Open Journal of Gastroenterology
Vol.3 No.2(2013), Article ID:32195,9 pages DOI:10.4236/ojgas.2013.32020
Prospective study evaluating the value of subjective global assessment and national risk score 2002 for post-operative risk detection in living related donor liver transplant recipients
![]()
1Endemic Medicine and Hepatology Department, Faculty of Medicine, Cairo University, Cairo, Egypt
2Clinical Nutrition Consultant National Hepatology and Tropical Medicine Research Institute, Cairo, Egypt
3Biostatistics and Biomedical Informatics, Faculty of Medicine, Cairo University, Cairo, Egypt
Email: *walfou2000@gmail.com
Copyright © 2013 A. Abd Elrehim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 19 February 2013; revised 4 April 2013; accepted 7 May 2013
Keywords: Malnutrition; Subjective Global Assessment; National Risk Assessment-2002; Chronic Liver Disease; Liver Transplantation
ABSTRACT
Background: Chronic liver disease may be associated with protein energy malnutrition. Those malnourished patients undergoing liver transplantation suffer great morbidities and even mortalities. Estimating the degree of malnutrition in patients with end stage liver disease is a difficult job, Subjective Global Assessment (SGA) and Nutritional Risk Score-2002 (NRS- 2002) are among many tools that can give an overview for the nutritional status of the patients. Aim: To detect the efficacy and the predictive validity of SGA and NRS 2002 for post-operative risk detection for liver transplant patients. Patients & Methods: 30 recipients of end stage liver disease had undergone a nutritional assessment by SGA score & NRS-2002 score, to be compared with the parameters of outcome of post-operative liver transplantation (ALT, AST, INR, Bilirubin, time spent in ICU, hospital infective episodes & number of antibiotic courses). Results: Patients declared as malnourished by SGA and NRS-2002 had higher post operative ALT & AST value, more prolonged INR, spent more time at ICU and hospital, suffered from more infective episodes and had more antibiotic courses in a significant statistical manner. Conclusion: SGA and NRS-2002 could be useful, simple and dependable tools to be used for risk detection of post-operative morbidities after liver transplantation.
1. INTRODUCTION
Chronic liver disease is frequently associated with malnutrition, its severity ranges from subclinical micronutrient deficiencies to frank protein-calorie malnutrition. [1].
Malnutrition is usually caused by several factors: loss of appetite, malabsorption, and increased requirements. [2] Previous studies have shown that a significant proportion of patients with advanced liver disease have protein-calorie malnutrition due to a combination of poor diet and inability of the diseased liver to metabolize nutrients [3].
The presence of protein-calorie malnutrition has been associated with increased shortand long-term mortality in patients with acute and chronic liver diseases, [4] including patients undergoing liver transplantation [5].
Preoperatively malnutrition has been reported to be associated with increased operative blood loss, length of stay in intensive care unit, [6] mortality, [7] and total hospital charges after liver transplantation [8].
Much of the difficulty in identifying an optimal method for nutritional assessment in patients with cirrhosis arises from the fact that many of the traditionally measured parameters of nutritional status vary with severity of liver disease, [9] since many of the traditionally measured parameters of nutritional status such as weight, anergy panels and biochemical values, vary with the severity of liver disease independently of nutritional status [10].
Multiple techniques have been proposed to detect malnutrition in patients with liver disease; [11,12] howeversome common nutritional parameters can be misleading in advanced liver disease because of water retention and ascites [13], compromised protein synthesis [14] and coexisting alterations in the renal function [15].
Subjective global assessment (SGA) based both on the physical signs of malnutrition and nutritional history, has been utilized to evaluate the nutritional status in patients with chronic liver disease and, in particular, those awaiting liver transplantation (LT) [16].
Subjective Global Assessment (SGA) uses clinical criteria to determine nutritional status without the use of objective measurements that has been validated in liver transplant candidates [17].
However there is no consensus on the best scoring system for assessing the nutritional status of hospitalized patients. For this reason Kondrup et al. 2003 established a scoring system as part of ESPEN guidelines for nutrition screening-2002 (NRS-2002) [18].
2. STUDY OBJECTIVES
The current study was carried out for detecting the efficacy and the predictive validity of SGA and NRS-2002 for post-operative risk detection for liver transplant patients.
