American Journal of Analytical Chemistry
Vol. 3 No. 8 (2012) , Article ID: 21911 , 4 pages DOI:10.4236/ajac.2012.38072
A Stability Indicating HPLC Method for Dronedarone in Bulk Drugs and Pharmaceutical Dosage Forms
1Research and Development Laboratory, United States Pharmacopeia-India Private Limited, Hyderabad, India
2Center for Pharmaceutical Sciences, IST, J.N.T. University, Hyderabad, India
3Dr. Reddy’s Laboratories Ltd. IPDO, Hyderabad, India
Email: nari_nit@yahoo.co.in
Received June 6, 2012; revised July 18, 2012; accepted July 26, 2012
Keywords: Dronedarone; HPLC; Forced Degradation; Validation; Stability Indicating
ABSTRACT
The objective of the current study was to develop a validated, specific and stability-indicating reverse phase HPLC method for the quantitative determination of Dronedarone and its related substances. The determination was done for active pharmaceutical ingredient and its pharmaceutical dosage forms in the presence of degradation products, and its process-related impurities. The drug was subjected to stress conditions of hydrolysis (acid and base), oxidation, photolysis and thermal degradation per International Conference on Harmonization (ICH) prescribed stress conditions to show the stability-indicating power of the method. Significant degradation was observed during acid, oxidative and photo stress studies. In the developed HPLC method, the resolution between Dronedarone and its process-related impurities was found to be greater than 2.0. Regression analysis shows an r value (correlation coefficient) of greater than 0.999 for Dronedarone and it’s all the five impurities. The chromatographic separation was achieved on a C8 stationary phase. The method employed a linear gradient elution and the detection wavelength was set at 288 nm. The stress samples were assayed against a qualified reference standard and the mass balance was found to be close to 99.6%. The developed HPLC method was validated with respect to linearity, accuracy, precision and robustness.
1. Introduction
Dronedarone is a drug mainly for the indication of cardiac arrhythmias, chemically as N-(2-Butyl-3-(p-(3-(dibutylamino)propoxy)benzoyl)-5-benzofuranyl)methanesulfonamide and its structural formu-la is C31H44N2O5S. Multaq is generic name for Dronedarone, is recommended as an alternative to amiodarone for the treatment of atrial fibrillation and atrial flutter in people whose hearts have either returned to normal rhythm or who undergo drug therapy or electric shock treatment to maintain normal rhythm [1].
In atrial fibrillation, atria beat more than 300 times per minute. The arrhythmatous condition needs to be controlled, as humans cannot withstand this rapid and chaotic beating of the heart. New investigational drugs like Dronedarone are being used. Dronedarone is the most recent antiarrhythmic drugs (AAD). It was approved by US-FDA and is available in the USA as Multaq tablets (400 mg). Dronedarone falls under the category of multiple ion channel blocker. It mainly targets the repolarization currents, making them less active and hence prolonging the action potential duration (APD). Dronedarone also exhibits antiadrenergic activity, thus reducing the pace of the pacemaker. Dronedarone has been proven to be a safe and efficacious AAD, evidenced by both animal and human studies. These studies showed that there was prolongation of the APD and absence of QT interval prolongation with long term administration of the drug. Also there was reduced thyroid hormone receptor expression. Dronedarone is significantly safer and effective in maintaining the sinus rhythm and reducing the ventricular proarrhythmias, justifying it for the long term treatment of atrial fibrillation compared to other antiarrhythmic drugs [2-5].
Few HPLC methods were available in literature for the analysis of Dronedarone includes, simultaneous determination of dronedarone and its active metabolite debutyldronedarone in human plasma by liquid chromatography-tandem mass spectrometry: Application to a pharmacokinetic study [6], Determination of the class III antiarrhythmic drugs dronedarone and amiodarone, and their principal metabolites in plasma and myocardium by high-performance liquid chromatography and UV-detection [7], RP-HPLC method development and validation of Dronedarone HCl in its Pure form and tablet dosage form-that speaks about the content of Dronedarone in bulk and pharmaceutical dosage forms [8]. No HPLC methods were reported in major pharmacopeia like USP, EP, JP and BP.
Extensive literature survey reveals there is no rapid stability-indicating HPLC method for determination of related substances and for quantitative estimation of dronedarone in bulk drugs and pharmaceutical dosage forms. The purpose of the present research work was to develop a suitable, single and rapid stability-indicating HPLC method for the determination of dronedarone and its related substances.
Hence, an attempt has been made to develop an accurate, rapid, specific and reproducible method for the determination of Dronedarone and all the five impurities in bulk drug samples and in pharmaceutical dosage forms along with method validation as per ICH norms. The stability tests were also performed on both drug substances and drug product as per ICH norms [9-12].
