Journal of Materials Science and Chemical Engineering
Vol.03 No.11(2015), Article ID:61374,7 pages
10.4236/msce.2015.311007
Synthesis and Characterization of Lithium Manganese Oxide with Different Ratio of Mole on Lithium Recovery Process from Geothermal Fluid of Lumpur Sidoarjo
Lukman Noerochim1*, Gita Akbar Satriawangsa1, Diah Susanti1, Amien Widodo2
1Department of Materials and Metallurgical Engineering, Sepuluh Nopember Institute of Technology, Surabaya, Indonesia
2Department of Geophysical Engineering, Sepuluh Nopember Institute of Technology, Surabaya, Indonesia

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).


Received 13 October 2015; accepted 19 November 2015; published 23 November 2015

ABSTRACT
Geothermal fluid of Lumpur Sidoarjo (Lusi) with lithium content as high as 5.81 mg/liter has a great potential as the source of lithium. Lithium recovery from geothermal liquid of Lusi is firstly investigated by adsorption methods with Lithium Manganese Oxide (LMO) as absorbent. LMO is considered as a promising candidate of adsorbent material due to non-toxic and low cost production. LMOs with different ratio of mole are prepared by solid state reactions method at temperature 500˚C for 5 hrs. XRD peaks of pre- and post-acid treatment LMO 1, 0.8 and 0.5 show a stable spinel crystal structure while LMO 2 has monoclinic structure. The highest lithium adsorption capability is obtained by LMO 1 with 6.6 mg/g.
Keywords:
Lithium Manganese Oxide, Adsorbent, Lithium Recovery, Geothermal Fluid, Lumpur Sidoarjo

1. Introduction
Nowadays, lithium is one among some other materials that currently have the highest demand in the world because of the rapid development of portable electronic devices such as mobile phones, tablets and laptops. The rising interest in electric cars in last few years also boosts the increasing consumption of lithium. The high demand for lithium is showed by the rate of consumption in 2012 that industry analysts and lithium producers estimate as many as 28,000 tons of lithium is used throughout the year. The number increased 10% compared to 2011 in worldwide lithium consumption [1] .
Lithium on natural resources is formed in several types ranging from brine to minerals such as spodumene. Brine is the most commonly used because of the high lithium content. There are also other alternative sources that are still in research progress such as sea water and geothermal fluids. Both of these sources actually have less lithium content, but judging from the abundance and ease in the production process these two sources have great potential to be an alternative production source of lithium.
One of location of geothermal fluid source in Indonesia is Lumpur Sidoarjo (Lusi). Lusi is a phenomenon where there is high temperature mud that flows continuously from inside earth crust. In 2011 the debit of the mud flow reaches 10,000 m3/day. This phenomenon will continue to occur approximately 25 years [2] . Investigation of lithium content inside Lusi has shown that there is lithium as high as 5 ppm (mg/liter) [3] . By doing a rough calculation as well as the assumption that the discharge of mud and the lithium content is constant, Lusi has a great potential to produce lithium as much as 18 tons annually.
Many researches had been conducted to find the best method to recover lithium. One such method is by adsorption method that is often used in sea water [4] -[6] . Inorganic adsorbent is used to absorb lithium ion from the source. One of the adsorbent compounds that are cheap, safe and non-toxic is lithium manganese oxide (LMO) [7] .
LMO has some crystal structures with different atomic configurations. Difference on the crystal structure will certainly have effect on how the LMO adsorbs lithium. In this study, four differences of Li/Mn mole ratio of LMO are synthesized by solid state reaction method with a mole ratio of Li/Mn 2, 1, 0.8 and 0.5. Lithium carbonate and manganese oxide is used as a precursor. Each as-prepared adsorbent will be studied systematically based on its crystal structure and ability to absorb lithium in Lusi.
2. Experimental Procedures
2.1. Preparation of Lithium Manganese Oxide
Lithium manganese oxide (LMO) was synthesized from LiCO3 and MnO2 by solid-state reaction process. The two reagents were dissolved and mixed well with different mole ratio in ethanol namely LMO 0.5, LMO 0.8, LMO 1 and LMO 2. The mixture was calcined at 500˚C for 5 hrs. After cooling down to room temperature, the prepared-LMO powder was dissolved in 0.5 M HCl solution for 24 hrs to elute lithium and to form a vacant site in the crystal structure of LMO. This process will produce an ion sieve type adsorbent to adsorb lithium ion in geothermal fluid of Lusi.
