Open Journal of Medicinal Chemistry
Vol.2 No.3(2012), Article ID:22929,7 pages DOI:10.4236/ojmc.2012.23012
Microwave Assisted and Al2O3/K2CO3 Catalyzed Synthesis of Azetidin-2-One Derivatives Containing Aryl Sulfonate Moiety with Anti-Inflammatory and Anti-Microbial Activity
1Department of Chemistry, Faculty of Science, Vaidyanath College, Parli-Vaijnath, India
2Division of Organic Chemistry, Faculty of Science, Dnyanopasak College, Parbhani, India
Email: bvkendre@yahoo.com, *bhusare71@yahoo.com
Received June 2, 2012; revised July 7, 2012; accepted July 19, 2012
Keywords: Aldimine; Azetidin-2-One; Anti-Inflammatory; p-Amino Benzoic Acid; 2-Amino Pyridine; p-Toluene Sulfonyl Chloride
ABSTRACT
We report the novel synthesis of azetidin-2-one derivatives containing aryl sulfonate moiety from the reaction of 2-hydroxy benzaldehyde with p-toluene sulfonyl chloride afforded firstly 2-formylphenyl 4-methylbenzene sulfonate (2). The compound (2) on reaction with p-aminobenzoic acid or 2-aminopyridine gave the corresponding aldimines (3). Furthermore, the aldimines are on reaction with chloroacetyl chloride gives corresponding azetidin-2-ones in good to moderate yield. Among the eight synthesized azetidin-2-ones, five selected compounds have been screened for the anti-inflammatory activity, few of them showed good anti-inflammatory activity compared with standard drugs. Antimicrobial activity of all synthesized compounds has been tested and most of the compounds showed good anti-bacterial and anti-fungal activities.
1. Introduction
The synthesis of new compounds and their biological screening is recently of enormous focus within the synthetic and medicinal chemistry communities. Azetidin- 2-ones are well known antibiotics containing β-lactam monocyclic ring and reported to exhibit interesting biological activities. They are mostly prescribed medicines to treat bacterial infections. They were found to possess powerful anti-microbial [1-2], anti-proliferative [3], IFN-gamma [4], cytotoxic [5], anticonvulsant, anti-tubercular [6], cholesterol absorption inhibitor [7], antiinflammatory and anti-degenerative activities [8]. Moreover, the β-Lactams derived from a carbapenem chiron are well known selective inhibitors of human fatty acid amide hydrolase [9]. On the other hand, the compounds containing aryl sulfonate moiety have been received considerable attention during last two decades as they are endowed with variety of biological activities like papillomavirus microbicidal [10], anti-human immunodeficiency virus-1[11], antineoplastic [12], and anticancer activity [13-14]. Therefore it was envisaged that the compounds containing both the chemical moieties would result in new compounds of interesting biological activities. Five selected compounds of this library were screened for the anti-inflammatory activity. All compounds synthesized in this series have been screened for anti-bacterial and anti-fungal activities.
2. Results and Discussions
Synthesis involves the initial formation of 2-formylphenyl 4-methylbenzene sulfonate (2a) by the efficient condensation of salicylaldehyde with p-toluene sulfonyl chloride in the presence of anhydrous potassium carbonate (K2CO3) under solvent free conditions by simple mortar pestle technique. The formation of product was judged by the ferric chloride functional group test for phenolic OH which was found negative. The structure was further confirmed by IR and 1H NMR spectroscopic methods. The IR spectrum of product showed absorption bands at 1345, 1712 cm–1 attributed to the presence of SO3 and CHO groups. The 1H NMR spectrum of this compound showed the presence of a singlet at δ 2.49 and 10.03 ppm attributing to the presence of CH3 and CHO groups, the appearance of multiple peaks in the region of δ 7.22 - 7.93 corresponding to the presence of aromatic ring systems in the molecule. A rapid formation of product, the generation of high purity compound, simple isolation procedure, excellent yield and eco-friendship are the key factors of this method. Secondly, the synthesis of aldimine was achieved by the reaction of 2-formylphenyl 4-methylbenzene sulfonate either with p-amino benzoic acid or 2-amino pyridine by conventional and microwave techniques using mild and low cost reagents. The yields obtained by the conventional method in the presence of absolute ethanol were 50% - 60% and time required for the completion of reaction was 4 - 5 hours. To solve this problem we moved our strategy towards the synthesis of aldimines under solvent free conditions using microwaves. The product formation was observed in short reaction time with excellent yields (80% - 87%). The aldimine formation was confirmed by IR, 1H NMR and 13CNMR spectroscopic methods. The aldimine obtained by the reaction of 2-formylphenyl 4-methylbenzene sulfonate with p-amino benzoic acid showed IR absorption bands at 3040 cm–1 - 3020 cm–1 for Ar C-H stretch, 1580 cm–1 - 1540 cm–1 for Ar C=C stretch, 1277 cm–1 for SO3 stretch and 1652 cm–1 for C=N stretching vibrations. The 1H NMR spectrum of this compound showed the presence of a singlet at δ 2.52 and δ 8.49 corresponding to the presence of CH3 and CH=N (azomethine) groups in the compound. 13C NMR spectroscopic characterization showed absorption at δ 21.672 and 157.124 for CH3 and CH=N carbons clearly confirms the formation of aldimine product. The formation of product was further confirmed by physical constant and functional group tests. A target molecule 4a (Scheme 1) was synthesized by the cyclocondensation reaction of aldimine 3a with chloroacetyl chloride under reflux2 in the presence of Et3N/1, 4-dioxane or solvent free conditions. The reflux method gave low yields (55% - 65%) of the products and took 4 - 5 hours for the completion. To overcome these drawbacks, we attempted to carry out above transformation under microwave irradiation. The equimolar mixture of reactants such as aldimine and chloroacetyl chloride in the presence of catalytic amount of basic and anhydrous Al2O3/K2CO3 was mixed well in mortar by pestle for 5 minutes.