2. Experimental
2.1. Chemicals
Samples of Dronedarone and its related impurities were obtained as gratis sample from Sebondscience Labs (Hyderabad, India) (Figure 1). Commercially available 400 mg of Dronedarone tablets (Multaq®) were purchased from Sanofi-Aventis, France. HPLC grade Acetonitrile, Methanol, analytical reagent grade Potassium dihydrogen phosphate, Tetra n-butyl ammonium hydrogen sulfate and potassium hydroxide were purchased from Merck,

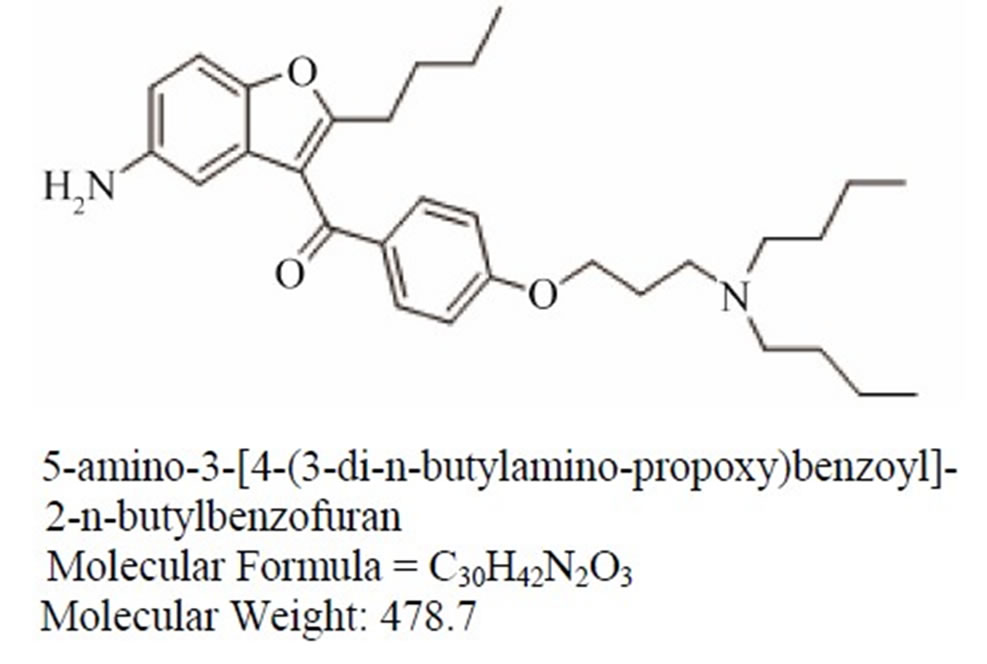

Dronedarone Imp-A Imp-B

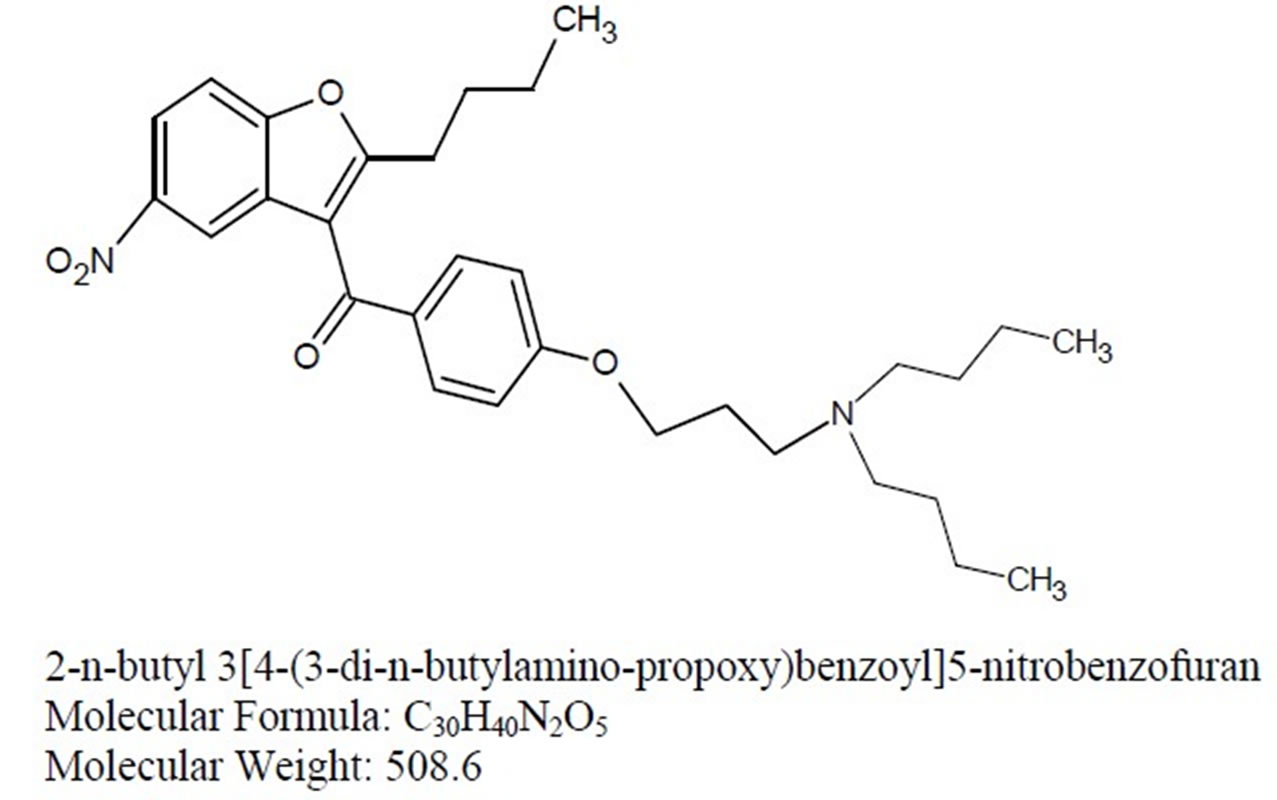

Imp-C Imp-D Imp-E
Figure 1. Chemical structures and labels of Dronedarone and its impurities.