3. PATIENTS AND METHODS
Thirty patients with End Stage Liver Disease enrolled in our study from those who were scheduled for living donor liver transplantation (LDLT) at Dar-Alfouad liver transplant center. Inclusion criteria included patients with end stage liver disease either due to cholestatic diseases or chronic Hepatitis B or C. Exclusion criteria included those with HIV infection, active alcohol or substance abuse, systemic infections, life limiting co-existing medical conditions: advanced heart, lung or neurologic conditions, uncontrolled psychiatric disorder and in those with inability to comply with preand post-transplant regimens. Each subject had passed into 2 phases of assessment:
3.1. Phase I: (Pre-Transplant)
All demographic data (date of birth, sex, height, weight), medical history (including a complete dietary history), physical examination, co morbidities, and concomitant medications had been documented, as well as laboratory investigations including complete blood picture, liver biochemical profile, renal function test and C-reactive protein).
Patients were assessed according to the following:
1) Nutritional status assessment a) Subjective Global Assessment [19]
SGA comprises a nutritional evaluation of height, current weight, weight before illness, and weight change in the previous 6 months; nutritional history (appetite, intake, gastrointestinal symptoms); physical appearance (subjective assessment of fat loss, edema, muscle wasting, and ascites), and existing conditions (infections, encephalopathy, renal insufficiency).
A = well-nourished.
B = moderately (or suspected of being) malnourished.
C = severely malnourished.
b) Nutritional Risk screening-2002: [20]
The purpose of the NRS-2002 system is to detect the presence of malnutrition and the risk of developing the malnutrition at the hospital setting [21]. It is a grading system of the severity of disease as a reflection of increased nutritional requirements [22].
Degree of malnutrition will be recorded according to a grading scale questionnaire ranging from zero up to six (points) indicating the severity of the nutritional status of the recipient.
c) MELD Score and CHILD-PUGH classification: [23].
3.2. Phase II: (Post-Transplant)
The following clinical and biochemical end-points are used:
Graft rejection, clinically significant infective episodes, defined as the patient becoming unwell with a fever requiring systemic antibiotics (for bacterial infection) or anti-viral therapy and level of (CRP); time spent on the intensive therapy unit (ITU), in addition to number of used antibiotics courses, time spent at hospital (ICU and Ward) and recipient graft function assessed by (Parameters of outcome): peak Aspartate and Alanine aminotransferases (AST) & (ALT), peak serum Total bilirubin, maximum International Normalized Ratio (INR) during the 1st and 2nd weeks after living donor liver transplant, [24] and C-reactive protein (CRP).
The thirty patients were divided according to the results of SGA into two groups moderate and severely malnourished groups. For NRS-2002 patients were categorized into severely malnourished group (score ≥ 4) and mild to moderate malnourished groups (score < 4).
4. RESULTS
Patients’ data were analyzed using SPSS 17.0 for windows 7. Quantitative variables were expressed by mean and SD (Standard deviation), compared using t-student, paired t-test, Wilcoxon Signed Ranks Test or ANOVA test when appropriate. Pearson correlation for correlating quantitative variables and regression were used when appropriate. Qualitative variables were expressed by number (Frequency) and percent. p value was considered significant if less than 0.05. All patients enrolled in our study were indicated for liver transplantation where 60% of the studied patients were due to hepatitis C virus, 10% were due to cryptogenic cirrhosis, 3.3% hepatitis B virus, 3.3% combined hepatitis B, C viruses and 3.3% autoimmune hepatitis & 16.7% were due to Combined HCV and HCC. All of the studied patients were males with a mean age of 50.3 ± 4.85 ranging between 40 to 58 years old (Tables 1 and 2).
The patients were divided according to SGA score into two groups moderate (n = 16 with a mean age of 51.13 ± 4.37) and severely (n = 14 with a mean age of 49.36 ± 5.35) malnourished patients. There was a significant increase in the liver function tests in both groups after liver transplantation (Table 3) (Figures 1 and 2), the same goes when dividing the patients according to NRS-2002 score into two groups one with moderate malnutrition (Table 4) (Figures 3 and 4).