The mixture was further subjected to microwave oven for 4 - 5 minutes under solvent free conditions. The progress of reaction was examined by TLC. The yield of isolated product was excellent. The spectral and analytical data was consistent with proposed structure of target molecule. The synthesized azetidin-2-one 4a was screened for antimicrobial and anti-inflammatory activities and its data was found to be significant. The above result motivated us to synthesize some more novel heterocyclic compounds with hope that the synthesized compounds will add more activeness as anti-microbial and anti-inflammatory agents. We tried to study the influence of substituent’s on the activities of molecules by substituting the groups at ortho and para positions on the aromatic ring system. The substitution of iodine, bromine and chlorine groups on aromatic ring system showed marginal effect on the activities of molecules. The iodine and bromine substituted azetidin-2-ones were found to be more potent anti-inflammatory agents (Table 1). Similarly, the iodine, bromine and chlorine substituted azetidin- 2-ones were tested for their antimicrobial activities and their influence on activity is reported in Table 2.
3. Anti-Inflammatory and Anti-Microbial Activity
3.1. Material, Methods and Reagents
Wistar albino rats with weight 25 gm - 50 gm were used for the study under the guidelines of Institutional Animal Ethics Committee (IAEC) at National Toxicology Centre, Pune. The standard drug indomethacin was purchased from Aldrich Co.
Culture medium the nutrient agar, sabouraud’s dextrose agar cultures, ampicilin and norcadine were of Spectrochem and Aldrich Co. The stock solutions of test compounds were prepared in dimethyl sulfoxide solvent.
3.2. Effect Synthesized Drugs (4a-h) on the Edema Volume of Injected Paws
