Open Journal of Thoracic Surgery
Vol.3 No.4(2013), Article ID:40751,3 pages DOI:10.4236/ojts.2013.34026
CT, MRI, and 18F-FDG PET-CT Findings of Pulmonary Benign Metastasizing Leiomyoma: A Case Report*
![]()
1Department of General Thoracic Surgery, Kawasaki Medical School, Kurashiki, Japan; 2Department of Pathology, Kawasaki Medical School, Kurashiki, Japan; 3Okayama Ohfuku Clinic, Okayama, Japan; 4Miyake Clinic, Okayama, Japan.
Email: #riki0716okita@yahoo.co.jp
Copyright © 2013 Riki Okita et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2013 are reserved for SCIRP and the owner of the intellectual property Riki Okita et al. All Copyright © 2013 are guarded by law and by SCIRP as a guardian.
Received September 10th, 2013; revised October 10th, 2013; accepted October 17th, 2013
Keywords: Benign metastasizing leiomyoma; CT; PET-CT; MRI
ABSTRACT
Here we report imaging studies of a patient with pulmonary benign metastasizing leiomyoma (BML). A 44-year-old woman who underwent a hysterectomy for uterine cellular leiomyoma presented with abnormal shadows on a chest X-ray. Chest computed tomography (CT) revealed multiple well-defined nodules in both lungs. Chest magnetic resonance imaging (MRI) indicated these nodules as T1-low/T2-high intensity lesions. Contrast-enhanced MRI indicated these nodules as well-enhanced lesions, while 18F-fluorodeoxyglucose positron emission tomography-CT revealed no abnormal accumulation in these nodules. Bilateral lung wedge resections were performed for the largest 2 lesions to confirm the diagnosis, and both nodules were histologically diagnosed as BML.
1. Introduction
Benign metastasizing leiomyoma (BML) is a rare disease that occurs in patients with benign leiomyomatous lesions, predominantly in women with a previous history of uterine leiomyoma [1]. Surgical resection is usually performed for histological diagnostic and/or curative purposes, while endocrine therapy [2] and a wait-and-see strategy [3] are also common approaches to this disease since the clinical course is typically indolent [4]. Clinically, it is important to distinguish between BML and metastatic leiomyosarcoma (LMS) because the therapeutic strategies and prognoses for BML and LMS are quite different; however, the understanding of typical imaging findings of BML is incomplete.
2. Case Report
A 44-year-old woman who had undergone a simple hysterectomy for uterine cellular leiomyoma 30 months earlier was found to have asymptomatic multiple pulmonary nodules, according to a chest X-ray. Chest computed tomography (CT) scans showed bilateral multiple pulmonary nodules (Figures 1(a)-(d)). Chest magnetic resonance imaging (MRI) revealed T1-low intensity/T2-high intensity nodules (Figures 2(a) and (b)), and contrastenhanced MRI demonstrated that the nodules were well enhanced (Figures 2(c) and (d)), which suggested that the nodules were blood flow-rich lesions. Interestingly, the nodules did not take up 18[F]-fluorodeoxyglucose (FDG) during an 18F-FDG positron emission tomography (PET)-CT scan (Figures 3(a),(b)). The largest 2 nodules were removed for histological diagnosis during videoassisted thoracic surgery, and both nodules were confirmed as pulmonary BML because non-atypical proliferating α-SMA-positive leiomyoma cells were observed without hemorrhage or necrosis (Figures 4(a)-(c)).
3. Discussion
Because of its rarity, typical imaging findings for pulmonary BML have not been established, with the exception of CT findings, which include multiple well-defined rounded nodules. Thus, we newly report that the lesions
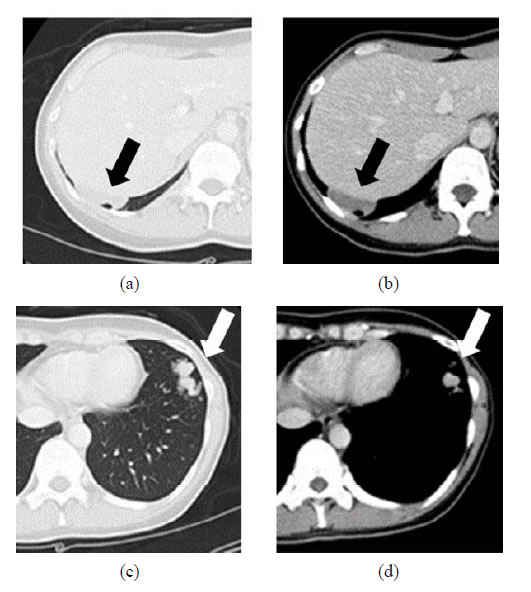
Figure 1. Chest CT scan showing multiple well-defined rounded bilateral lung nodules. (a) representative lesions; (b) a mass lesion that measured 46 mm in right S9; (c) and (d) grouped small nodules in the left S8.
