Open Journal of Gastroenterology
Vol.4 No.1(2014), Article ID:42396,5 pages DOI:10.4236/ojgas.2014.41007
Early peak of hydrogen during lactose breath test predicts intestinal motility
![]()
1Department of Internal Medicine, Catholic University, Rome, Italy
2Department of Surgery, Catholic University, Rome, Italy
Email: veronica.ojetti@tin.it
Copyright © 2014 Veronica Ojetti et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Veronica Ojetti et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received 6 December 2013; revised 6 January 2014; accepted 18 January 2014
ABSTRACT
Lactose breath test (LBT) is considered the gold standard for the diagnosis of lactose malabsorption. The test is considered positive for a peak of hydrogen (H2) ≥ 20 parts per million (ppm) above the baseline. Some patients (pts) showed a rapid peak between 30 and 90 minutes after lactose ingestion. The aim of this study was to evaluate the predictive value of an early peak during a LBT and an accelerated oro-cecal transit time (OCTT). We retrospectively analyzed all pts who referred to our Gastroenterology unit for Irritable Bowel Syndrome, from January to September 2012, who performed LBT, glucose and lactulose breath test. We consider a positive LBT for a peak of H2 > 20 ppm, a positive GHBT for a peak >12 ppm and we considered a normal OCCT a peak of H2 ≥ 10 ppm between 75 ± 105 min after lactulose load. The correlation between LBT and OCTT was evaluated by Pearson score. 93 pts (65 F/28 M mean age 47 ± 6 years) with a positive LBT, without small intestinal bacterial overgrowth were analyzed: 46 pts (32 F/14 M; mean age 48 ± 6 years) with an early peak (<90 min) of H2 (≥20 ppm) were enrolled as case, and 47 pts matched for sex and age with a peak of H2 after 90 min were enrolled as controls. 72% (33/46) of the group with an early peak showed an accelerated, 17% (8/46) a normal and 11% (5/46) a delayed OCTT. Meanwhile, in control group 40.4% (19/47) showed a normal, 57.5% (27/47) a delayed and just 1 pts an accelerated OCTT. The specificity and sensibility of LBT for an accelerated OCTT were 97.9% and 71.7% respectively. The positive predictive value of LBT for an accelerated OCTT is 97.1%; the negative predictive value is 78%. There is a significant correlation between LBT and OCTT (p wang#Bracket## 0.05). The presence of an early peak of H2 between 30 and 90 min after the ingestion of 25 gr of lactose could predict the presence of an accelerated OCTT in 97% of pts. If confirmed by further study, in this subset of pts, lactulose breath test for evaluating OCTT could be avoided.
KEYWORDS
Lactose Breath Test; Transit Time; IBS; Hydrogen
1. INTRODUCTION
Lactose malabsorption is a very common condition in which lactase, the enzyme of the brush border membrane of enterocytes that hydrolyzes lactose into galactose and glucose, is deficient [1]. Approximately 1/3 of patients with hypolactasia complain gastrointestinal symptoms such as abdominal pain, flatulence and diarrhea, configuring the framework of lactose intolerance. Lactose intolerance is present in up to 15% of people of Northern European descent, up to 80% of Black Latinos, and up to 100% of American Indians and Asians [2].
The lactose breath test (LBT) is the most common test used in clinical practice for the diagnosis of lactose malabsorption; it is a very simple, low-cost and useful test with a good specificity (89% - 100%) and sensitivity (69% - 100%) [3,4]. The LBT is commonly performed with an oral lactose load of 25 gr after measuring a baseline breath sample. Every 30 minutes after the ingestion of carbohydrate for four hours, breath samples are taken and analyzed for the presence of hydrogen and/or methane in parts per million (ppm) via gas-chromatograph. The absolute rise in hydrogen varies, but the test is considered abnormal if hydrogen rises more than 20 ppm above baseline [5].
The breath test is based on the assumption that if all the lactose is absorbed in the small intestine, there should be no spill over of lactose into the colon [6]. In lactasedeficient subjects, the colonic flora metabolizes lactose into hydrogen and short-chain fatty acids. The hydrogen can traverse the intestinal mucosa and be absorbed into the systemic circulation, and then excreted troughs the lungs [3,4,7,8].