Darmstadt, Germany. High purity water was prepared by using Millipore Milli-Q plus water purification system. All samples and impurities used in this study were of greater than 99.0% purity.
2.2. Equipment
The HPLC system, used for method development, forced degradation studies and method validation was Waters 2695 binary pump plus auto sampler and a 2996 photo diode array detector with Empower software (Waters Corporation, MA, USA). The output signal was monitored and processed using Empower software on Pentium computer (Digital equipment Co). Water bath equipped with temperature controller was used to carry out degradation studies for all solution. Photo stability studies were carried out in a photo stability chamber (Mack Pharmatech, Hyderabad, India). Thermal stability studies were performed in a dry air oven (Mack Pharmatech, Hyderabad, India).
2.3. Chromatographic Conditions
The chromatographic column used was Agilent Zorbax RX C8 column (150 × 4.6) mm with 5 µm particles. Buffer consists of a mixture of 10 mM Potassium dihydrogen phosphate and 10 mM Tetra n-butyl ammonium hydrogen sulfate, pH adjusted to 3.2 using potassium hydroxide. The mobile phase A consists of buffer and mobile phase B consists of acetonitrile. The flow rate of the mobile phase was 1.0 mL·min–1. The HPLC gradient program was set as: time (min)/% solution B: 0/35, 25/80, 25.1/35 and 30/35. The column temperature was maintained at 25˚C and the detection was monitored at a wavelength of 288 nm. The injection volume was 20 μL. Methanol was used as diluent. The concentration is 250 µg·mL–1 for related substances method and 50 µg·mL–1 for Assay method.
2.4. Preparation of Solutions
2.4.1. Preparation of Standard Solutions
A stock solution of Dronedarone (2.5 mg·mL–1) was prepared by dissolving appropriate amount in the methanol. Working solutions were prepared from above stock solution for related substances determination and assay determination, respectively. A stock solution of impurities (mixture of imp-1, imp-2 imp-3 imp-4 and imp-5) at a concentration of 250 µg·mL–1 was also prepared in methanol.
2.4.2. Preparation of Sample Solutions
Multaq® tablets contain 400 mg of Dronedarone. The inactive ingredients present in Multaq® were hypromellose, starch—maize, crospovidone, poloxamer, lactose, silica—colloidal anhydrous, magnesium stearate, titanium dioxide, macrogol 6000 and carnauba wax. Twenty Multaq tablets (400 mg) were weighed and the average weight was calculated. The tablets were powdered in a mortar and a sample of the powder equivalent to 25 mg of the active pharmaceutical ingredient (Dronedarone) was transferred to 100 mL volumetric flask. Approximately 75 mL methanol was added and the flask was placed on rotatory shaker for 10 min and sonicated for 10 min to dissolve the material completely. The solution was then diluted to 100 mL and centrifuged at 3000 rpm for 10 min. The supernatant was collected and filtered through a 0.22 µm pore size Nylon 66-membrane filter. The filtrate was used as sample solution.
2.5. Specificity
Specificity is the ability of the method to measure the analyte response in the presence of its potential impurities. Stress testing of the drug substance can help to identify the likely degradation products, which can in turn help to establish the degradation pathways and the intrinsic stability of the molecule and validate the stability indicating power of the analytical procedures used.
The specificity of the Dronedarone in the presence of its impurities namely imp-A, imp-B, imp-C, imp-D, impE and degradation products was determined by developed HPLC method. Forced degradation studies were also performed on Dronedarone to provide an indication of the stability indicating property and specificity of the proposed method [9-12]. The stress conditions employed for degradation study includes light (carried out as per ICH Q1B), heat (105˚C), acid hydrolysis (5 N HCl), base hydrolysis (0.1 N NaOH) and oxidation (5% H2O2). For heat study period was 2 days and for light studies, study period was to illuminate the sample for 1.2 million Lux hours, where as for acid, base and peroxide hydrolysis the test period was 48 h. Peak purity of stressed samples of Dronedarone was checked by using 2996 Photo diode array detector of Waters Corporation, MA, USA.
2.6. Analytical Method Validation
The developed chromatographic method was validated for linearity, precision, accuracy, sensitivity, robustness and system suitability.
2.6.1. Precision
The precision of the related substance method was checked by injecting six individual preparations of (250 µg·mL–1) Dronedarone spiked with 0.10% each imp-A, imp-B, imp-C, imp-D and imp-E. The %RSD area of each imp-A, imp-B, imp-C, imp-D and imp-E was calculated. Precision study was also determined by performing the same procedures on a different day (interday precision).