The effect of synthesized compounds 4a-h and standard drug indomethacin was evaluated on the in vitro inhibition of cyclooxygenase-2 enzymes after 6 h. All the screened compounds were found to inhibit inflammation caused by cyclooxygenase-2 enzymes. The normal control, indomethacin and test compounds were administered to the rats 30 minutes before the injection of 0.1 ml of 1% carrageenan suspension in normal saline. The test drugs 50 mg/kg and the standard drug 10 mg/kg were dosed to the animals. The animals were divided into eight groups containing six animals in each group. Male and female adult Wistar albino rats marked H, B, T having weight 25 gm - 50 gm were used for the study. The animals were kept overnight on fasting. The antiinflammatory activity was studied by Winter et al. method [15]. The experimental procedures were carried out under the guidelines of Institutional Animal Ethics Committee (IAEC) at National Toxicology Centre, Pune. The no. 26 gauge needle was used to inject the carrageenan suspension into the sub planar region of the right hind paw. Immediately thereafter the edema volume of the injected paws were measured plethysmographically by water displacement method. For the comparison purpose volume of edema at various prefixed time intervals 1 h, 2 h, 4 h and 6 h was measured. The difference between paw volumes of the treated animals was measured and the mean edema volume was calculated. Percentage
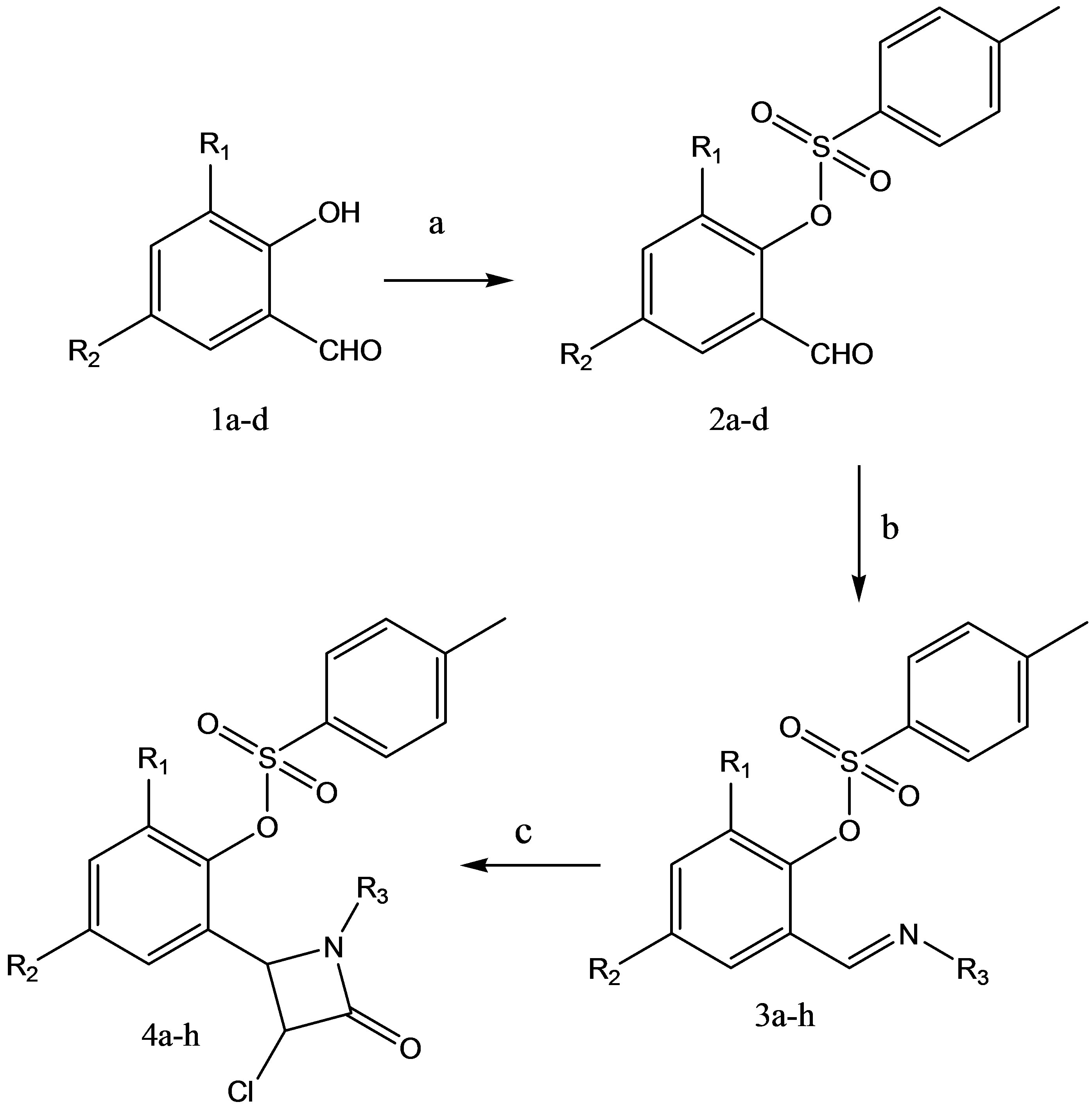
Scheme 1. Synthesis of azetidin-2-ones 4a-h.
Reagents and conditions: a—P-toluene sulfonyl chloride, anhydrous K2CO3, grind 7 min - 8 min; b—4-amino benzoic acid OR 2-amino pyridine, MW 3 min - 4 min; c—Chloroacetyl chloride, Et3N, reflux 4 h - 5 h, 1, 4-dioxane OR Al2O3/K2CO3, MW 4 min - 5 min.; R1=H, I, Br; R2=H, I, Br, Cl; R3 =-C6H4 (4-COOH),-C5H4N.
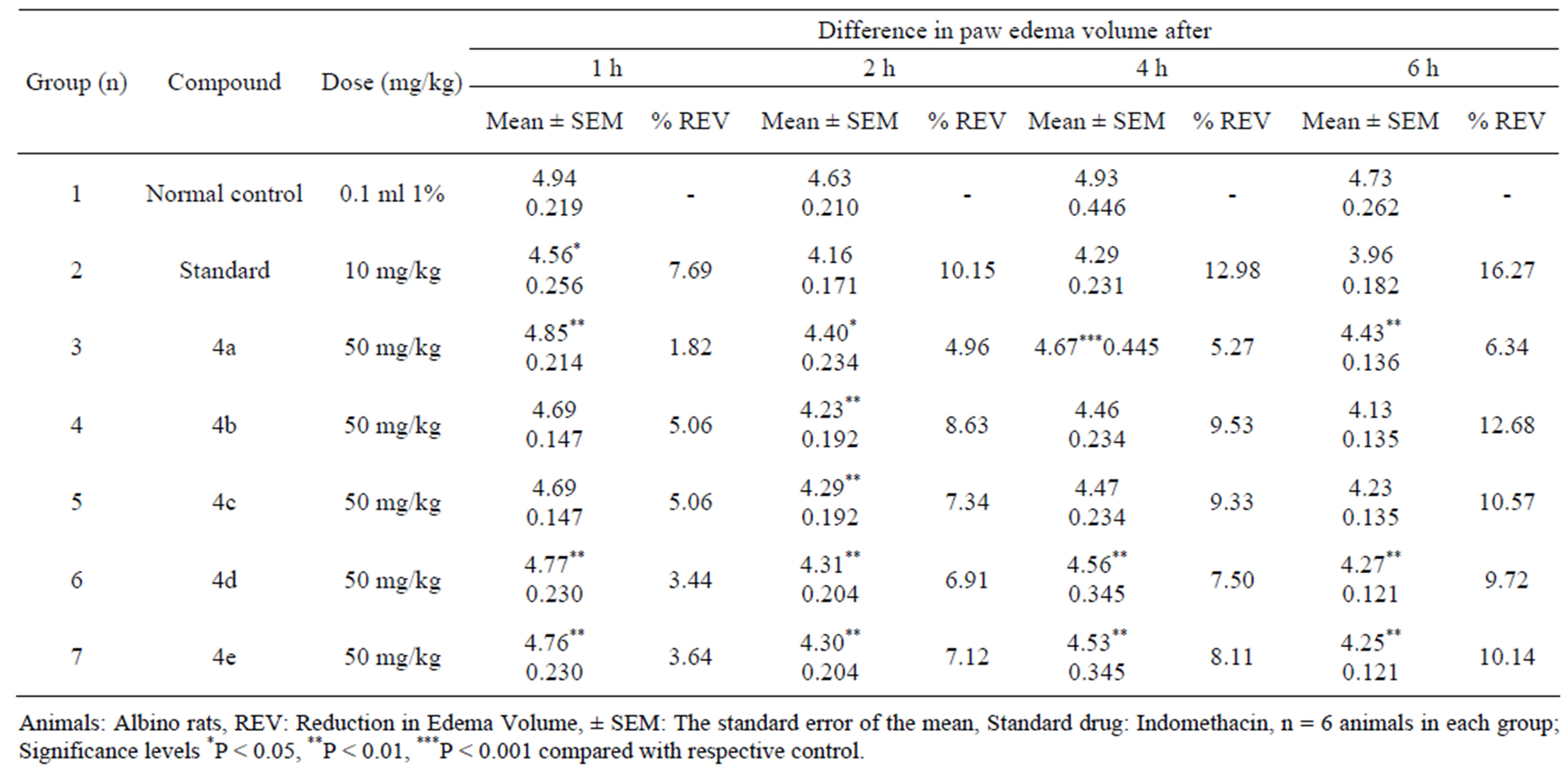
Table 1. Anti-inflammatory activity of azetidin-2-ones.