The peak of hydrogen after administration of lactose varies over time, and depends largely on gastric emptying and oro-cecal transit time (OCTT); usually there is an increase higher than 20 ppm after 120 minutes.
The OCTT can be determined through various methods: scintigraphic, radiological, with the use of isotopically labelled 13C breath test or through the determination of hydrogen exhaled [9].
The scintigraphic method with chromium ethylenediamine tetraacetic acid (EDTA) is considered the gold standard even though it may be done in a few specialized centres with the departments of nuclear medicine. The evaluation of the intestinal transit time through the oral administration of radiopaque markers is a simple method but requires time and is poorly available in the centres of radiology [10].
The breath test using 13C-lactose-ureide is a valid alternative to scintigraphic techniques for measuring OCTT. In particular, breath samples were obtained every 10 - 15 min for 10 h and measured by isotope ratio mass spectrometry [11].
Glucose breath test (GHBT) is commonly used for the diagnosis of small intestinal bacterial overgrowth (SIBO) [12]. Lactulose hydrogen breath test (LHBT) is widely used as a non-invasive test for the detection of OCTT. The main criticism of this test is that the exact location of the intestinal segment, where the substrate is metabolized, is unclear. OCTT is defined as the time elapsing between lactulose ingestion and a sustained increase in H2 excretion of >10 ppm above the baseline value, which is about 90 ± 15 min in normal subjects. Therefore, OCTT is calculated on the basis of the colic peak of H2 excretion [13].
The aim of this study was to evaluate the predictive value of an early peak during a LBT and an accelerated OCTT.
2. METHODS
We retrospectively analyzed all patients (pts) who referred to our Gastroenterology Unit, from January to September 2012, for irritable bowel syndrome (IBS) who were submitted to LBT, GHBT and LHBT.
The diagnosis of IBS was performed according to Rome III criteria.
93 pts (65F/28M; mean age 47 ± 6 yrs) with a positive LBT, without SIBO were analyzed: 46 pts (32F/14M; mean age 48 ± 6 yrs) with an early peak (before 90 min) of H2 (≥20 ppm) were used as case, 47 pts matched for sex and age with a peak of H2 (≥20 ppm) after 90 min were used as controls.
2.1. Lactose Breath Test (LBT)
To minimize the basal hydrogen excretion, patients were asked to have a carbohydrate-restricted dinner on the day before the test and to be fasting for at least 12 h on the testing day. Before starting the test, patients did a mouth wash with 20 ml of chlorhexidine 0.05%. Smoking and physical exercise were not allowed for 30 min before and during the test. End-alveolar breath samples were collected immediately before lactose ingestion. Then a dose of 25 g of lactose was administered and breath samples were taken every 30 min for 4 h using a two-pack system. The two bags system includes a mouthpiece, a T valve and two bags, the first collects dead-space exhaled air, the latter takes alveolar air. The air samples were aspirated from the latter bag with a 30 ml plastic syringe and immediately analyzed using a model Quintron Gas Chromatograph (Breath Traker Quintron Instrument Company, Milwaukee, WI, USA). Results were expressed as parts per million (ppm).
LBT was considered positive for lactose malabsorption when an increase in H2 value more than 20 ppm over the baseline value was registered [13].
2.2. Glucose Breath Test (GHBT)
The procedures for preparing the test are the same as for the LBT.
After providing a baseline breath sample, subjects ingested 50 g of glucose in 200 ml water. Breath samples were taken every 15 minutes for 2 hours.
The method of collection of breath and the dosage was the same used for the lactose breath test.
The criteria for a positive SIBO was represented by a rise in H2 > 12 ppm [13].
2.3. Lactulose Breath Test (LHBT)
The procedures for preparing the test are the same as for the LBT.
After providing a baseline breath sample, subjects ingested 10 g of lactulose in 200 ml water. Breath samples were taken every 15 minutes for 4 hours.