By comparing both moderate and severely malnourished patients, according to both SGA and NRS-2002, regarding number of infective episodes, number of antibiotics used, time spent in ICU after transplantation, it was found that the severely malnourished patients are significantly higher regarding these parameters than those of the moderately malnourished patients (Tables 5 and 6).
It was also found that SGA can predict the number of infective episode with r2 = 0.378 and p-value = 0.00 while NRS can predict it with r2 = 0.8 and p-value = 0.00, SGA can predict the number of antibiotics used during hospital stay after transplantation with r2 = 0.282 and p-value = 0.003, while NRS can predict it with r2 = 0.504 and p-value = 0.00, SGA can’t predict the time spent in ICU after transplantation where r2 = 0.008 and p-value = 0.6, while NRS can predict it with r2 = 0.258 and p-value = 0.004 (Tables 5 and 6) (Figure 5).
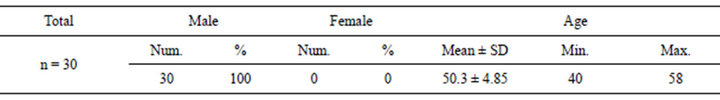
Table 1. Demographic features of the studied patients.
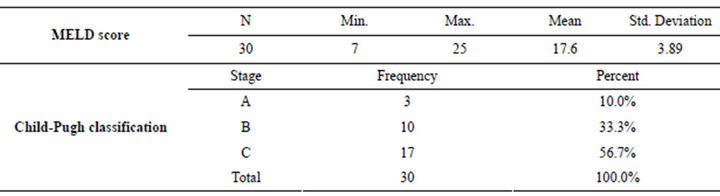
Table 2. Clinical scoring of studied patients.
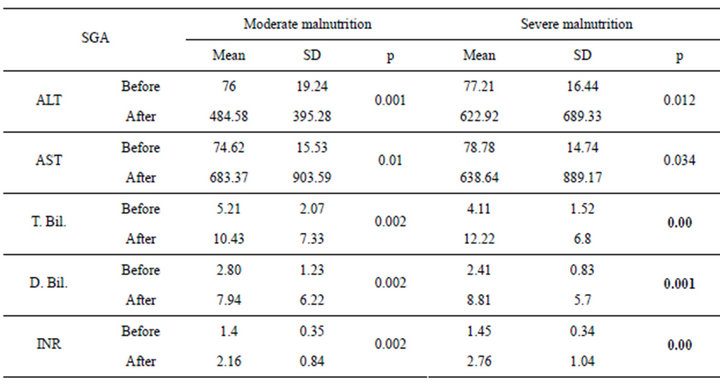
Table 3. SGA in relation to liver functions in pre and post transplantation.
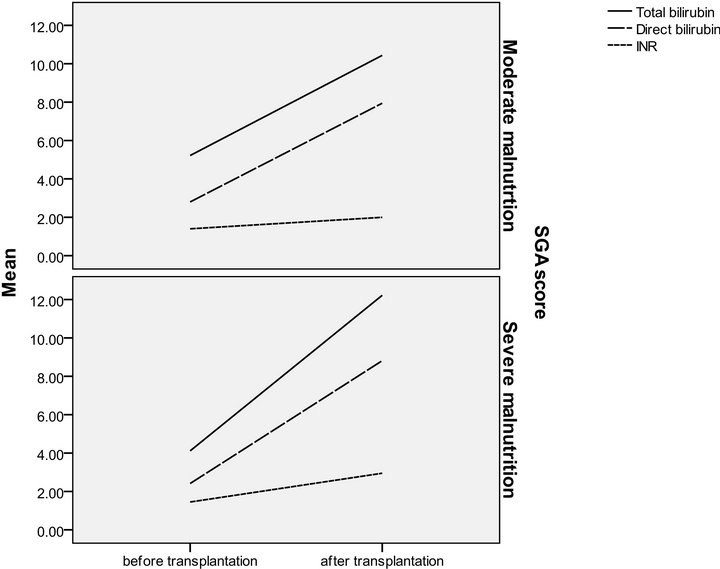
Figure 1. SGA in relation to serum bilirubin and INR in pre and post transplantation.