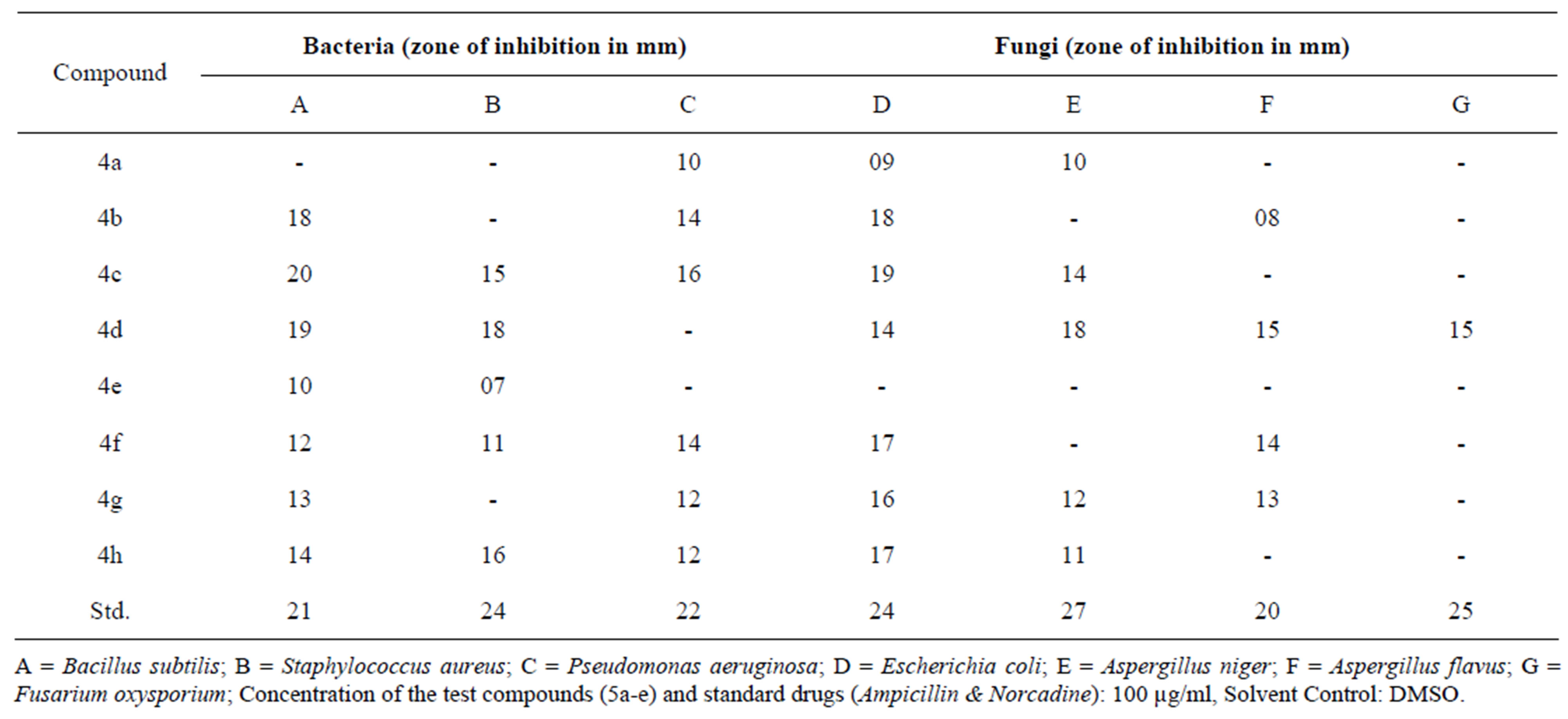
Table 2. Antibacterial and antifungal activity of azetidin-2-ones.
reduction in edema volume was calculated by using the formula, % reduction = 100 × Vo-Vt/Vo. Where, Vo = Volume of the paw of control at time “t”. Vt = Volume of the paw of drug treated at time “t”. From the obtained data, the mean edema volume and percentage reduction in edema was calculated. The results are presented in Table 2. The SD and SEM were calculated by using ANOVA, Dunnets “t” test. Compounds 4b, 4c and 4e were found excellent COX-2 inhibitors. On the other hand, the other synthesized compounds 4a and 4d have been showed moderate inhibitory effects on COX-2 enzymes. It is observed that the compounds containing iodine and bromine groups on the aromatic ring system showed stronger anti-inflammatory effects than the chlorine substituent. It is also clear that the iodine and bromine substituted azetidin-2-ones showed highest COX-2 enzyme inhibitory effects than the other compounds.
