Natural Resources
Vol.4 No.4(2013), Article ID:35632,5 pages DOI:10.4236/nr.2013.44043
Green Turtle, Chelonia mydas, Nesting and Temperature Profile of the Nesting Beach at Huyong Island, the Similan Islands in Andaman Sea
![]()
1Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok, Thailand; 2Naval Special Warfare Command, Royal Thai Fleet, Royal Thai Navy, Sattahip, Thailand; 3Center of Excellence in Biodiversity, Chulalongkorn University, Bangkok, Thailand.
Email: *noppadon.k@chula.ac.th
Copyright © 2013 Sarawoot Gomuttapong et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 3rd, 2013; revised May 17th, 2013; accepted May 30th, 2013
Keywords: Conservation; Nesting Ecology; Sea Turtle; Thermal Profile
ABSTRACT
Global change in temperature is regarded as a serious natural disaster that may cause extinction of organisms. Green turtle, Chelonia mydas, is not only an endangered species but also a species with temperature dependent sex determination that could be affected by the global warming. In this study, nesting and temperature profile of C. mydas nesting beach at Huyong Island, the most important nesting site of C. mydas in Andaman Sea of Thailand, were studied in order to monitor a potential effect of regional climate change on the green turtle nesting activity and habitat. Nesting activities were surveyed during May-August 2011 and temperature profiles of the nesting beach were monitored for 58 days during the normal incubation period of this species. Total of 25 nests with clutch size of 105 ± 25 eggs was found during this study period suggesting normal nesting activity of the green turtle. Temperature profile of the nesting beach showed similar trend among nests with no clear influence of the vegetation cover. Mean nest temperature at the middle-third period, corresponding to the temperature-sensitive period of C. mydas, ranged from 28.3˚C to 28.9˚C, suggesting a slightly male-biased sex ratio of the offspring. Overall, temperature profile of the nesting beach showed little or no indication of adverse effects of regional increase in temperature on nesting activity and egg incubation of the green turtle at this period.
1. Introduction
The green turtle, Chelonia mydas, is regarded as an endangered species by the International Union for Conservation of Nature and Natural Resources (IUCN) due to extensive population declines as a result of overexploitation of eggs and adult turtles as well as degradation of marine and nesting habitats [1]. With an unequivocal warming of the climate system according to the Intergovernmental Panel on Climate Change [2], it is highly possible that the green turtle habitats are being affected by regional climate changes, particularly temperature increases.
Since every species of sea turtles possesses a unique mode of reproduction and development known as temperature-dependent sex determination (TSD) [3], sex of their offspring depends on incubating temperature. Sea turtle eggs incubated at 26˚C or below develop into 100% male offspring, while eggs incubated at 32˚C or higher develop into 100% female offspring [3]. A 1:1 sex ratio of the offspring could be obtained upon incubation at the pivotal temperature [4]. In nature, eggs in one nest could expose to range of 26˚C - 32˚C and give rise to different ratio of male-female offspring.
It is believed that temperature-dependent sex determination is ancestral condition and simply maintained in extant species because it is currently adaptive neutral [5]. The notion of adaptive neutral (no net evolutionary advantage of disadvantage to species) is applicable to stable environment. However, with an inevitable increase in temperature [2], the TSD animals may end up having a single sex population in the long run. In case of sea turtle, the slight change in temperature could result in all female population, and may lead to extinction of the species in the near future. The scenario of high incubating temperature resulted in single sex turtle has been tested in laboratory [4], however, there is still no evidence in the natural habitat.
In eastern part of the Indian Ocean, Huyong Island is known as a nesting site of the green turtles [6]. During 1997-2001, surveys of C. mydas nesting reported that 54 ± 30 nests with an average clutch size of 111 ± 3 eggs were found in each year [7]. Since an 800-m beach of the island is regarded as the most important nesting site for C. mydas in Andaman Sea of Thailand, the Royal Thai Navy has used this island for the sea turtle conservation project since 1995 [7]. Integrity of this nesting site thus plays crucial role in the success of the sea turtle conservation project of Thailand In this study, nesting and temperature profile of C. mydas nesting beach at Huyong Island were studied during the 2011 nesting season in order to monitor a potential effect of regional climate change on the green turtle nesting activity and habitat.