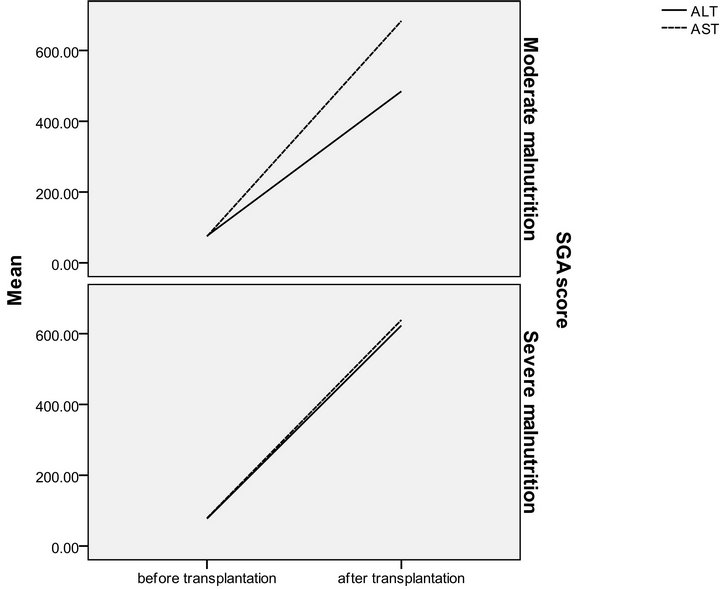
Figure 2. SGA in relation to serum ALT and AST in pre and post transplantation.
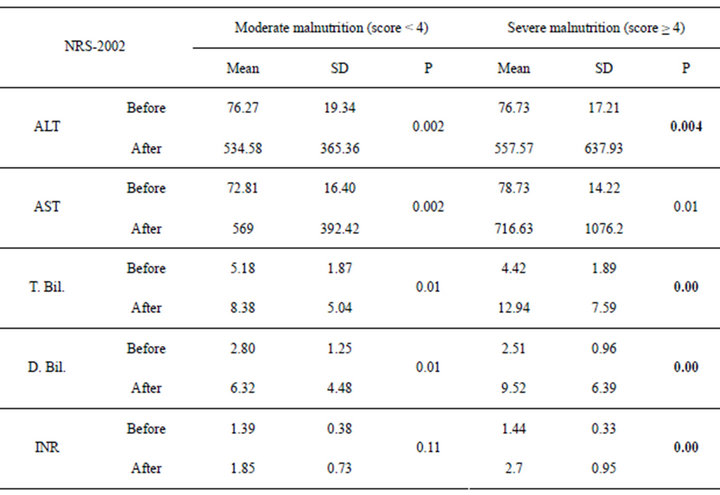
Table 4. NRS-2002 in relation to liver functions in pre and post transplantation.
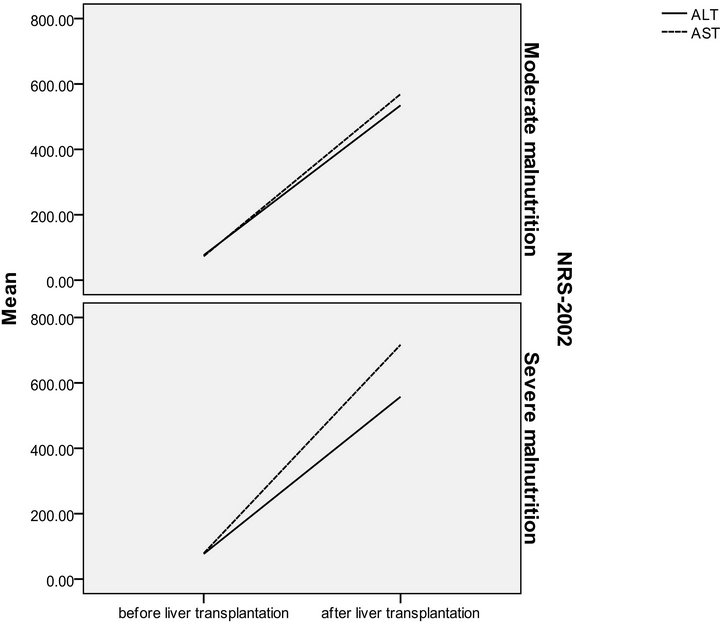
Figure 3. NRS-2002 in relation to ALT and AST in pre and post transplantation.