The method of collection of breath and the dosage was the same used for the lactose breath test.
We considered a normal OCTT a peak of hydrogen ≥10 ppm between 75 ± 105 min after lactulose load [13].
2.4. Gastrointestinal Symptoms
76 out of 93 pts (80% of overall pts) were lactose intolerant. As regards bowel habits, 93% of our cases present an IBS-Diarrhea; meanwhile 73% of control presents an IBS-Constipation.
2.5. Statistical Analysis
The correlation between LBT and OCTT was evaluated by Pearson score. P values <0.05 were considered to be significant. Data concerning H2 excretion and clinical score were expressed as means ± SD. All scatter plots were carried out using Statistical Package for Social Sciences (SPSS12.0) for Windows.
3. RESULTS
46 pts (32F/14M; mean age 48 ± 6 yrs) with early peak of H2 (≥20 ppm) were enrolled as cases (group A), and 47 pts, matched for sex and age, with a peak of H2 (≥20 ppm) after 90 min were enrolled as controls (group B). We excluded in all patients, the presence of SIBO through the GHBT.
In the group A: 72% (33/46) showed an accelerated (mean 52 ± 12.2), 17% (8/46) a normal (mean 84.6 ± 9.08) and 11% (5/46) a delayed (mean 227.4 ± 25.8) OCTT. Meanwhile, in group B: 40.4% (19/47) showed a normal (mean 93 ± 12), 57.5% (27/47) a delayed (mean 177 ± 35.1) and just 1 pts (2.1%) an accelerated OCTT (Table 1; Figure 1).
The specificity and sensitivity of LBT for determining an accelerated OCTT was 97.9% and 71.7% respectively.
The positive predictive value of LBT of determining an accelerated OCCT is 97.1%; the negative predictive value is 78%. There is a significant correlation between LBT and OCTT (p < 0.05).