2.2. Physical and Chemical Analysis
The change in the spinel structure of each sample before and after the acid treatment was examined by using X-ray diffraction (XRD, Philips Xpert with a Cu-Kα radiation tube), the morphology of particles were observed by using the scanning electron microscopy (SEM, Philips FEI). The concentration of Li in the supernatant solution was determined through inductively coupled plasma atomic emission spectrophotometer (ICP-AES, 138 Ultrace, Jobin-Yvon) and the extractabilities of the metal ions were calculated accordingly.
2.3. Lithium Adsorption and Desorption
Lithium adsorption and desorption of LMO adsorbent was started with preparation of membrane reservoir made of polypropylene (Kimtex®). The membrane reservoir was subsequently filled with the powder of LMO as much as 0.1 grams. After the introducing LMO adsorbent, all sides of membrane reservoir were sealed. The filled-membrane reservoir was placed in the 1 L of 0.5 M HCl aqueous solution for 24 hrs. At the 2nd day, the filled-membrane reservoir was dipped into 1 L of the extractive Lusi solution for 24 hrs. Desorption of lithium was performed in the 1 L of 0.5 M HCl aqueous solution for 24 hrs. Amount of lithium in the solution was measured by inductively coupled plasma atomic emission spectropho-tometer (ICP- AES, 138 Ultrace, Jobin- Yvon).
The uptake capability of each LMO samples was calculated by using the formula below.
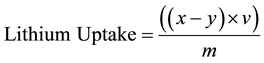 (1)
(1)
x = Lithium content in Lusi pre-adsorption (mg/liter);
y = Lithium content in Lusi post-adsorption (mg/liter);
v = Lusi Volume (liter);
m = Adsorbent Mass (gr).
3. Results and Discussion
SEM images of the prepared-LMO as shown in Figures 1(a)-(d) depicted that the prepared-LMO powders had irregular shape with particles size varying between 3 μm - 60 μm. There is no much different morphology from LMO 0.5 sample to LMO 2.
XRD results are shown from each LMO samples in Figure 2. LMO 0.5 and LMO 0.8 are successfully indexed as Li2MnO3 with spinel crystal structure (PDF Card No. 00-035-0782). LMO 1 shows spinel structure


Figure 1. SEM images of LMO 0.5 (a); LMO 0.8 (b); LMO 1 (c); LMO 2 (d).
Figure 2. XRD patterns of each LMO samples.
with some monoclinic phase of Li2MnO3 (the peak at 2-theta 20.92; 37.1 and 65.6). LMO 2 diffraction pattern shows that this adsorbent has monoclinic structure of Li2MnO3 and there are also some diffraction peaks belonging to lithium carbonate due to incomplete process during synthesis.
XRD results show that LMO 0.5, LMO 0.8 and LMO 1 have the spinel structure. Despite having the same crystal structure, each LMO has the difference of location or distribution of lithium atom in the crystal structure. LMO 0.5 has lithium atom in the tetrahedral site and manganese atom in the octahedral site (Figure 3). The increasing ratio of Li/Mn in the LMO will have an effect on position of lithium atom to occupy the position previously occupied by the manganese atom in octahedral [8] . The distribution of these cations can be seen in Table 1 with illustrations that can be seen in Figure 3.
XRD test after acid treatment for LMO adsorbent is shown in Figure 4. Generally, the shape of the diffraction pattern of LMO 0.5, 0.8 and 1 that are undergoing the acid treatment process is still remained with the diffraction pattern before acid treatment. The only difference is the peaks slightly shifted toward the right side to higher 2-theta values. This indicates that the Li ion extraction reaction proceed topotactically, involving a decrease of lattice constant of the spinel structure (Table 2).