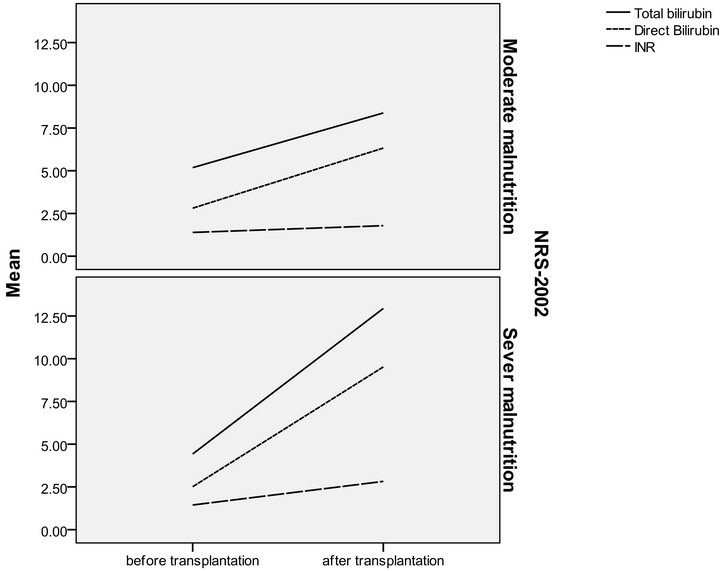
Figure 4. NRS-2002 in relation to serum bilirubin and INR in pre and post transplantation.
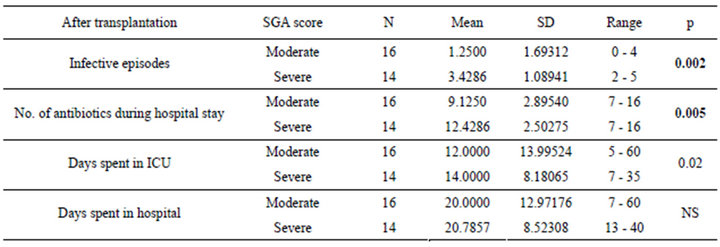
Table 5. Relation between SGA and post liver transplantation assessment parameters.
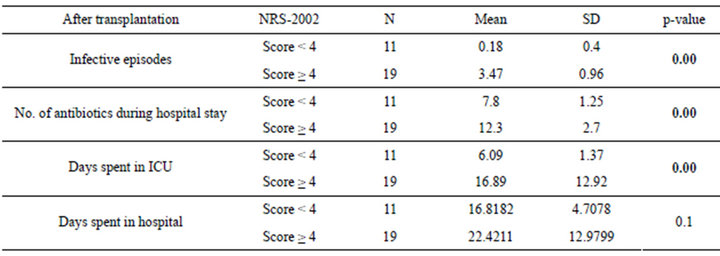
Table 6. Relation between NRS-2002 and post liver transplantation assessment parameters.
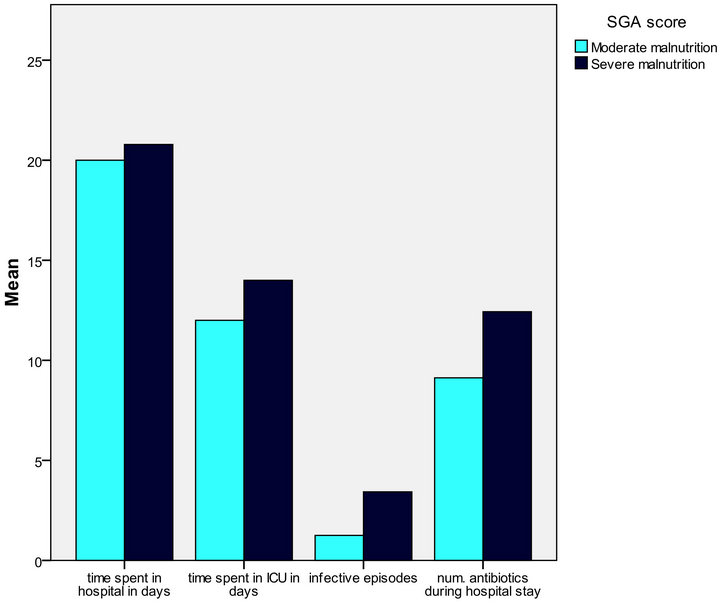
Figure 5. Relation between SGA and post liver transplantation assessment parameters (infective episodes and No. of antibiotics used in hospital stay was highly significant).