The intermediate precision (ruggedness) of the method was also evaluated using different analyst, different column and different instrument in the same laboratory.
Assay method precision was evaluated by carrying out six independent assays of test sample of Dronedarone against qualified reference standard. The %RSD of six assay values obtained was calculated. The intermediate precision of the assay method was evaluated by different analyst and by using different instrument from the same laboratory.
2.6.2. Sensitivity
Sensitivity was determined by establishing the Limit of detection (LOD) and Limit of quantitation (LOQ) for imp-A, imp-B, imp-C, imp-D and imp-E estimated at a signal-to-noise ratio of 3:1 and 10:1 respectively, by injecting a series of dilute solutions with known concentration. The precision study was also carried out at the LOQ level by injecting six individual preparations of imp-A, imp-B, imp-C, imp-D and imp-E, calculated the %RSD for the areas of each impurity.
2.6.3. Linearity and Range
Linearity test solutions for assay method has prepared from stock solution at five concentration levels from 50 to 200% of assay analyte concentration (25, 37.5, 50, 75 and 100 µg·mL–1).
A linearity test solution for related substance method was prepared by diluting the impurity stock solution to the required concentrations. The solutions were prepared at seven concentration levels. From LOQ to 200% of the permitted maximum level of the impurity (i.e. LOQ, 0.0375%, 0.075%, 0.1125%, 0.15%, 0.225% and 0.3%) was subjected to linear regression analysis with the least square method. Calibration equation obtained from regression analysis was used to calculate the corresponding predicted responses. The residuals and sum of the residual squares were calculated from the corresponding predicted responses.
Linearity was checked for three consecutive days in the same concentration range for both assay and related substance method and calculated the %RSD value of the slope and Y-intercept of the calibration curve. Upper and lower levels of range were also established.
2.6.4. Accuracy
The accuracy of the assay method was evaluated in triplicate at five concentration levels, i.e. 25, 37.5, 50, 75 and 100 µg·mL–1 in bulk drugs and pharmaceutical dosage forms. At each concentration, three sets were prepared and injected in triplicate. The percentage of recovery was calculated at each level.
The accuracy of the related substance method was evaluated in triplicate at 0.075%, 0.1125%, 0.15%, 0.225% and 0.3% of the analyte concentration (250 µg·mL–1). The percentage of recoveries for imp-1, imp-2, imp-3, imp-4 and imp-5 were calculated.
2.6.5. Robustness
To determine the robustness of the developed method, experimental conditions were deliberately changed and the resolution (Rs) between Dronedarone, imp-A, imp-B, imp-C, imp-D and imp-E were evaluated. The flow rate of the mobile phase was 1.0 mL·min–1. To study the effect of flow rate on the developed method, 0.1 units of flow was changed (i.e. 0.9 and 1.1 mL·min–1). The effect of column temperature on the developed method was studied at 20˚C and 30˚C instead of 25˚C. The effect of pH on resolution of impurities was studied by varying ±0.1 pH units (i.e. buffer pH altered from 3.2 to 3.1 and 3.3). In the all above varied conditions, the components of the mobile phase were held constant.
2.6.6. Solution Stability and Mobile Phase Stability
The solution stability of Dronedarone in the assay method was carried out by leaving the test solutions of samples in tightly capped volumetric flasks at room temperature for 48 h. The same sample solutions were assayed at 6 h intervals up to the study period against freshly prepared standard solution. The mobile phase stability was also carried out by assaying the freshly prepared sample solutions against freshly prepared reference standard solutions at 6 h intervals up to 48 h. Mobile phase prepared was kept constant during the study period. The %RSD of assay of Dronedarone was calculated for the study period during mobile phase and solution stability experiments.
The solution stability of Dronedarone and its related impurities were carried out by leaving both spiked sample and un-spiked sample solution in tightly capped volumetric flask at room temperature for 48 h. imp-A, imp-B, imp-C, imp-D and imp-E was determined at every 6 h interval, up to the study period.
Mobile phase stability was also carried out for 48 h by injecting the freshly prepared sample solutions, for every 6 h interval. Content of imp-A, imp-B, imp-C, imp-D and imp-E was checked in the test solutions. Mobile phase prepared was kept constant during the study period.