LMO 2 diffraction pattern (Figure 4(d)) shows that the shape of the diffraction pattern is significantly changed. This indicates that the monoclinic crystal structure owned by Li2MnO3 was unstable when subjected to acid treatment process. Discharge of ion Lithium from the crystal structure of LMO 2 results in a change in the crystal structure, which is not a desired property in an adsorbent, because it will affect the performance of the adsorbent in an ongoing process.
Diffraction pattern of LMO 0.5, 0.8 and 1 were in the shift of each peak to the right side. This shift is due to the ion exchange and redox mechanism at the acid treatment process. The position of each peak shifted to the right after the acid treatment can be indicated that there are changes in lattice parameters.
The reaction occurred during acid treatment has already explained by many researchers [9] . In LMO 0.5 the reaction can be explained in redox mechanism, when Mn3+ was disproportionate to Mn4+ and Mn2+, this reaction resulted in λ-MnO2 that have vacant site in tetrahedral position, but from this reaction some manganese ion was dissolved into HCl [9] .
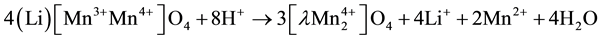 (2)
(2)
At LMO 1 and 0.8 the mechanism is different. the reaction happens in ion exchange manner between Li+ ion and H+ [10] , there are no redox mechanisms in the exchange process, this happens because at LMO 1 and

Figure 3. Spinel crystal structure of (Li)[Mn2]O4 (a) dan (Li)[Li0.33Mn1.67]O4 (b) [11] .
Figure 4. XRD pattern of all samples LMO before and after acid treatment (a) LMO 0.5, (b) LMO 0.8, (c) LMO 1, and (d) LMO 2.
LMO 0.8 there is stable Mn4+. This mechanism is more desired by some researcher due to its better ability to hold their crystal structure better and lithium uptake capability higher.
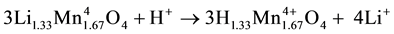 (3)
(3)
Lithium Uptake Analysis
Table 3 shows the results of the ICP test for all samples of LMO. LMO 0.5, 0.8 and 1 have successfully adsorbed the lithium from Lusi. While at LMO 2 the lithium somehow increases as high as 7.92 mg/L, this is show that no absorption of lithium ions occurs.
The result shows that the increasing of the mole ratio of Li/Mn will result in the greater ability of the adsorbent to absorb lithium. However, this does not apply to LMO 2, as is known from the XRD diffraction pattern that LMO 2 does not have the ability to retain its crystal structure which could lead to the absence of the ability to absorb lithium from Lusi.
The difference absorption capability of the LMO 0.5 - 1 (Figure 5) can be attributed to the increasing number of sites that can attract lithium ions in the crystal structure of the adsorbent which has greater Li/Mn mole ratio. As discussed in XRD results, the higher mole ratio of Li/Mn in the adsorbent, the more the manganese position is replaced by Lithium. This will result in the more sites that can attract lithium at greater Li/Mn Mole ratio.
Table 1. Cation distribution at spinel type LMO [5] [10] [12] .
Table 2. Calculation results of lattice parameter before and after acid treatment [13] .
Table 3. Lithium uptake.
Figure 5. Lithium uptake vs. Li/Mn mole ratio from each LMO.
4. Conclusion
Ion-exchange-type manganese oxide LMO adsorbent was successfully prepared through the solid-state reaction process. Lithium adsorption process from Lumpur Sidoarjo (Sidoarjo mud) was performed by using the LMO- based adsorbents. LMO 1, 0.8 and 0.5 have stable spinel crystal structure, while the LMO 2 has an unstable monoclinic crystal structure. Adsorbent LMO with the crystal structure of spinel has the highest ability to absorb Li. LMO with the most high lithium uptake capability is LMO 1 with 6.6 mg/g. It can be attributed to the increasing number of sites that can attract lithium ions in the crystal structure of the adsorbent which has greater Li/Mn mole ratio. LMO 2 with monoclinic structure shows that this type of LMO does not have the ability to absorb lithium. Further studies on producing a large-capacity adsorbent powder based on the results obtained in this study will be conducted.