5. DISCUSSION
Chronic liver disease (CLD) is a major health problem affecting millions of people and resulting in large health care expenditures and economic losses [25]. Improvements in survival after liver transplantation have broadened the indications for its use as a proven therapy for ESLD, rapidly increasing the number of transplant candidates [26].
Protein energy malnutrition (PEM) is common in patients with ESLD and is highly prevalent in all forms of chronic liver disease, regardless of etiology and increases the risk of death [27].
Previous reports have focused their attention on the role of malnutrition as an independent risk factor on the outcome of LT [28,29] however, the results were controversial.
In our study, we found that patients with ESLD and severe malnutrition as defined by SGA had significantly longer lengths of stay in the ICU, and hospital after liver transplantation, in addition to higher number of infective episodes and hence, more frequent antibiotic courses were used. This could be explained by the lower immunity in malnourished recipients, and this goes with researches stressing on the importance of the immune nutrition for hepatic patients [30]. On the other hand, Figueiredo et al. 2000, found that the SGA alone is not able to identify patients with low body cell mass, an important index of malnutrition [31]. In addition to being subjective, indefinite, better to describe the nutritional status rather to be a risk screening tool and relies on the experience of the operator [32].
When using NRS-2002, we found that recipients who had higher scores (≥4) had longer ICU stay; more infective episodes, and more frequent antibiotics were used. In addition to this, it was significantly related to some post-operative measures that tend to track malnutrition like total bilirubin and INR.
Shaw and colleagues examined the effect of a number of variables on 6-months survival after liver transplantation in 160 patients. There were six factors having prognostic significance: operative blood loss, coma score, malnutrition (subjectively assessed on a three point scale), serum bilirubin, PT and date of transplant. The most significant of these that could potentially be corrected was nutritional status [33].
Perioperative mortality was low in our series; no correlation could therefore be proven between malnutrition and mortality. Episodes of infections, the period spent in ICU and the total days spent in hospital were all strongly influenced by the recipients’ nutritional status. The main shortcoming of our study was the relatively small number of patients, which may thus be subject to an inadvertent selection bias. Selberg et al. 1997 found that patients with a better nutritional status at transplantation had improved survival rates after LT [34].
A series of cirrhotic patients with a prevalence of cholestatic liver disease, showed that malnourished cases were at a higher risk for post-operative complications; however, the time spent in the ICU and the total time spent in the hospital were not different in well-nourished and in malnourished patients [35].
Regarding the predictive ability, SGA predicted the postoperative number of infective episodes and the number of antibiotics used during postoperative stay while NRS can predict the postoperative number of infective episodes, the number of antibiotics used during postoperative stay and the time spent in ICU after transplantation. Deluis et al. 2006 had seen no predictive value [29] while Merli et al. 2010 showed a predictive ability of SGA for postoperative infective episodes, hospital stay and ICU admission duration [36]. Also, Stephenson et al. 2001 had detected a significant predictive value of SGA with hospital stay and intraoperative blood usage [16].
We couldn’t detect any correlation between Child and MELD scores with any of the parameters of post-operative outcome. This might be related to the fact that both scores are devoid from any nutritional parameters, which are considered very powerful predictive factors and evidently affects the outcome.
6. CONCLUSION
SGA and NRS-2002 could be useful, simple and dependable tools to be used for risk detection of post-operative morbidities after liver transplantation.
7. ACKNOWLEDGEMENTS
Dar Al Fouad liver transplantation unit.
REFERENCES
- Mendenhall, C.L., Moritz, T.E., Roselle, G.A., et al. (1995) Protein energy malnutrition in severe alcoholic hepatitis: Diagnosis and response to treatment. Journal of Journal of Parenteral and Enteral Nutrition, 19, 258-265. doi:10.1177/0148607195019004258
- Kondrup, J. (2007) Nutritional support in chronic liver disease. ESPEN, Module 13.2.