3.3. Effect of Drugs on the Growth of Microorganisms
The antibacterial activity of the test samples 4a-h was determined by agar cup plate [16] method using ampicillin (100 μg/ml) as standard drug and four pathogens such as Bacillus subtilis, Staphylococcus aureus, and Escherichia coli and Pseudomonas aeruginosa. This method was based on diffusion of antibacterial component from reservoir bore to the surrounding inoculated nutrient agar medium so that the growth of microorganisms was inhibited as circular zone around the bore. The concentration of test compounds was 100 μg/ml and was prepared in dimethyl sulfoxide as solvent. The test samples and standard drug were placed in a bore made in Petri dishes which contains different pathogens and were incubated at 37˚C for 24 hours. The zone of inhibitions around the bore was measured after 24 hours. The antibacterial activity was classified as standards (>22 mm), highly active (15 mm - 22 mm), moderately active (10 mm - 15 mm), least active (7 mm - 10 mm) and less than 7 mm was taken as inactive. The antibacterial and antifungal activity data are recorded in Table 2. The antifungal activity of synthesized compounds was determined by using Aspergillus niger, Aspergillus flavus and Fusarium oxysporium pathogens. Dimethyl sulphoxide was used as control and dextrose agar as culture medium for antifungal activity. Norcadine (100 μg/ml) was used as standard drug for the comparison and determination of their antifungal activities. Among the synthesized compounds, 4b, 4c, 4d and 4h were showed highest antimicrobial activity against various pathogens. The other tested compounds were exhibited minimum inhibitory effects on the bacterial and fungal strains. It is clear that iodine, bromine and chlorine groups were found to be crucial for the inhibition of microbial strains.
4. Experimental
4.1. Materials
2-Hydroxy benzaldehyde, 4-chloro 2-hydroxy benzaldehyde, p-toluene sulfonyl chloride, chloro acetyl chloride, and triethyl amine were obtained from Spectrochem and Aldrich companies. All the chemicals used were of analytical grade. Melting points were performed in open capillary tubes and were uncorrected. IR spectra were recorded in KBR pellets on a 250 MHz spectrometer and 1H NMR spectra in DMSO-d6 on Perkin Elmer 200 MHz spectrometer using TMS as an internal standard and chemical shifts in ppm. 13C NMR spectra were recorded on Avance 50 MHz spectrometer in CDCl3. Anti-inflammatory activity of all compounds was tested at National Toxicology Centre, Pune. The target compounds were synthesized as shown in Scheme 1.
4.2. General Procedure
Synthesis of 2-formylphenyl-4-methylbenzene sulfonate: A mixture of 2-hydroxy benzaldehyde (1.22 g, 0.01- mol), p-toluene sulfonyl chloride (1.91 g, 0.01 mol) and anhydrous K2CO3 (2.07 g, 0.015 mol) was grinded well in mortar for 6 - 7 minutes. The reaction mixture was then left aside for one hour at room temperature. It was then poured into ice cold water and stirred for 10 minutes. The solid obtained was filtered, washed with water, dried and crystallized from ethanol to get pure product in 94% yield. The purity of compounds was monitored by thin layer chromatography. The obtained compound was purified by column chromatography using ethyl acetate/ chloroform (2:8) as solvents.
Synthesis of aldimines by microwave method: A mixture of 2-formylphenyl 4-methylbenzene sulfonate (2.76 g, 0.01 mol) and p-amino benzoic acid (1.37 g, 0.01 mol) in dry ethanol (4 mL) was stirred well for five minutes in 50 ml beaker and dried in air. A beaker covered with watch glass was subjected to micro wave oven for 2 - 3 minutes at low power (40%, 100˚C). The product formation was monitored by TLC. A mixture was cooled to room temperature and crystallized with ethyl alcohol to produce a pure aldimine in 90% yield. The obtained compounds were purified by column chromatography using acetone/chloroform (2:8) as solvent. The iodine, bromine and chlorine substituted aldimines were similarly synthesized by the similar procedure.
Synthesis of 2-azetidinones (4a-h) by conventional method: A mixture of aldimine 3a (3.95 g, 0.01 mol) and triethylamine (10 mL) in dry 1, 4-dioxane (10 mL) was stirred well at 0˚C - 5˚C temperature. To this mixture chloroacetyl chloride (1.13 g, 0.01 mol) was added drop wise for half an hour. The mixture was then stirred for additional 5 hours at 60˚C temperature and left 24 hours at room temperature. The mixture was concentrated, cooled, poured into ice cold water, filtered, washed with cold water and then dried. The product was crystallized with acetone: n-hexane mixture. The obtained compounds were purified by column chromatography using acetone/n-hexane mixture.
Synthesis of azetidin-2-ones (4a-h) by microwave method: A mixture of aldimine 3a (3.95 g, 0.01 mol), chloroacetyl chloride (1.13 g, 0.01 mol) and 1:1 amount of anhydrous K2CO3 (0.69 g, 0.005 mol) and basic alumina (0.51 g, 0.005 mol) was mixed well into mortar. The whole quantity was transferred to 100 ml beaker and subjected to domestic microwave oven for 3 - 4 minutes at medium-high power. The mixture was cooled to room temperature, to this 10 ml ice cooled water was added and it was then stirred for 5 minutes. The solid obtained was filtered, washed by water, dried and crystallized with ethyl alcohol. The obtained compounds were purified by column chromatography using acetone/chloroform as solvent.