2. Materials and Methods
2.1. Study Site
Huyong Island (8˚28ʹN, 97˚38ʹE) of the Similan Islands, Phang-Nga province, Thailand was used as a study site. The island is regarded as the most important nesting site of green turtle on Andaman Sea. The island is currently protected by the Royal Thai Navy and used for sea turtle conservation project [7]. One sandy beach (~800 meter long) is available for turtle nesting in the eastern part of the island. Dominant vegetation cover of the beach includes screw pine (Pandanus tectorius) and beach naupaka (Scaevola taccada).
2.2. Nesting Survey
Field surveys were conducted during May to August 2011. The highest nesting activity was reported during this period of the year [6]. Nesting activity of the green turtle was surveyed visually along the beach every two hours between 8:00 PM to 5:00 AM. Nests were marked for further egg relocation. If nesting turtle was found, turtle identity was individually checked from PIT tag. Size of the nesting turtle including curve carapace length (CCL) and width (CCW) was measured. After nesting was completed, eggs were excavated and relocated for incubation in artificial nests. Maximum depth of natural nest and clutch size were also recorded.
2.3. Nesting Beach Temperature
Five nests of C. mydas were randomly chosen as representative for this study. After egg relocation, data loggers (Trix-8, LogTag Recorders, Auckland, NZ) were buried at the maximum depth of each nest. Nesting beach temperature was recorded on hourly basis for 58 days which is an approximate incubation time of C. mydas egg. For further analysis, nest temperature pattern was separated into 3 periods of incubation including the first-third, the middle-third, and the last-third periods.
2.4. Statistical Analysis
All parameters were tested for normality and homogeneity of variance prior to analysis with parametric statistics. Descriptive statistics (mean and standard deviation) was used to describe nest characteristics. Mean nesting beach temperature was compared among periods using one-way ANOVA followed by Tukey’s HSD test.
3. Results
3.1. Nest Characteristics
There were 25 nests of C. mydas found during May to August 2011. Nesting turtles were found and identified at 19 nests, while only turtle tracks were found in the remaining nests. These 19 nests were belonged to 9 female turtles with curve carapace length of 90.5 - 100.5 cm and curve carapace width of 83.6 - 70.2 cm. Clutch sizes of these nests ranged from 80 to 130 eggs (Mean ± SD: 105 ± 25). Depth to the first egg was 44.78 - 63.72 cm, and depth to the bottom was 67.84 - 76.78 cm, respectively. Majority of these nests (60%) were not covered by any vegetation (Table 1).
Five nests were randomly selected from these nests and used for recording temperature profiles of the nesting beach. Characteristics of these 5 representative nests are shown in Table 2.
3.2. Nesting Beach Temperature Profile
Temperature profiles of five representative nests (Figure
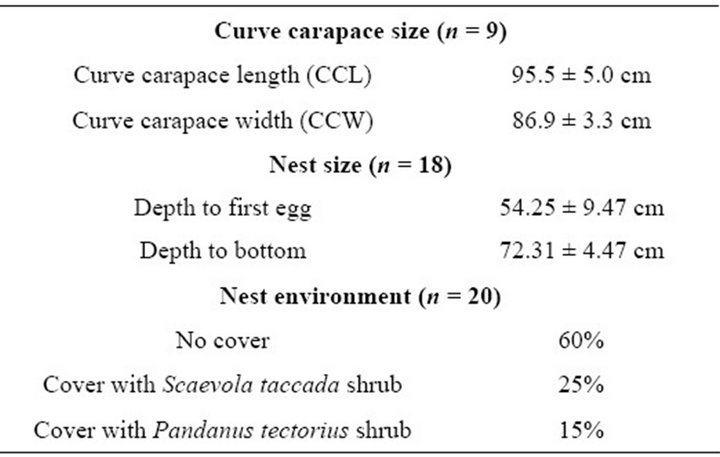
Table 1. Curve carapace size, nest characteristics and clutch size of female Chelonia mydas nested during MayAugust, 2011 at Huyong Island, the Similan Islands.