3. Results and Discussion
3.1. Method Development and Optimization
The HPLC method carried out in this study aimed at developing chromatographic system capable of eluting and resolving Dronedarone from its process related impurities and degradation products that comply with the general requirements for system suitability. Initial trials were done on Inertsil ODS 2 C18 column (150 mm × 4.6 mm i.d., particle size 5 µm) with mobile phase, 0.1% Formic acid: Acetonitrile with the gradient as (time (min)/% solution B): 0/25, 5/25, 45/30, 50/30, 51/75, 55/75 at flow rate 1.0 mL·min–1. Longer retention times and poor peak shape of Dronedarone was problem with the above method. Different columns such as YMC Pack ODS AM, Hypersil BDS and different buffers such as potassium dihydrogen phosphate, Trifluoroacetic acid were also tried with different gradient methods to achieve the best chromatographic separation. But long retention times and poor peak shapes were still unavoidable. With 0.1% trifluoroacetic acid, IMP-B and IMP-C are co-eluting and long retention times are seen. Studied the separation and peak shape by varying pH from 2.5 to 7.0 with phosphate buffer, and observed that, as the pH is increasing towards 7.0, peaks were strongly retaining. Also at higher pH, Dronedarone and IMP-A are coeluting. Added triethylamine to the mobile phase to study the separation on a C18 column at 6.5 pH. The peak shapes significantly improved but Dronedarone and IMP-A are still co-eluting. Changed the column to Agilent Zorbax SB CN and obtained better separations and peak shapes with Solution A as 0.01 M Potassium phosphate and 1 mL·Lt–1 Triethylamine at 6.5 pH and Solution B as Acetonitrile. But the Acid degradation impurity was not separating from the Dronedarone peak, making the method to modify further. The use of ion pair agent in acidic pH as buffer along with acetonitrile improved the peak shape of Dronedarone and obtained good resolution between all the impurities and Dronedarone. The % of acetonitrile played a key role in the retention times and resolution between impurities.
After many logical trials, chromatographic condition was established such that which could be suitable for separation of drug-degradation products and drug-five known impurities.
Using the optimized conditions, Dronedarone and its known impurities were well separated with a resolution of greater than 2. The system suitability results are given in Table 1.
3.2. Results of Forced Degradation Studies
3.2.1. Degradation in Acidic Solution
The drug was exposed to 5 N HCl at 65˚C for 4 h. Dronedarone has shown significant sensitivity towards the treatment of 5 N HCl. The drug gradually undergone degradation with time in 5 N HCl and prominent degradation was observed (~6%).
3.2.2. Degradation in Basic Solution
The drug was exposed to 0.1 N NaOH at 65˚C for 48 h. No significant degradation was observed until the study period.

Table 1. System suitability report.
3.2.3. Oxidative Conditions
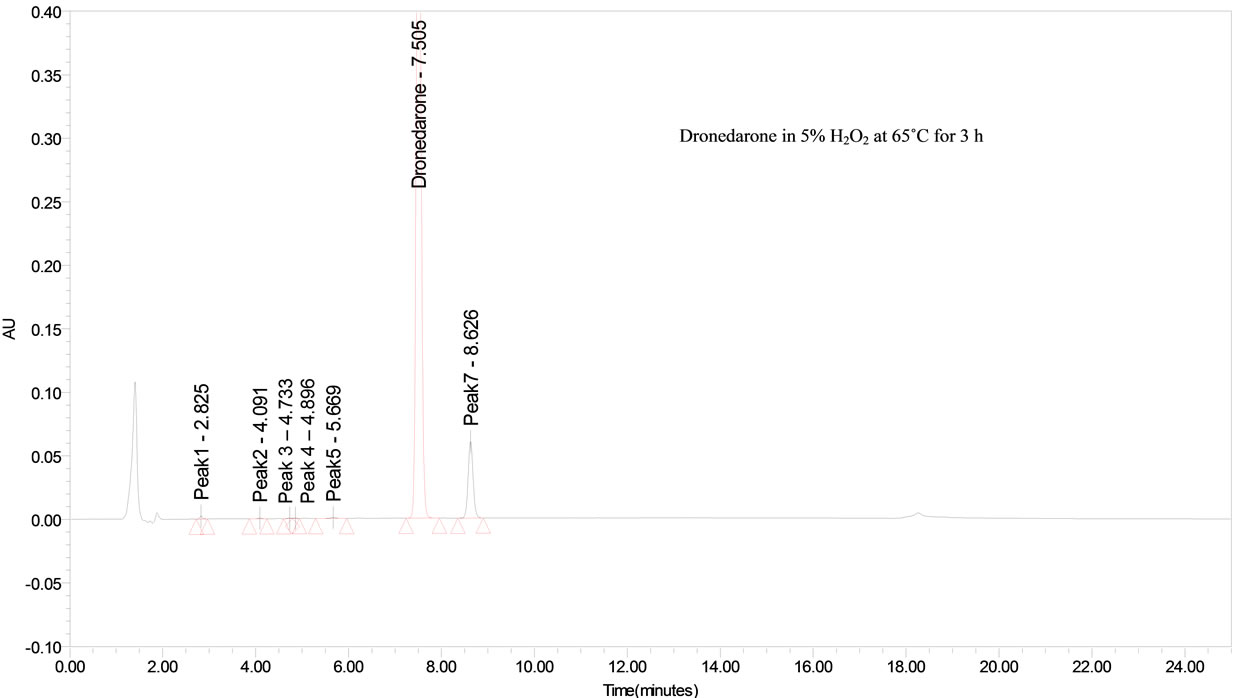
The drug was exposed to 5% hydrogen peroxide at 65˚C for 3 h. Dronedarone has shown significant sensitivity towards the treatment of 5% hydrogen peroxide and the drug gradually undergone prominent degradation (~10%).