4. CONCLUSIONS
Our study showed for the first time a correlation between an early peak of hydrogen on lactose breath test and an accelerated OCTT.
In fact, the presence of an early peak of hydrogen within 90 minutes after ingestion of 25 grams of lactose has a positive predictive value of an accelerated OCTT of 97%.
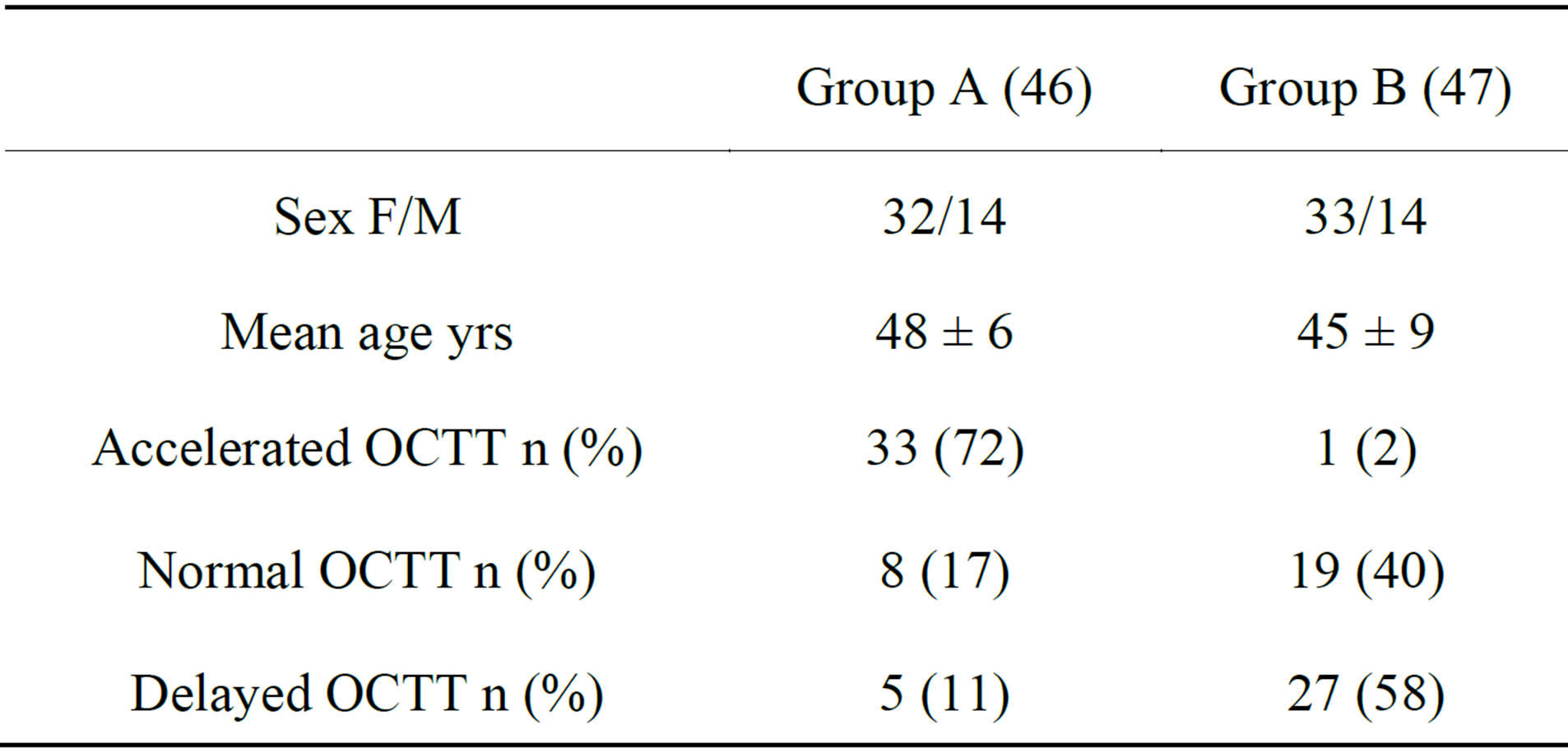
Table 1. Demographic characteristics of analyzed pts.
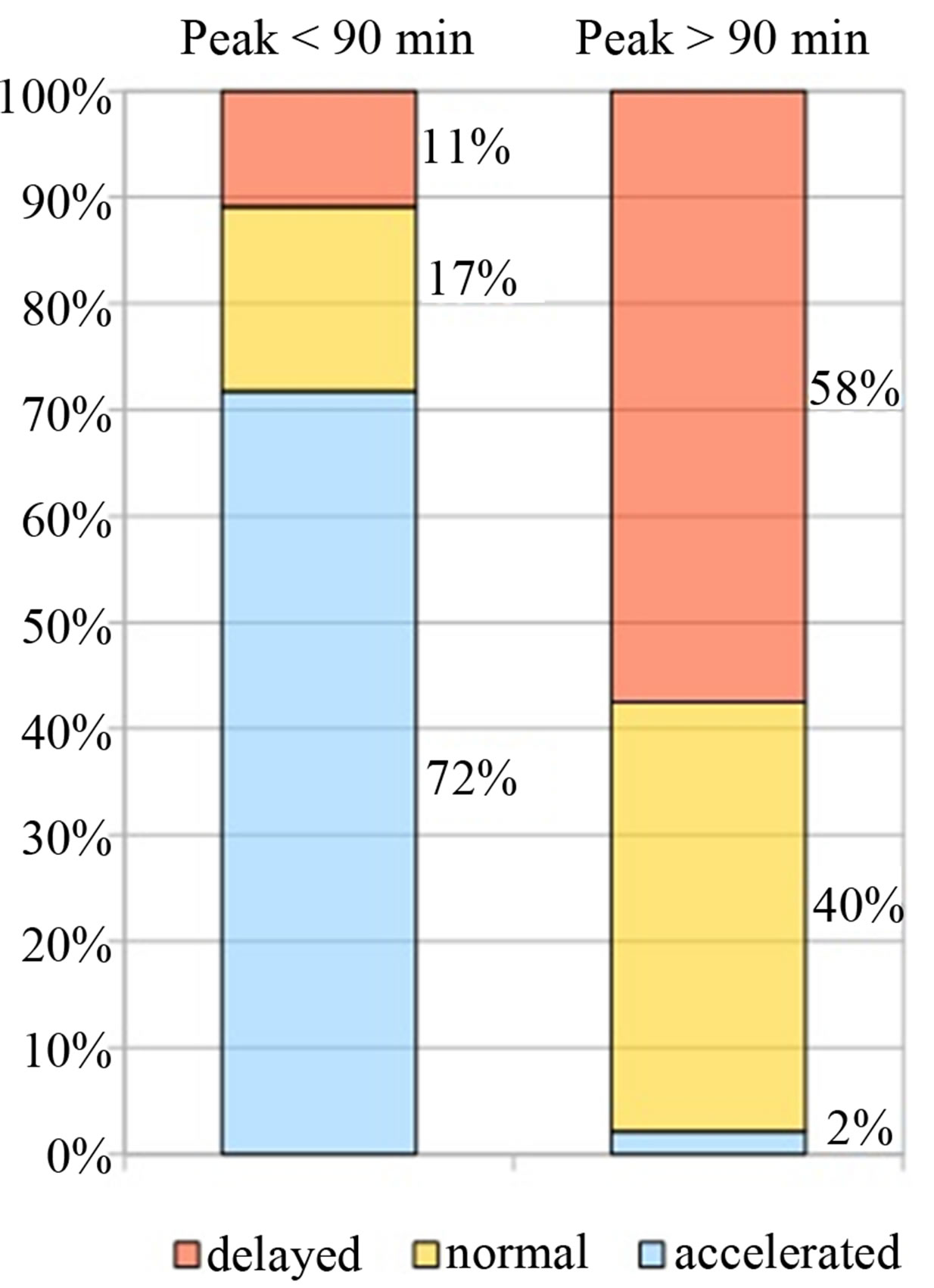
Figure 1. Correlation between H2 peak and OCTT.
In a Gastroenterology Unit many patients with IBS came with the prescription of many tests such as lactose, lactulose and glucose breath tests to better define their pathology. The possibility of having information on lactose malabsorption and OCTT with the execution of a single test should be able, if confirmed, to reduce waiting times and costs for the National Health System.
Many studies [4] in literature had evaluated the correlation between OCTT and gastric emptying with the development of lactose intolerance symptoms during a breath test [14,15]. It is well known, in fact, that gastric emptying rate and intestinal transit time alters the time in which lactose is exposed to intestinal lactase.
In particular an interesting paper, who evaluated the relationship between lactose digestion, gastrointestinal transit time and symptoms in lactose malabsorbers after dairy consumption, reveals a strong inverse correlation between gastrointestinal symptoms displayed and OCTT. The authors suggest that when intestinal transit time increases, lactose digestion and tolerance symptoms improve [15].
Other studies confirm a direct link between motility and symptoms; in fact, the slowing down of OCTT was associated with less severe symptoms of lactose intolerance [16,17].
Glucose breath test is a simple test commonly used for the non invasive diagnosis of SIBO.
We excluded, in our study, pts with a diagnosis of SIBO because it is well described in the literature [18] that during bacterial overgrowth, subjects may have lactose intolerance not by enzyme deficiency but to premature exposure of lactose to bacteria in the small bowel before there is time for a correct absorption [19-22]. This was further validated by Nucera et al., who demonstrated in 98 subjects with IBS that small intestinal bacterial overgrowth eradication, as confirmed by negative lactulose breath test, caused a significant reduction in lactose breath test positivity [23].
We have therefore deliberately excluded this confounder.
A recent study showed some interesting correlations between the results obtained during the lactulose and lactose or fructose breath test.
They showed that the peak and magnitude of hydrogen after lactulose load significantly correlated with the peak response both to fructose and lactose ingestion, moreover the time of the first rise during lactulose breath test, might also give insight into transit time. In clinical practice, this can affect the duration of the subsequent breath tests, especially in patients with a late rise (later than 120 min) in which the sugar tests must be prolonged [13].