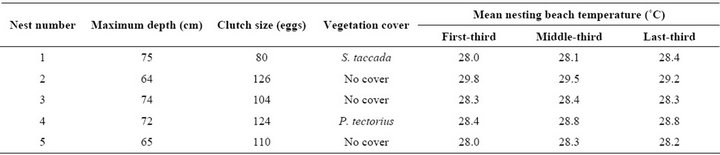
Table 2. Characteristics of five representative nests of Chelonia mydas nested during May-August, 2011 at Huyong Island, the Similan Islands.
1(a)) and average temperature profile (Figure 1(b)) showed similar pattern of fluctuation throughout the 58- day period. Temperature of a nest with no vegetation cover (No. 2), seemed to be higher than other nests. However, the role of vegetation cover on beach temperature is not yet conclusive since other two nests (No. 3 and 5) with no vegetation cover showed no different temperature profile from nests with vegetation cover (No. 1 and 4).
Based on average daily temperature, temperature during the middle-third period or a temperature-sensitive period ranged from 28.3˚C ± 0.6˚C to 28.9˚C ± 0.5˚C (Figure 1(b)). Mean nesting beach temperature during the first-third, the middle-third and the last-third of incu- bation period was 28.5˚C, 28.6˚C, and 28.6˚C, respec- tively. There was no significant difference among periods (ANOVA, p > 0.05; Figure 2).
4. Discussion
Compared to previous records of an annual nesting rate during 1997-2001 (54 ± 30 nests with an average clutch size of 111 ± 3 eggs [7]), nesting activity of C. mydas during this 4-month period in 2011 (25 nests with an average clutch size of 105 ± 25) was within a normal range for this site. However, long term monitoring program should be carried out to verify whether the nesting activity at this location would be affected by regional climate change.
Similar trend of temperature profiles in these 5 nests suggested that variation of nest depths from 64 to 75 cm mildly affected the temperature profile of the nesting beach. This is similar to previous studies that recorded nest temperature at 50-cm depth and found small degree of fluctuation [8-10]. The role of vegetation cover on nesting beach temperature profile was inconclusive in this study. However, a study by Van De Merwe et al. [10] showed that temperature profiles between two different shading areas (70% vs. 100%) were not significantly different. It seemed that difference in nest environment did not influence the nest temperature pattern. As a result, further study on contribution of other physical factors
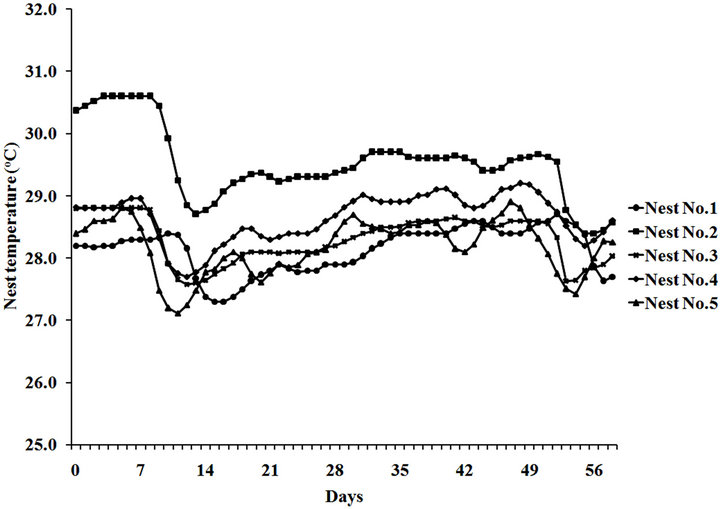 (a)
(a)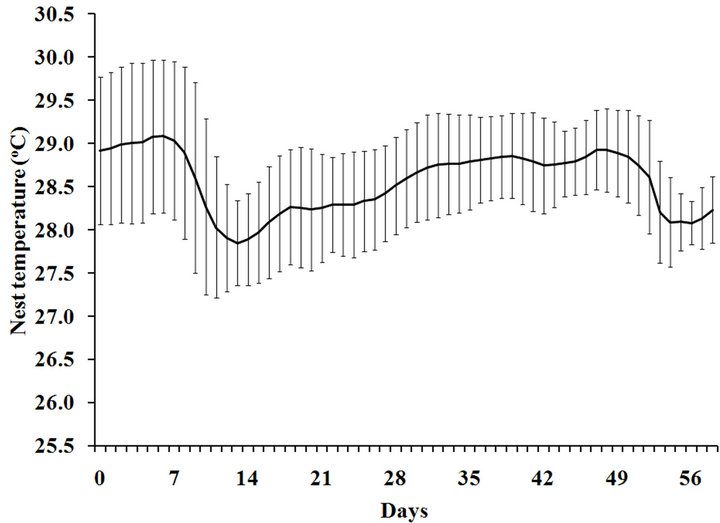 (b)
(b)
Figure 1. Temperature profile at the maximum depth of five representative nests (a) and an average profile (b) of the nesting beach used by Chelonia mydas during MayAugust, 2011 at Huyong Island, the Similan Islands.