3.2.4. Photo Stress Stability
The drug was exposed to 33,000 Lux hours. Dronedarone has shown significant sensitivity towards the illumination of UV light and the drug gradually undergone prominent degradation (~7%).
Dronedarone was stable under forced thermal degradation. No common degradation products were observed in all the above conditions (See Figure 2).
From the degradation studies, Peak purity test results derived from PDA detector, confirmed that the Dronedarone peak was homogeneous and pure in all the analyzed stress samples. The mass balance of stressed samples was close to 99.6%. No degradants were observed after 25 min in the extended runtime of 20 min of all the Dronedarone samples. The developed HPLC method was found to be specific in the presence of imp-A, imp-B, imp-C, imp-D and imp-E and its degradation products confirm the stability indicating power of the developed method.
3.3. Method Validation
3.3.1. Precision
The %RSD of assay of Dronedarone during assay method precision study and intermediate precision study was 0.4 and the %RSD of area of imp-A, imp-B, imp-C, imp-D and imp-E in related substance method precision study were within 2.0, confirming the good precision of the developed analytical method.
3.3.2. Sensitivity
The limit of detection of imp-A, imp-B, imp-C, imp-D and imp-E were 0.003%, 0.003%, 0.002%, 0.004% and 0.002% (of analyte concentration, i.e., 250 µg·mL–1) respectively for 20 mL injection volume. Under the same conditions, the LOQ were 0.011%, 0.009%, 0.006%, 0.012% and 0.007% (of analyte concentration, i.e. 250 µg·mL–1) respectively.
The precision at LOQ concentration for imp-A, imp-B, imp-C, imp-D and imp-E were below 2%.
3.3.3. Linearity and Range
Linear calibration plot for assay method was obtained over the calibration ranges tested, i.e. 25 - 100 µg·mL–1 and the correlation coefficient obtained was greater than 0.999. The result shows an excellent correlation existed between the peak area and concentration of the analyte.
Linear calibration plot for related substance method was obtained over the calibration ranges tested, i.e. LOQ to 0.3 % for imp-A, imp-B, imp-C, imp-D and imp-E. The correlation coefficient obtained was greater than 0.999
 (a)
(a)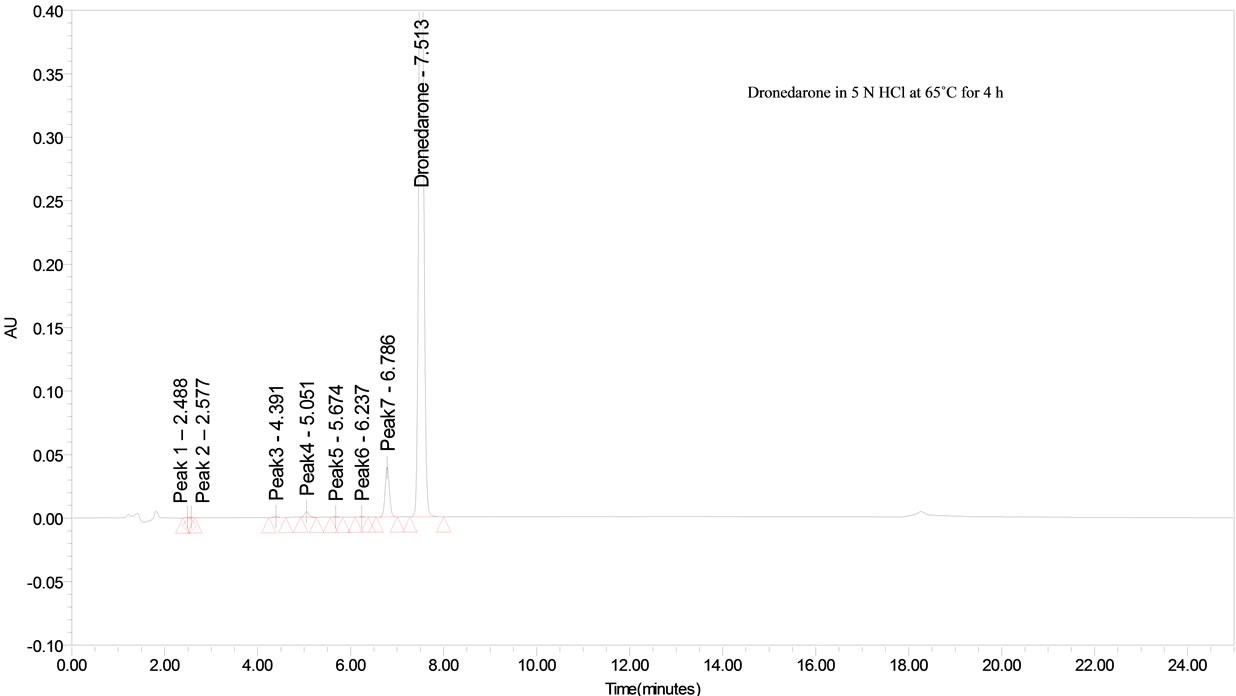 (b)