- Porayko, M.K., DiCecco, S. and O’Keefe, S.J. (1991) Impact of malnutrition and its therapy on liver transplantation. Seminars in Liver Disease, 11, 305-314. doi:10.1055/s-2008-1040448
- Mendenhall, C., Tosch, T., Weesner, R. (1986) VA cooperative study on alcoholic hepatitis: Prognostic significance of protein calorie malnutrition. American Journal of Clinical Nutrition, 43, 213.
- Selberg, O., Bottcher, J., Tusch, G., Pichlmayr, R., Henkel, E. and Muller, M. (1997) Identification of highand low-risk patients before liver transplantation: A prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology, 25, 652. doi:10.1002/hep.510250327
- Ricci, P., Therneau, T., Malinchoc, M., et al. (1997) A prognostic model for the outcome of liver transplantation in patients with cholestatic liver disease. Hepatology, 25, 672. doi:10.1002/hep.510250330
- Lautz, H., Selberg, O., Korber, M. and Muller, M. (1992) Protein-calorie malnutrition in liver cirrhosis. Journal of Clinical Investigation, 70, 478. doi:10.1007/BF00210228
- Kim, W.R., Therneau, T.M., Dickson, E.R. and Evans, R.W. (1995) Preoperative predictors of resource utilization in liver transplantation. Clinical Transplantation, 1, 315.
- Harrison, J., McKiernan, J. and Neuberger, J.M. (1997) A prospective study on the effect of recipient nutritional status on outcome in liver transplantation. Transplant International, 10, 369-374. doi:10.1111/j.1432-2277.1997.tb00931.x
- De Luis, D.A., Izaola, O.M., Velicia, C, Antolín, G.S., et al. (2006) Impact of dietary intake and nutritional status on outcomes after liver transplantation. Revista Espanola de Enfermedades Digestivas (Madrid), 98, 6-13.
- Figueiredo, F.A., Dickson, E.R., Tousif, P., et al. (2000) Impact of nutritional status on outcomes after liver transplantation. Transplantation, 70, 1347-1352. doi:10.1097/00007890-200011150-00014
- Morgan, M.Y., Madden, A.M., Jennings, G., Elia, M. and Fuller, N.J. (2006) Two components models are of limited value for the assessment of body composition in patients with cirrhosis. American Journal of Clinical Nutrition, 84, 1151-1162.
- Plauth, M, Cabré, E., Riggio, O., et al. (2006) ESPEN guidelines on enteral nutrition: Liver disease. Clinical Nutrition, 25, 285-294. doi:10.1016/j.clnu.2006.01.018
- McCullough, A.J., Mullen, K.D. and Kalhan, S.C. (1991) Measurements of total body and extracellular water in cirrhotic patients with and without ascites. Hepatology, 14, 1102-1108. doi:10.1002/hep.1840140626
- Merli, M., Romiti, A., Riggio, O. and Capocaccia, L. (1987) Optimal nutritional indexes in chronic liver disease. Journal of Parenteral and Enteral Nutrition, 1, 130-134. doi:10.1177/014860718701100521
- Alberino, F., Gatta, A., Amodio, P., et al. (2001) Nutrition and survival in patients with liver cirrhosis. Nutrition, 17, 445-450. doi:10.1016/S0899-9007(01)00521-4
- Pikul, J., Sharpe, M.D., Lowndes, R. and Ghent, C.N. (1994) Degree of preoperative malnutrition is predictive of postoperative morbidity and mortality in liver transplant recipients. Transplantation, 57, 469-472. doi:10.1097/00007890-199402150-00030
- Kondrup, J., Rasmussen, H.H., Hamberg, O., et al. (2003) Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clinical Nutrition, 22, 321-336. doi:10.1016/S0261-5614(02)00214-5
- Covinsky, K.E., Martin, G.E., Beyth, R.J., et al. (1999) The relationship between clinical assessments of nutriational status and adverse outcomes in older hospitalized medical patients. Journal of the American Geriatrics Society, 47, 532-538.