including sand properties, moisture content, etc. on thermal profile of the nesting beach should be further examined.
Many research suggested that mean nest temperature at the middle-third period of incubation was the best parameter for prediction of sex ratio since it coincides with an occurrence of temperature sensitive period of embryo [11]. In this study, mean nest temperature at the middlethird period ranged from 28.3˚C to 28.9˚C. Compared to
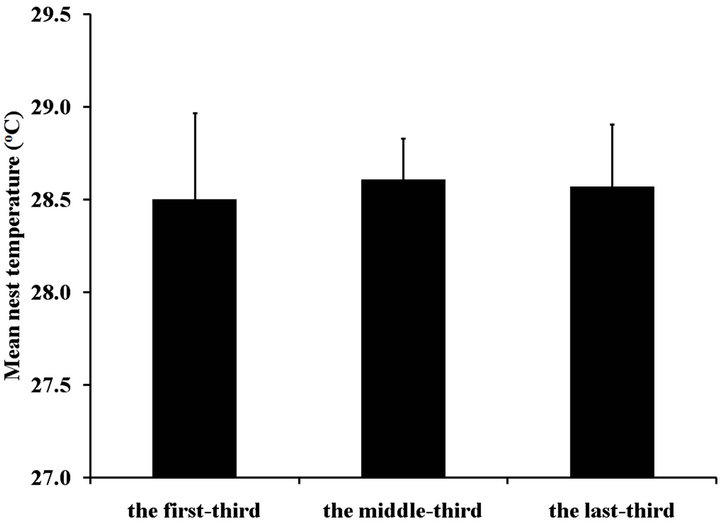
Figure 2. Mean nesting beach temperature at three incubation periods of Chelonia mydas eggs during May-August, 2011 at Huyong Island, the Similan Islands. No significant difference was found among these incubation periods (ANOVA, p > 0.05).
the pivotal temperature of C. mydas population at the Suriname (29.2˚C - 29.5˚C) [12], it could be deduced that slightly male-biased sex ratio would occur with the Huyong Island population. However, in turtle with temperature-dependent sex determination, the pivotal temperature for sex determination could be varied among species, populations, and nests of turtles [3,12,13]. Further study to find the exact pivotal temperature of the green turtle at Huyong Island is needed in order to predict sex ratio with more accuracy. Since sex identification based on external morphology of hatchling is unlikely [4], combination of direct observation from gonadal histology and indirect sexing technique based on hormone measurement [14] should be pursued in order to provide tools for the study of this biological consequence from global and regional climate change.
5. Conclusion
Compared to the earlier records during 1997-2001, normal nestings of C. mydas were found at Huyong Island, the Similan Islands during May to August 2011. Temperature profile of the nesting beach showed similar trend among nests with no clear influence of the vegetation cover. Mean nest temperature at the middle-third period or corresponding to the temperature-sensitive period ranged from 28.3˚C to 28.9˚C, suggesting a slightly male-biased sex ratio of the offspring. Overall, temperature profile of the nesting beach indicated little or no effects of regional increase in temperature on nesting activity and egg incubation of the green turtle at this period.