2-(3-Chloro-1-(4-carboxyphenyl)-4-oxoazetidin-2-yl) phenyl 4-methyl benzene sulfonate (4a): White solid, Yield (MW), 85%, m.p. 140˚C - 142˚C. IR (KBr, ʋmax/cm–1): 3072 (C-H, aromatic ring str), 2886 (C-H, str), 1710 (C=O, str), 1625 - 1518 (C=C, aromatic ring str), 1398 (SO3, str), 1171 (C-O-S, str), 1084 (C-N, str), 690 (C-Cl, str). 1H NMR (200MHz, DMSO-d6): δ 2.54 (3H, s, CH3), 5.56 (1H, d, CH-N), 5.57 (1H, d, CH-Cl), 7.52 - 8.30 (10H, m, Ar-H), 11.58 (1H, s, COOH). 13C NMR (50 MHz, CDCl3)): δ 21.641, 52.406, 60.420, 111.216, 119.056, 124.389, 126.678, 128.870, 129.826, 130.201, 130.806, 131.033, 146.075, 147.026, 149.525, 160.410, 169.425.
2-(3-Chloro-1-(4-carboxyphenyl)-4-oxoazetidin-2-yl)-4, 6-diiodophenyl 4-methyl benzene sulfonate (4b): Pale brown solid, Yield (MW), 90%, m.p. 128˚C - 130˚C. IR (KBr, ʋmax/cm–1): 3052 (C-H, aromatic ring str), 2900 (C-H, str), 1675 (C=O, str), 1610 - 1500 (C=C, aromatic ring str), 1390 (SO3, str), 1176 (C-O-S, str), 1080 (C-N, str), 690 (C-Cl, str), 554 (C-I, str). 1H NMR (200MHz, DMSO-d6): δ 2.46 (3H, s, CH3), 5.52 (1H, d, CH-N), 5.53 (1H, d, CH-Cl), 7.52 - 8.54 (10H, m, Ar-H), 11.56 (1H, s, COOH). 13C NMR (50 MHz, CDCl3): δ 21.642, 52.406, 60.420, 89.058, 90.025, 119.151, 125.056, 127.676, 127.850, 129.829, 130.201, 130.846, 132.829, 134.241, 145.575, 147.058, 149.550, 159.425, 169.342.
2-(3-Chloro-1-(4-carboxyphenyl)-4-oxoazetidin-2-yl)-4, 6-dibromophenyl 4-methyl benzene sulfonate (4c): White solid, Yield (MW), 87%, m.p. 153˚C-155˚C. IR (KBr, ʋmax/cm–1): 3054 (C-H, aromatic ring str), 2900 (C-H, str), 1698 (C=O, str), 1600 - 1495 (C=C, aromatic ring str.), 1375 (SO3, str), 1170 (C-O-S, str), 1072 (C-N, str), 670 (C-Cl, str), 574 (C-Br, str). 1H NMR (200MHz, DMSO-d6): δ 2.51 (3H, s, CH3), 5.53 (1H, d, CH-N), 5.54 (1H, d, CH-Cl), 7.51 - 8.37 (10H, m, Ar-H), 11.45 (1H, s, COOH). 13C NMR (50 MHz, CDCl3): δ 21.421, 52.402, 60.620, 112.058, 115.025, 123.155, 125.056, 127.678, 127.870, 129.858, 130.205, 130.856, 132.826, 134.845, 145.585, 147.454, 149.654, 160.025, 169.409.
4-Chloro-2-(3-chloro-1-(4-carboxyphenyl)-4-oxoazetidin-2-yl) phenyl 4-methyl benzene sulfonate (4d): White solid, Yield (MW), 82%, m.p. 135˚C - 137˚C. IR (KBr, ʋmax/cm–1): 3061 (C-H, aromatic ring str), 2877 (C-H, str), 1709 (C=O, str), 1620-1500 (C=C, aromatic ring str), 1389 (SO3, str), 1174 (C-O-S, str), 1064 (C-N, str.), 685 (C-Cl, str). 1 H NMR (200MHz, DMSO-d6): 2.45 (3 H, s, CH3), 5.36 (1 H, d, CH-N), 5.61(1 H, d, CH-Cl), 6.64 - 7.28 (6 H, m, Ar-H), 7.88 - 7.76 (3 H, m, Ar-H), 8.40 - 8.33 (2 H, m, pyridine ring). 13C NMR (50MHz, CDCl3): δ 21.634, 52.601, 61.014, 116.243, 124.554, 127.441, 128.611, 129.569, 130.528, 130.531, 132.152, 145.305, 156.714, 166.015.
2-(3-chloro-4-oxo-1-(pyridin-2-yl) azetidin-2-yl) phenyl 4-methylbenzenesulfonate Compound (4e): White solid, Yield (MW), 80%, m.p. 114˚C - 117˚C. IR (KBr, ʋmax/cm–1): 3055 (C-H, aromatic ring str.), 2866 (C-H, str), 1702 (C=O, str), 1610 - 1524 (C=C, aromatic ring str), 1367 (SO3, str), 1170 (C-O-S, str), 1071 (C-N, str.), 701 (C-Cl, str). 1H NMR (200MHz, DMSO-d6): 2.43 (3H, s, CH3), 5.29 (1 H, d, CH-N), 5.54 (1 H, d, CH-Cl), 6.60 - 7.33 (7 H, m, Ar-H), 7.86 - 7.90 (3 H, m, Ar-H), 8.47 - 8.51 (2 H, m, pyridine ring). 13C NMR (50 MHz, CDCl3): δ 21.601, 52.500, 60.441, 115.016, 119.867, 124.559, 127.072, 128.610, 129.846, 130.231, 130.503, 131.143, 146.205, 147.116, 157.312, 166.227.