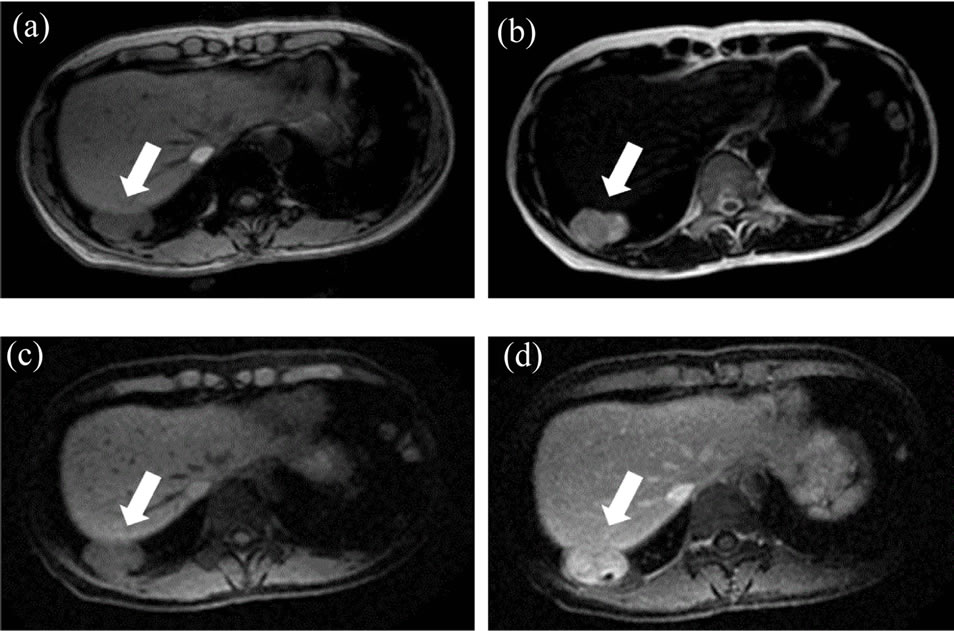
Figure 2. MRI findings. The mass lesion in right S9 was shown as a T1-low/T2-high intensity lesion (a), (b), and contrast-enhanced MRI showed it as a well-enhanced mass (c), pre-enhanced phase and (d), enhanced phase.
 (a) (b)
(a) (b)
Figure 3. (a) and (b) Bilateral pulmonary nodules with no 18F-FDG uptake.
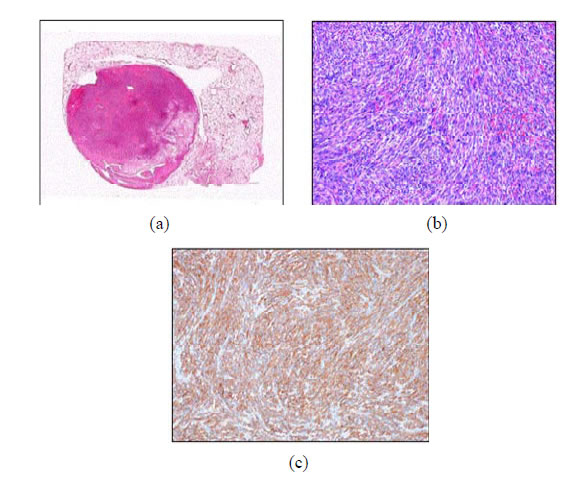
Figure 4. (a) Macroscopically, the tumor in the right S9 presented as an isolated rounded mass and (b) microscopically, hematoxylin-eosin staining showing non-atypical spindleshaped cells that proliferated in a complex arrangement without hemorrhage or necrosis. The mitotic index is 7/50 HPF. (c) Immunohistochemical staining showing αSMA-positive spindle-shaped cells.
in the present case appeared as T1-low/T2-high intensity nodules on MRI and as blood flow-rich tumors on contrast-enhanced MRI. A few studies reported 18F-FDG PET-CT findings in pulmonary BML, and all appeared as avid-mild accumulating nodules [5-8]. In accordance with previous reports, the lesions in the present case did not take up 18F-FDG, suggesting that BML is a blood flow-rich tumor with low metabolic activity.