6. Acknowledgements
This research was supported by the Plant Genetic Conservation Project under the Royal Initiative of HRH Princess Maha Chakri Sirindhorn—Replied by Chulalongkorn University. We would like to thank the Royal Thai Navy for transportation, accommodation and other supports during our stay at Huyong Island.
REFERENCES
- International Union for Conservation of Nature and Natural Resources (IUCN), “IUCN Red List of Threatened Species, Version 2012.2,” 2012. http://www.iucnredlist.org
- Intergovernmental Panel on Climate Change (IPCC), “Climate Change 2007: Synthesis Report, Contribution of Working Group I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change,” IPCC, Geneva, 2007, 104 p.
- E. D. Standora and J. R. Spotila, “Temperature Dependent Sex Determination in Sea Turtles,” Copeia, Vol. 1985, No. 3, 1985, pp. 711-722. doi:10.2307/1444765
- T. Wibbels, “Critical Approaches to Sex Determination of Sea Turtles,” In: P. L. Lutz, J. A. Musick and J. Wyneken, Eds, The Biology of Sea Turtles, Vol. 2, CRC Press, Boca Raton, 2003, pp. 103-134.
- F. J. Janzen and P. C. Phillips, “Exploring the Evolution of Environmental Sex Determination, Especially in Reptiles,” Journal of Evolutionary Biology, Vol. 19, No. 6, 2006, pp. 1775-1784. doi:10.1111/j.1420-9101.2006.01138.x
- K. Kittiwattanawong, “Biology and Conservation of Green Turtle Chelonia mydas in Thailand,” DSc. Dissertation, Kyoto University, Kyoto, 2004.
- W. Klom-In, “Nesting Location of the Green Sea Turtle (Chelonia mydas) at the Huyong Island: Biology and Conservation,” Naval War College, Institute of Advanced Naval Studies, Nakhon Pathom, 2002, 103 p. (in Thai)
- R. A. Ackerman, “The Nest Environment and the Embryonic Development of Sea Turtles,” In: P. L. Lutz and J. A. Musick, Eds, The Biology of Sea Turtles, Vol. 1, CRC Press, Boca Raton, 1997, pp. 83-106.
- Y. Kaska, R. Downie, R. Tippett and R. W. Furness, “Natural Temperature Regimes for Loggerhead and Green Turtle Nests in the Eastern Mediterranean,” Canadian Journal of Zoology, Vol. 76, No. 4, 1998, pp. 723-729.
- J. Van De Merwe, K. Ibrahim and J. Whittier, “Effect of Nest Depth, Shading, and Metabolic Heating on Nest Temperatures in Sea Turtle Hatcheries,” Chelonian Conservation and Biology, Vol. 5, No. 2, 2006, pp. 210-215. doi:10.2744/1071-8443(2006)5[210:EONDSA]2.0.CO;2
- J. D. Miller and C. Limpus, “Ontogeny of Marine Turtle Gonads,” In: P. L. Lutz, J. A. Musick and J. Wyneken, Eds, The Biology of Sea Turtles, Vol. 2, CRC Press, Boca Raton, 2003, pp. 199-224.
- M. H. Godfrey and N. Mrosovsky, “Pivotal Temperature for Green Sea Turtle, Chelonia mydas, Nesting in Suriname,” Herpetological Journal, Vol. 16, No. 1, 2006, pp. 55-61.
- A. C. Broderick, B. J. Godley, S. Reece and J. R. Downie, “Incubation Periods and Sex Ratios of Green Turtles: Highly Female Biased Hatchling Production in the Eastern Mediterranean,” Marine Ecology Progress Series, Vol. 202, 2000, pp. 273-281. doi:10.3354/meps202273
- Z. Xia, P. Li, H. Gu, J. J. Fong and E. Zhao, “Evaluating Noninvasive Methods of Sex Identification in Green Sea Turtle (Chelonia mydas) Hatchlings,” Chelonian Conservation and Biology, Vol. 10, No. 1, 2011, pp. 117-123. doi:10.2744/CCB-0852.1
NOTES
*Corresponding author.