Our data also confirm a close correlation between the time of the peak of hydrogen after the administration of lactose and the peak after administration of lactulose.
One of the most important limitation of our study is that it’s a single centre and retrospective study. Surely these data need further studies carried out on a larger number of patients and confirmed with the aid of further specific tests for the determination of the oro-cecal transit time.
In conclusion, our study addressed important issues in the interpretation of lactose breath test, which have attracted a resurgence of interest in fact an early peak of hydrogen >20 ppm within 90 minutes after lactose ingestion correlate significantly with an accelerated OCTT.
REFERENCES
- Campbell, A.K., Waud, J.P. and Matthews, S.B. (2009) The molecular basis of lactose intolerance. Science Progress, 92, 241-287. http://dx.doi.org/10.3184/003685005783238408
- Vesa, T.H., Marteau, P. and Korpela, R. (2000) Lactose intolerance. Journal of the American College of Nutrition, 19, 165S-175S. http://dx.doi.org/10.1080/07315724.2000.10718086
- Metz, G., Jenkins, D.J., Peters, T.J., Newman, A. and Blendis, L.M. (1975) Breath hydrogen as a diagnostic method for hypolactasia. The Lancet, 1, 1155-1157. http://dx.doi.org/10.1016/S0140-6736(75)93135-9
- Pimentel, M., Kong, Y. and Park, S. (2003) Breath testing to evaluate lactose intolerance in irritable bowel syndrome correlates with lactulose testing and may not reflect true lactose malabsorption. The American Journal of Gastroenterology, 98, 2700-2704. http://dx.doi.org/10.1016/S0002-9270(03)01703-9
- Ghoshal, U.C. (2011) How to interpret hydrogen breath tests. Journal of Neurogastroenterology and Motility, 17, 312-317. http://dx.doi.org/10.5056/jnm.2011.17.3.312
- Law, D., Conklin, J. and Pimentel, M. (2010) Lactose intolerance and the role of the lactose breath test. The American Journal of Gastroenterology, 105, 1726-1728. http://dx.doi.org/10.1038/ajg.2010.146
- Hovde, O. and Farup, P.G. (2009) A comparison of diagnostic tests for lactose malabsorption—Which one is the best? BMC Gastroenterology, 9, 82. http://dx.doi.org/10.1186/1471-230X-9-82.
- Beyerlein, L., Pohl, D., Delco, F., Stutz, B., Fried, M. and Tutuian, R. (2008) Correlation between symptoms developed after the oral ingestion of 50 g lactose and results of hydrogen breath testing for lactose intolerance. Alimentary Pharmacology & Therapeutics, 27, 659-665. http://dx.doi.org/10.1111/j.1365-2036.2008.03623.x
- Yu, D., Cheeseman, F. and Vanner, S. (2011) Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut, 60, 334-340. http://dx.doi.org/10.1136/gut.2009.205476
- Szarka, L.A. and Camilleri, M. (2012) Methods for the assessment of small-bowel and colonic transit. Seminars in Nuclear Medicine, 42, 113-123. http://dx.doi.org/10.1053/j.semnuclmed.2011.10.004
- Christian, M., Morrison, D., Dodson, B., Preston, T., Amarri, S., Franchini, F., Edwards, C. and Weaver, L. (2002) Measurement of oro-cecal transit time in young children using lactose [13C] ureide requires further validation. Journal of Pediatric Gastroenterology & Nutrition, 34, 570-571; author reply 571. http://dx.doi.org/10.1097/00005176-200205000-00024
- Rana, S.V., Sharma, S., Kaur, J., Sinha, S.K. and Singh, K. (2012) Comparison of lactulose and glucose breath test for diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Digestion, 85, 243-247. http://dx.doi.org/10.1159/000336174