Cite this paper
LukmanNoerochim,Gita AkbarSatriawangsa,DiahSusanti,AmienWidodo, (2015) Synthesis and Characterization of Lithium Manganese Oxide with Different Ratio of Mole on Lithium Recovery Process from Ge-othermal Fluid of Lumpur Sidoarjo. Journal of Materials Science and Chemical Engineering,03,56-62. doi: 10.4236/msce.2015.311007
References
- 1. Jaskula, B.W. (2013) Lithium: U.S. Geological Survey Mineral Commodity Summaries. USGS National Minerals Information Center, Virginia, 94-95.
- 2. David, R.J., Simon, S.A., Swarbrick, R.E. and Tingay, M.J. (2010) Probabilistic Longevity Estimate for the LUSI Mud Volcano, East Java. Journal of the Geological Society, 168, 517-523.
- 3. Tanikawa, W. (2011) The Mechanism of Overpressure Generation in the LUSI Mud Volcano. E-Proceeding Symposium on Future Lusi, BPLS.
- 4. Ooi, K., Miyai, Y. and Katoh, S. (1986) Recovery of Lithium from Seawater by Manganese Oxide Adsorbent. Separation Science and Technology, 21, 755-766.
- 5. Chitrakar, R., Kanoh, H., Miyai, Y. and Ooi, K. (2001) Recovery of Lithium from Seawater Using Manganese Oxide Adsorbent (H1.6Mn1.6O4) Derivied from Li1.6Mn1.6O. Industrial & Engineering Chemistry Research, 40, 2054-2058.
http://dx.doi.org/10.1021/ie000911h - 6. Kitajou, A., Suzuki, T., Nishihama, S. and Yoshizuka, K. (2003) Selective Recovery of Lithium From Seawater Using a Novel MnO2 Adsorbent II-Enhancement of Lithium Ion Selectivity of The Adsorbent. Ars Separatoria Acta, 2, 97-106.
- 7. Wang, L., Ma, W., Liu, R., Li, H.Y. and Meng, C.C. (2006) Correlation between Li+ Adsorption Capacity and the Preparation Conditions of Spinel Lithium Manganese Precursor. Solid State Ionics, 177, 1421-1428.
http://dx.doi.org/10.1016/j.ssi.2006.07.019 - 8. David, W.I.F., Thackeray, M.M., Bruce, P.G. and Goodenough, J.B. (1984) Lithium Insertion Into β-MnO2 and The Rutile-Spinel Transformation. Materials Research Bulletin, 19, 99-106.
http://dx.doi.org/10.1016/0025-5408(84)90015-1 - 9. Chung, K.-S., Lee, J.-C., Kim, W.-K., Kim, S.B. and Cho, K.Y. (2008) Inorganic Adsorbent Containing Polymeric Membrane Reservoir for the Recovery of Lithium from Seawater. Journal of Membrane Science, 325, 503-508.
http://dx.doi.org/10.1016/j.memsci.2008.09.041 - 10. Hunter, J.C. (1981) Preparation of a New Crystal Form of Manganese Dioxide: λ-MnO2. Journal of Solid State Chemistry, 3, 142-147.
http://dx.doi.org/10.1016/0022-4596(81)90323-6 - 11. Chung, K., Lee, J., Kim, E., Lee, K., Kim, Y. and Ooi, K. (2004) Recovery of Lithium from Seawater Using Nano-Manganese Oxide Adsorbents Prepared by Gel Process. Materials Science Forum, 449-452, 277-280.
http://dx.doi.org/10.4028/www.scientific.net/MSF.449-452.277 - 12. Ammundsen, B., Aitchison, P.B., Burns, G.R., Jones, D.J. and Roziere, J. (1997) Proton Insertion and Lithium-Proton Exchange in Spinel Lithium Manganates. Solid State Ionics, 97, 269-276.
http://dx.doi.org/10.1016/S0167-2738(97)00065-9 - 13. Feng, Q., Miyai, Y., Kanoh, H. and Ooi, K. (1992) Li+ Extraction/Insertion with Spinel-Type Lithium Manganese Oxides, Characterization of Redox-Type and Ion-Exchange-Type Sites. Langmuir, 8, 1861-1867.
http://dx.doi.org/10.1021/la00043a029
NOTES
*Corresponding author.








