035
- A. Mitakos and I. Panderi, “Determination of the Carboxylic Acid Metabolite of Clopidogrel Inhuman Plasma by Liquid Chromatography-Electrospray Ionization Mass Spectrometry,” Analytica Chimica Acta, Vol. 505, No. 1, 2004, pp. 107-114. doi:10.1016/S0003-2670(03)00019-9
- S. S. Singh, K. Sharma, D. Barot, P. R. Mohan and V. B. Lohray, “Estimation of Carboxylic Acid Metabolite of Clopidogrel in Wistar Rat Plasma by HPLC and Its Application to a Pharmacokinetic Study,” Journal of Chromatography B, Vol. 821, No. 2, 2005, pp. 173-180. doi:10.1016/j.jchromb.2005.05.013
- E. Souri, H. Jalalizadeh, A. Kebriaee-Zadeh, M. Shekarchi and A. Dalvandi, “Validated HPLC Method for Determination of Carboxylic Acid Metabolite of Clopidogrel in Human Plasma and Its Application to a Pharmacokinetic Study,” Biomedical Chromatography, Vol. 20, No. 12, 2006, pp. 1309-1314. doi:10.1002/bmc.697
- A. Depta, T. Giese, M. Johannsen, G. Brunner and J. Chroatgr, A 865(1999) 175.
NOTES
*Corresponding author.