- Gasbarrini, A., Corazza, G.R., Gasbarrini, G., Montalto, M., Di Stefano, M., Basilisco, G., Parodi, A., Usai-Satta, P., Vernia, P., Anania, C., Astegiano, M., Barbara, G., Benini, L., Bonazzi, P., Capurso, G., Certo, M., Colecchia, A., Cuoco, L., Di Sario, A., Festi, D., Lauritano, C., Miceli, E., Nardone, G., Perri, F., Portincasa, P., Risicato, R., Sorge, M., Tursi, A. and 1st Rome H2-Breath Testing Consensus Conference Working Group (2009) Methodology and indications of H2-breath testing in gastrointestinal diseases: The Rome Consensus Conference. Alimentary Pharmacology & Therapeutics, 29, 1-49. http://dx.doi.org/10.1111/j.1365-2036.2009.03951.x
- Savarino, E., Mei, F., Parodi, A., Ghio, M., Furnari, M., Gentile, A., Berdini, M., Di Sario, A., Bendia, E., Bonazzi, P., Scarpellini, E., Laterza, L., Savarino, V. and Gasbarrini, A. (2013) Gastrointestinal motility disorder assessment in systemic sclerosis. Rheumatology (Oxford), 52, 1095-1100. http://dx.doi.org/10.1093/rheumatology/kes429
- Labayen, I., Forga, L., González, A., Lenoir-Wijnkoop, I., Nutr, R. and Martínez, J.A. (2001) Relationship between lactose digestion, gastrointestinal transit time and symptoms in lactose malabsorbers after dairy consumption. Alimentary Pharmacology & Therapeutics, 15, 543-549. http://dx.doi.org/10.1046/j.1365-2036.2001.00952.x
- Vesa, T.H., Marteau, P.R., Briet, F.B., Boutron-Ruault, M.C. and Rambaud, J.C. (1997) Raising milk energy content retards gastric emptying of lactose in lactoseintolerant humans with little effect on lactose digestion. The Journal of Nutrition, 127, 2316-2320.
- Lomer, M.C., Parkes, G.C. and Sanderson, J.D. (2008) Review article: Lactose intolerance in clinical practice— Myths and realities. Alimentary Pharmacology & Therapeutics, 27, 93-103. http://dx.doi.org/10.1111/j.1365-2036.2007.03557.x
- Bate, J.P., Irving, P.M., Barrett, J.S. and Gibson, P.R. (2010) Benefits of breath hydrogen testing after lactulose administration in analysing carbohydrate malabsorption. European Journal of Gastroenterology & Hepatology, 22, 318-326. http://dx.doi.org/10.1097/MEG.0b013e32832b20e8
- Vernia, P., Di Camillo, M. and Marinaro, V. (2001) Lactose malabsorption, irritable bowel syndrome and selfreported milk intolerance. Digestive and Liver Disease, 33, 234-239. http://dx.doi.org/10.1016/S1590-8658(01)80713-1
- Goldstein, R., Braverman, D. and Stankiewicz, H. (2000) Carbohydrate malabsorption and the effect of dietary restriction on symptoms of irritable bowel syndrome and functional bowel complaints. The Israel Medical Association Journal, 2, 583-587.
- Pimentel, M., Chow, E.J. and Lin, H.C. (2003) Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: A doubleblind, randomized, placebo-controlled study. The American Journal of Gastroenterology, 98, 412-419. http://dx.doi.org/10.1016/S0002-9270(02)05902-6
- Parker, T.J., Woolner, J.T., Prevost, A.T., Tuffnell, Q., Shorthouse, M. and Hunter, J.O. (2001) Irritable bowel syndrome: Is the search for lactose intolerance justified? European Journal of Gastroenterology & Hepatology, 13, 219-225. http://dx.doi.org/10.1097/00042737-200103000-00001
- Nucera, G., Gabrielli, M., Lupascu, A., Lauritano, E.C., Santoliquido, A., Cremonini, F., Cammarota, G., Tondi, P., Pola, P., Gasbarrini, G. and Gasbarrini, A. (2005) Abnormal breath tests to lactose, fructose and sorbitol in irritable bowel syndrome may be explained by small intestinal bacterial overgrowth. Alimentary Pharmacology & Therapeutics, 21, 1391-1395. http://dx.doi.org/10.1111/j.1365-2036.2005.02493.x