2-(3-Chloro-4-oxo-1-(pyridin-2-yl) azetidin-2-yl)-4, 6-diiodophenyl 4-methyl benzene sulfonate (4f): Pale brown solid, Yield (MW), 85%, m.p. 122˚C - 123˚C. IR (KBr, ʋmax/cm–1): 3066 (C-H, aromatic ring str), 2914 (C-H, str), 1680 (C=O, str), 1610 - 1520 (C=C, aromatic ring str.), 1388 (SO3, str), 1178 (C-O-S, str), 1070 (C-N, str), 682 (C-Cl, str), 550 (C-I, str). 1H NMR (200MHz, DMSO-d6): 2.47 (3H, s, CH3), 5.27 (1H, d, CH-N), 5.55 (1H, d, CH-Cl), 7.08 (1H, t, Pyridine ring), 7.27 (3H, m, Ar-H), 7.71 (1H, s, Ar-H), 7.83 - 7.88 (3H, m, Ar-H), 8.46 - 8.50 (2H, m, pyridine ring). 13C NMR (50 MHz, CDCl3): δ 21.631, 52.363, 60.481, 89.153, 90.127, 119.160, 125.386, 127.776, 127.852, 129.727, 130.231, 130.645, 132.809, 134.234, 145.770, 147.058, 159.215, 167.041.
2-(3-Chloro-4-oxo-1-(pyridin-2-yl) azetidin-2-yl)-4, 6-dibromophenyl 4-methyl benzene sulfonate (4g): White solid, Yield (MW), 88%, m.p. 131˚C - 133˚C. IR (KBr, ʋmax/cm–1): 3074 (C-H, aromatic ring str), 2918 (C-H, str), 1700 (C = O, str), 1605-1490 (C = C, aromatic ring str), 1374 (SO3, str), 1175 (C-O-S, str), 1080 (C-N, str), 670 (C-Cl, str), 570 (C-Br, str). 1H NMR (200MHz, DMSO-d6): 2.44 (3H, s, CH3), 5.26 (1H, d, CH-N), 5.53 (1H, d, CH-Cl), 7.05 (1H, t, Pyridine ring), 7.23 (3H, m, Ar-H), 7.73 (1H, s, Ar-H), 7.84 - 7.85 (3H, m, Ar-H), 8.41 - 8.47 (2H, m, pyridine ring). 13C NMR (50 MHz, CDCl3): δ 21.503, 52.442, 60.721, 111.928, 115.225, 124.135, 125.150, 127.670, 127.679, 129.758, 130.005, 130.516, 132.622, 134.860, 145.489, 147.554, 160.121, 166.401.
4-Chloro-2-(3-chloro-4-oxo-1-(pyridin-2-yl) azetidin- 2-yl) phenyl 4-methyl benzene sulfonate (4g): White solid, Yield (MW), 85%, m.p. 110˚C - 112˚C. IR (KBr, ʋmax/cm–1): 3051 (C-H, aromatic ring str), 2880 (C-H, str), 1712 (C=O, str), 1615 - 1518 (C=C, aromatic ring str), 1392 (SO3, str), 1164 (C-O-S, str), 1056 (C-N, str), 702 (C-Cl, str). 1H NMR (200MHz, DMSO-d6): 2.41 (3H, s, CH3), 5.30 (1H, d, CH-N), 5.56 (1H, d, CH-Cl), 6.61 - 7.30 (6 H, m, Ar-H), 7.83 - 7.84 (3 H, m, Ar-H), 8.45 - 8.49 (2 H, m, pyridine ring). 13C NMR (50 MHz, CDCl3): δ 21.611, 52.521, 60.314, 115.047, 124.458, 127.542, 128.713, 129.542, 130.258, 130.533, 131.640, 145.215, 157.515, 166.312
5. Conclusion
In conclusion, a simple, efficient and cost-effective procedure was developed for the synthesis of azetidin-2-one derivatives by using inexpensive and commercially available K2CO3/Al2O3 as catalyst under microwave conditions. The compounds 4a-e was investigated for their anti-inflammatory activity. The all synthesized compounds showed significant anti-inflammatory activity when compared with standard drug. The compounds were also evaluated for their anti microbial activity against various gram positive & gram negative bacterial and fungal strains. Compounds 4b-d, and 4h were found to be more potent antibacterial and antifungal agents when compared with standard (Ampicillin and Norcadine).
6. Acknowledgements
We thank UGC, New Delhi for providing the financial assistance to complete this work. We are thankful to Dr. P. L. More, Principal, DSM College, Parbhani, and Dr. R. K. Ippar, Principal, Vaidyanath College, Parli-vaijnath, for providing laboratory facilities. Authors are also thankful to Dr. K. G. Apte, National Toxicology Centre, Pune, for providing anti-inflammatory activity evaluation data.
REFERENCES
- Y. Rokade and N. Dongare, “Synthesis and Antimicrobial Activity of Some Azetidinone Derivatives with the β- naphthol,” Rasayan Journal of Chemistry, Vol. 3, No. 4, 2010, pp. 641-645.