- Reilly, H.M., Martineau, J.K., Moran, A. and Kennedy, H. (1995) Nutritional screening evaluation and implementation of a simple nutrition risk score. Clinical Nutrition, 14, 269-273. doi:10.1016/S0261-5614(95)80063-8
- Kondrup, J., Rasmussen, H.H., Hamberg, O., et al. (2003) Nutritional risk screening (NRS-2002): A new method based on an analysis of controlled clinical trials. Clinical Nutrition, 22, 321-336. doi:10.1016/S0261-5614(02)00214-5
- Kondrup, J., Allison, S.P., Elia, M., et al. (2003) ESPEN guidelines for nutritional screening-2002. Clinical Nutrition, 22, 415-421. doi:10.1016/S0261-5614(03)00098-0
- Wiesner, R.H., McDiarmid, S.V., Kamath, P.S., Edwards, E.B., et al. (2001) MELD and PELD: Application of survival models to liver allocation. Liver Transplantation, 7, 567-580. doi:10.1053/jlts.2001.25879
- Akerman, P.A., Jenkins, R.L. and Bistrian, B.R. (1993) Preoperative nutritional assessment in liver transplantation. Nutrition, 9, 350-356.
- Gopalan, S., Saran, S. and Sengupta, R. (2000) Practicalities of nutrition support in chronic liver disease. Current Opinion in Clinical Nutrition & Metabolic Care, 3, 227- 229. doi:10.1097/00075197-200005000-00011
- Vintro, A.Q., Krasnoff, J.B. and Painter, P. (2002) Roles of nutrition and physical activity in musculoskeletal complications before and after liver transplantation. AACN Clinical Issues, 13, 333-347. doi:10.1097/00044067-200205000-00016
- McCullough, A.J. and Bugianesi, E. (1997) Protein calorie malnutrition and the etiology of cirrhosis. American Journal of Gastroenterology, 92, 734-738.
- Shahid, M., Johnson, J., Nightingale, P. and Neuberger, J. (2005) Nutritional markers in liver allograft recipients. Transplantation, 79, 359-362. doi:10.1097/01.TP.0000150022.64564.C2
- De Luis, D.A., Izaola, O., Velicia, M.C., et al. (2006) Impact of dietary intake and nutritional status on outcomes after liver transplantation. Revista Espanola de Enfermedades Digestivas (Madrid), 98, 6-13.
- Plank, L.D., McCall, J.L., Gane, E.J., et al. (2005) Preand postoperative immunonutrition in patients undergoing liver transplantation: A pilot study of safety and efficacy. Clinical Nutrition, 24, 288-296. doi:10.1016/j.clnu.2004.11.007
- Figueiredo, F.A., Dickson, E.R., Pasha, T.M., et al. (2000) Utility of standard nutritional parameters in detecting body cell mass depletion in patients with end-stage liver disease. Liver Transplantation, 6, 575-581. doi:10.1053/jlts.2000.9736
- Barbosa-Silva, M.C. and Barros, A.J. (2006) Indications and limitations of the use of subjective global assessment in clinical practice: An update. Current Opinion in Clinical Nutrition & Metabolic Care, 9, 263-269. doi:10.1097/01.mco.0000222109.53665.ed
- Shaw, B.W., Wood, R.P., Gordon, R.D., et al. (1985) Influence of selected patient variables and operative blood loss on six-month survival following liver transplantation. Seminars in Liver Disease, 5, 385-393. doi:10.1055/s-2008-1040637
- Selberg, O., Böttcher, J., Tusch, G., et al. (1997) Identification of highand low-risk patients before liver transplantation. A prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology, 25, 652-657. doi:10.1002/hep.510250327
- Harrison, J., McKiernan, J. and Neuberger, J.M. (1997) A prospective study on the effect of recipient nutritional status on outcome in liver transplantation. Transplant International, 10, 360-374. doi:10.1111/j.1432-2277.1997.tb00931.x
- Merli, M., Giusto, M., Gentili, F., Novelli, G., et al. (2010) Nutritional status: Its influence on the outcome of patients undergoing liver transplantation. Liver International, 30, 208-214. doi:10.1111/j.1478-3231.2009.02135.x
NOTES
*Corresponding author.