(b)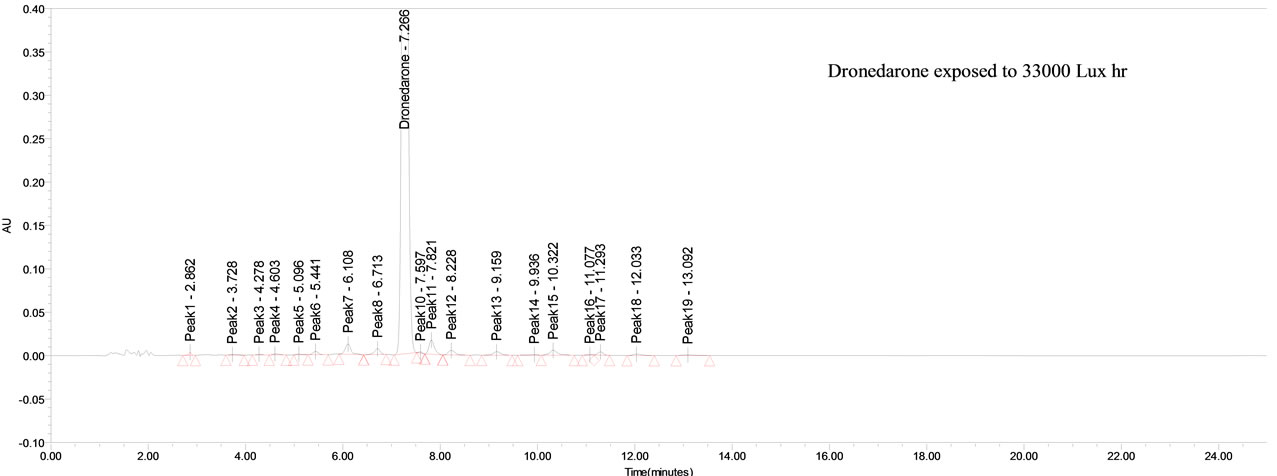 (c)
(c)
Figure 2. Typical chromatogram of stressed Dronedarone samples.
for all five impurities. The result shows an excellent correlation existed between the peak area and concentration of imp-A, imp-B, imp-C, imp-D and imp-E.
At all concentration levels, standard deviation of peak area was significantly low and RSD was below 1.0%. Analysis of residuals indicated that residuals were scattered within ±2% with respect to 100% concentration response. Linearity was checked for related substances over the same concentration ranges on three consecutive days and the %RSD of the slopes and Y-intercept of the calibration plots were with in 2.3 and 5.0 respectively. The range of the method was found from LOQ to 0.3% of the analyte concentration (250 µg·mL–1).
3.3.4. Accuracy
The percentage recovery of Dronedarone in bulk drug samples ranged from 98.8% - 100.6% and in pharmaceutical dosage forms ranged from 100.3% - 102.2% (Table 2). The percentage recovery of imp-A, imp-B, imp-C, imp-D and imp-E in bulk drug samples ranged from 99.6% to 101.0% (Table 3). HPLC chromatograms of spiked sample with all five impurities in Dronedarone bulk drug sample are shown in Figure 3.
3.3.5. Robustness
Close observation of analysis results for deliberately changed chromatographic conditions (flow rate, pH and column temperature) revealed that the resolution between closely eluting impurities, namely imp-A, imp-B, imp-C, imp-D and imp-E was always greater than 2.0, illustrating the robustness of the method.
3.3.6. Solution Stability and Mobile Phase Stability
The %RSD of assay of Dronedarone during solution stability and mobile phase stability experiments was within 1.0. No significant changes were observed in the content

Table 2. Results of accuracy study for bulk drugs and pharmaceutical dosage forms.
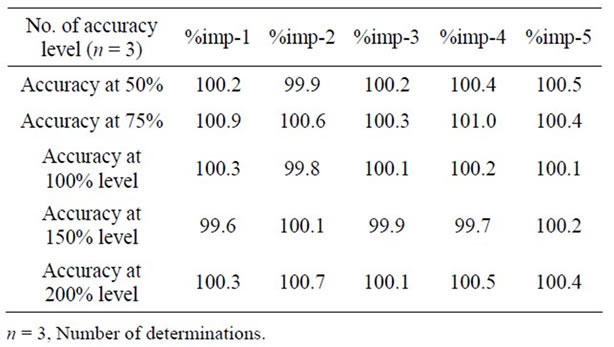
Table 3. Results of accuracy study for impurities.
of imp-A, imp-B, imp-C, imp-D and imp-E during solution stability and mobile phase stability experiments. The solution stability and mobile phase stability experiments data confirms that sample solutions and mobile phase used during assay and related substance determination were stable up to the study period of 48 h.