- R. S. Keri, K. M. Hosamani, R. V. Shingalapur and H. R. S. Reddy, “2-Azetidinone Derivatives: Design, Synthesis, in Vitro Anti-Microbial, Cytotoxic Activities and DNA Cleavage Study,” European Journal of Medicinal Chemistry, Vol. 44, No. 12, 2009, pp. 5123-5130. doi:10.1016/j.ejmech.2009.09.011
- N. M. O’Boyle, M. Carr, L. M. Greene, N. O. Keely, A. J. S. Knox, T. McCabe, D. G. Lloyd, D. M. Zisterer and M. J. Meegan, “Synthesis, Biochemical and Molecular Modelling Studies of Antiproliferative Azetidinones Causing Microtubule Disruption and Mitotic Catastrophe,” European Journal of Medicinal Chemistry, Vol. 46, No. 9, 2011, pp. 4595-4607. doi:10.1016/j.ejmech.2011.07.039
- B. M. Brooks, C. A. Hart and J. W. Coleman, “Differential Effects of Beta-Lactams on Human IFN-Gamma Activity,” The Journal of Antimicrobial Chemotherapy, Vol. 56, No. 6, 2005, pp. 1122-1125. doi:10.1093/jac/dki373
- S. R. Keri, K. M. Hosamani, R. V. Shingalapur and H. R. S. Reddy, “2-Azetidinone Derivatives: Design, Synthesis, in Vitro Anti-microbial, Cytotoxic Activities and DNA Cleavage Study,” European Journal of Medicinal Chemistry, Vol. 44, No. 12, 2009, pp. 5123-5130. doi:10.1016/j.ejmech.2009.09.011
- B. Raga, L. Amith, T. VijayKumar, M. Havangirao and C. H. Upendra, “Synthesis and Antitubercular Activities of Azetidinone and Thiazolidinone Derivatives from 5-Chloro- 3-Methylbenzofuran,” International Journal of ChemTech Research, Vol. 2, No. 3, 2010, pp. 1764-1770.
- B. Xu, “New Azetidinone Cholesterol Absorption Inhibitors,” Expert Opinion on Therapeutic Patents, Vol. 17, No. 7, 2007, pp. 791-797. doi:10.1517/13543776.17.7.791
- J. B. Doherty, C. P. Dorn, P. L. Durette, P. E. Finke, M. MacCoss, S. G. Mills, S. K. Shah, S. P. Sahoo, S. A. Polo and W. K. Hagmann, “Substituted Azetidinones as Anti- Inflammatory and Antidegenerative Agents,” Wild Ones, Vol. 94, No. 10, 1994, p. 143.
- M. Feledziak, C. Michaux, A. Urbach, G. Labar, G. G. Muccioli, D. M. Lambert and J. Marchand-Brynaert, “β-Lactams Derived from a Carbapenem Chiron are Selective Inhibitors of Human Fatty Acid Amide Hydrolase Versus Human Monoacylglycerol Lipase,” Journal of Medicinal Chemistry, Vol. 52, No. 22, 2009, pp. 7054- 7068. doi:10.1021/jm9008532
- N. D. Christensen, C. A. Reed, T. D. Culp, P. L. Hermonat, M. K. Howett, R. A. Anderson and L. J. Zaneveld, “Papillomavirous Microbicidal Activities of High-Molecular-Weight Cellulose Sulphate, Dextrane Sulphate and Polystyrene Sulfonate,” Antimicrob Agents Chemother, Vol. 45, No. 12, 2001, pp. 3427-3432. doi:10.1128/AAC.45.12.3427-3432.2001
- S. Rusconi, M. Moonis, D. P. Merrill, P. V. Pallai, E. A. Neidhardt, S. K. Singh, M. S. Osburne, A. T. Profy, J. C. Jenson and M. S. Hirsch, “Naphthalene Sulfonate Polymers with CD-4 Blocking and Anti-Human Immunodeficiency Virus Type 1 Activities,” Antimicrob Agents Chemother, Vol. 40, No. 1, 1996, pp. 234-236.
- M. A. Hanna, M. M. Girges and M. A. Berghot, “Sulfonate Ester-Containing (Imidazol-1-yl)-N-Substituted Benzenesulfonamides of Anticipated Antineoplastic Activity,” Phosphorus, Sulfur, and Silicon and the Related Elements, Vol. 61, No. 3-4, 1991, pp. 239-246. doi:10.1080/10426509108036803
- L. M. Betts, N. C. Tam, S. M. H. Kabir, R. F. Langler and I. Crandall, “Ether Aryl Sulfonic Acid Esters with Improved Antimalarial/Anticancer Activities,” Australian Journal of Chemistry, Vol. 59, No. 4, 2006, pp. 277-282. doi:10.1071/CH04299
- L. Cyr, R. Langler and C. Lavigne, “Cell Cycle Arrest and Apoptosis Responses of Human Breast Epithelial Cells to the Synthetic Organosulfur Compound p-Methoxyphenyl p-Toluenesulfonate,” Anticancer Research, Vol. 28, No. 5A, 2008, pp. 2753-2764
- C. A. Winter, E. A. Risely and G. W. Nuss, “Carregeenin Induced Oedema in Hind Paw of the Rat as Assay for Antiinflammatory Drugs,” Proceedings of the Society for Experimental Biology and Medicine, Vol. 111, 1962, pp. 544-547.
- “Indian Pharmacopoeia, Microbiological Assay and Test, ed,” Vol. 2, 1996, A-100-107.
NOTES
*Corresponding author.