The main clinical interest is to distinguish BML from metastatic LMS. Ogawa and his collaborator reported that pulmonary BML with malignant transformation showed high 18F-FDG uptake with a maximum SUV of 18.8 [9], suggesting that 18F-FDG PET-CT might be a useful tool to distinguish BML from LMS. On the other hand, it was reported that uterine leiomyoma showed 99mTc uptake [10] and that pulmonary BML showed high 99mTc uptake but low 18F-FDG uptake [8], suggesting that 18F-FDG PET-CT combined with 99mTc scintigraphy might be a useful diagnostic option to distinguish BML from other diseases.
In conclusion, we experienced a case of BML. The lesions were well enhanced on contrast-enhanced MRI, while no metabolic activity was indicated on 18F-FDG PET-CT, suggesting that BML is a blood flow-rich tumor with low metabolic activity.
REFERENCES
- P. E. Steiner, “Metastasizing Fibroleiomyoma of the Uterus: Report of a Case and Review of the Literature,” American Journal of Pathology, Vol. 15, No. 1, 1939, pp. 89-110.
- J. A. Rivera, S. Christopolus, D. Small and M. Trifiro, “Hormonal Manipulation of Benign Metastasizing Leiomyomas: Report of Two Cases and Review of the Literature,” The Journal of Clinical Endocrinology & Metabolism, Vol. 89, No. 7, 2004, pp. 3183-3188. http://dx.doi.org/10.1210/jc.2003-032021
- K. Hoetzenecker, H. J. Ankersmit, C. Aigner, M. Lichtenauer, S. Kreuzer, S. Hacker, W. Hoetzenecker, G. Lang and W. Klepetko, “Consequences of a Wait-and-See Strategy for Benign Metastasizing Leiomyomatosis of the Lung,” The Annals of Thoracic Surgery, Vol. 87, No. 2, 2009, pp. 613-614. http://dx.doi.org/10.1016/j.athoracsur.2008.06.052
- S. Abramson, R. C. Glikeson, J. D. Goldstein, P. K. Woodard, R. Eisenberg and N. Abramson, “Benign Metastasizing Leiomyoma: Clinical, Imaging, and Pathologic Correlation,” AJR, Vol. 176, No. 6, 2001, pp. 1409-1413. http://dx.doi.org/10.2214/ajr.176.6.1761409
- F. Spamaz, M. Ergin, O. Katrancioglu, T. Gonlugur, U. Gonlugur and S. Elagoz, “Benign Metastasizing Leiomyoma,” Lung, Vol. 186, No. 4, 2008, pp. 271-273. http://dx.doi.org/10.1007/s00408-008-9084-8
- V. Scioscio, P. Feraco, L. Miglio, F. Toni, D. Malvi, A. M. Pacilli, L. Fasano, M. Fabbri and M. Zompatori, “Benign Metastasizing Leiomyoma of the Lung: PET Findings,” Journal of Thoracic Imaging, Vol. 24, No. 1, 2009, pp. 41-44. http://dx.doi.org/10.1097/RTI.0b013e31818a0840
- X. Lin, W. Fan, P. Lang, Y. Hu, X. Zhang and X. Sun, “Benign Metastasizing Leiomyoma Identified Using 18FFDG PET/CT,” International Journal of Gynecology & Obstetrics, Vol. 110, No. 2, 2010, pp. 154-156. http://dx.doi.org/10.1016/j.ijgo.2010.03.017
- X. Jin, Y. Meng, Z. Zhu, H. Jing and F. Li, “Elevated 99mTc 3PRGD2 Activity in Benign Metastasizing Leiomyoma,” Clinical Nuclear Medicine, Vol. 38, No. 2, 2013, pp. 117-119. http://dx.doi.org/10.1097/RLU.0b013e318279f14d
- M. Ogawa, M. Hara, Y. Ozawa, S. Moriyama, M. Yano, S. Shimizu and Y. Shibamoto, “Benign Metastasizing Leiomyoma of the Lung with Malignant Transformation Mimicking Mediastinal Tumor,” Clinical Imaging, Vol. 35, No. 5, 2011, pp. 401-404. http://dx.doi.org/10.1016/j.clinimag.2010.11.003
- O. L. Manfredi and J. E. Aruny, “The Role of 99mTcBound Phosphates and Grey Scale Echography in the Differentiation of Pelvic Tumors,” Clinical Nuclear Medicine, Vol. 4, No. 3, 1979, pp. 99-107. http://dx.doi.org/10.1097/00003072-197903000-00004
NOTES
*Conflict of interest: All authors have no conflict of interest.
#Corresponding author.