3.3.7. Assay Analysis
Analysis was performed for different batches of Dronedarone in both bulk drug samples (n = 3) ranged from
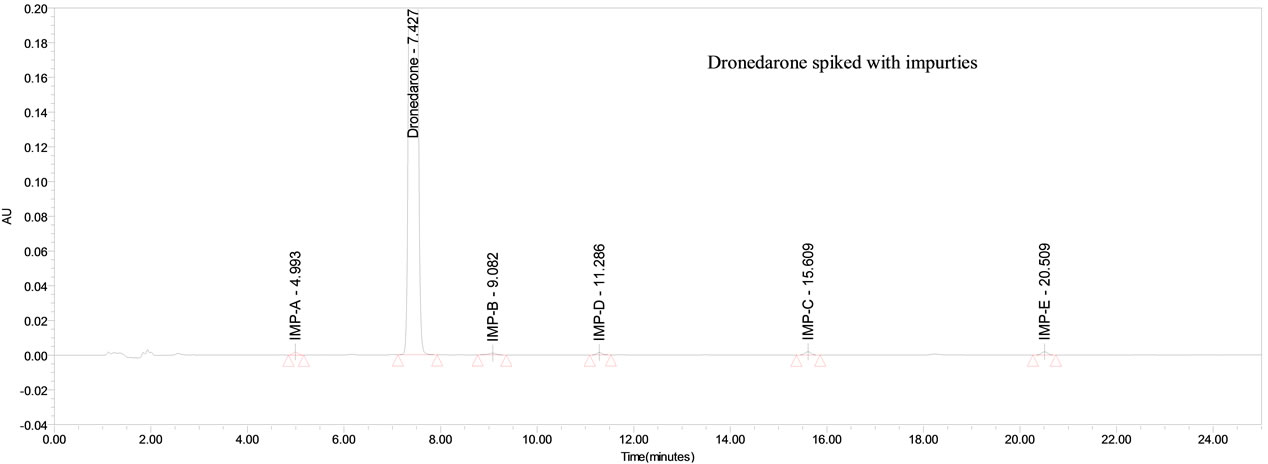
Figure 3. Typical chromatogram of Dronedarone spiked with impurities at 0.1% specification level.
99.91% - 99.95% and dosage forms (n = 3) ranged from 100.6% - 102.9 %.
4. Conclusion
The HPLC method developed for quantitative and related substance determination of Dronedarone in both bulk drugs and pharmaceutical dosage forms are precise, accurate and specific. The method was completely validated showing satisfactory data for all the method validation parameters tested. The developed method is stability indicating and can be used for the routine analysis of production samples and also to check the stability of Dronedarone samples.
5. Acknowledgements
The authors wish to thank the management of United States Pharmacopeia laboratory—India for supporting this work.
REFERENCES
- FDA “FDA Approves Multaq to Treat Heart Rhythm Disorder,” 2009.
- T. S. Mohamed Saleem, K. Bharani, C. Madhusudhana Chetty, et al., “Dronedarone in the Management of Atrial Fibrillation,” Open Access Emergency Medicine, Vol. 2010, No. 2, 2010, pp. 17-23. doi:10.2147/OAEM.S8988
- S. H. Hohnloser, H. J. G. M. Crijns, M. van Eickels, C. Gaudin, R. L. Page, C. Torp-Pedersen and S. J. Connolly, “Effect of Dronedarone on Cardiovascular Events in Atrial Fibrillation,” The New England Journal of Medicine, Vol. 360, 2009, pp. 668-678.
- R. N. Fogoros, “Dronedarone for Atrial Fibrillation, Like Amiodarone But without the Toxicity?” About.com Guide, 2011.
- N. Penugonda, A. Mohmand-Borkowski and J. F. Burke, “Dronedarone for Atrial Fibrillation: How Does It Compare with Amiodarone?” Cleveland Clinic Journal of Medicine, Vol. 78, No. 3, 2011, pp. 179-185.
- C. Xie, S. L. Yang, D. F. Zhong, X. J. Dai and X. Y. Chen, “Simultaneous Determination of Dronedarone and Its Active Metabolite Debutyldronedarone in Human Plasma by Liquid Chromatography-Tandem Mass Spectrometry: Application to a Pharmacokinetic Study,” Journal of Chromatography B, Vol. 879, No. 28, 2011, pp. 3071-3075. doi:10.1016/j.jchromb.2011.09.004
- R. W. Bolderman, J. J. Rob Hermans and J. G. Maessen, “Determination of the Class III Antiarrhythmic Drugs Dronedarone and Amiodarone, and Their Principal Metabolites in Plasma and Myocardium by High-Performance Liquid Chromatography and UV-Detection,” Journal of Chromatography B, Vol. 877, No. 18-19, 2009, pp. 1727-1731.
- A. Patel and J. Akhtar, “RP-HPLC Method Development and Validation of Dronedarone HCl in Its Pure Form and Tablet Dosage Form,” Journal of Chemical and Pharmaceutical Research, Vol. 4, No. 4, 2012, pp. 2173-2179.
- ICH, “Stability Testing of New Drug Substances and Products Q1A (R2),” International Conference on Harmonization, IFPMA, Geneva, 2003.
- USP, United States Pharmacopoeia, “Validation of Compendial Methods,” 31st Edition, United States Pharmacopeial Convention, Rockville, 2008.
- J. T. Carstensen and C. T. Rhodes, “Drug Stability Principles and Practices,” 3rd Edition, 2000.
- M. Bakshi and S. Singh, “Development of Validated Stability-Indicating Assay Methods—Critical Review,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 28, No. 6, 2002, pp. 1011-1040. doi:10.1016/S0731-7085(02)00047-X

