Open Journal of Forestry
Vol.4 No.4(2014), Article
ID:48349,14
pages
DOI:10.4236/ojf.2014.44041
Ecological Study of Two Grasses: Cenchrus ciliaris and Digitaria commutata Endangered Autochthonous of the Dry Zone of Tunisia
Imen Dhib1, Abdessalem Abdessamad1, Mustapha Ksontini1, Ali Ferchichi2
1Laboratoire de sylviculture forstière, National Institute of Searches in Rural Genius, Water and Forest, Ariana, Tunis
2Laboratoire d’arido culture et culture oasiènne, Institute of the Dry Regions Medenine Route du Djorf Km, Medenine, Tunisie
Email: dhibimen@ymail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/
![]()


Received 28 March 2014; revised 6 May 2014; accepted 29 May 2014
ABSTRACT
The Saharian ecosystems present an important intensity of rising sensitization to: Erosion, and desertification whose impacts are irreversible. On the one hand, the weakness of the yield and the poverty of soil lead to a limited biodiversity. In addition to these difficulties, hydra is the main cause of the rarification of certain pastoral species such as Cenchrus ciliaris and Digitaria commutata. The ecological study underlines a distribution of these species which are very dependent on water resources in the dry regions of Tunisia. The bioclimatic (temperature, pluviometry) variations lead to modifications to these species from one area to another which are translated through several parameters. Concerning the number of bundles, the difference is important. The national park of Bouhedma records the highest number, compared with Bni khdach, Jerba, Khanguit aicha and Matmata respectively. The variation inter-site of study also is considerable by the study of the morphological parameters (height, number and length of leaves by bundle, number of ear) whose bundles of the national park Bouhedma occupy the first class.
Keywords:Ecological Study, Grasses, Endangered, Dry Zone Of Tunisia

1. Introduction
The socioeconomic changes which affected numerous countries, disrupted the system of use of various natural resources such as the intensive grazing, the clearing, and the invasion of the cereal culture because of the big need in food, together with the climate changes which have engendered a fast regression of pasture land, a quantitative and qualitative degradation of the phytogenetics resources (Ouled Belgacem, 2001). Yet, the Mediterranean south region, often suffers from a big deficit in plant species (Khedim et al., 2004) increased with the water shortage, which is considered as the major constraint for the development of the various pastoral species in these lands. So, grasses such as Digitaria commutata and Cenchrus ciliaris in spite of their resistance to the lack of water, are endangered at the level of Tunisian dry areas. Such a threat makes it an urgent need to study their phenology in different sites of studies by determining certain morphological and ecological parameters.
2. Material and Methods
With a view to carry out this work we have resort to two grasses species Cenchrus ciliaris and Digitaria commutata, wish are distributed in the dry zones of the South of Tunisia, Table1
There are many researches dealing with the study of the phenology of grasses by the measure of the strain of the air shoots such as Chaieb, 1993 who showed that it is the foliar extension which establishes the most characteristic parameter of the behavior to xenophiles grasses it is advocated by Soriano and Salted, 1983, in Chaeib, 1993. At the level of this study we implied this parameter to better identify the phenology of both studied species. The measure of the foliar extension is controlled during one year, according to the phenology cycle of the species, thus our principle consists in:
1) The counting, number of the emitted green leaves;
2) The measure, the length of some leaves, the length of inflorescence and the distance of knots;
3) The counting of the number of ear, the number of seeds and number of knots. These measures are made every month, since the beginning of the vegetative activity (in December 2010) until the stop of this activity (in June 2011). Every month we allocate an average for all the moderate parameters.
This method allows us to compare the performances of every species according to the site of studies where the bioclimatic and geographical characteristics differ from site to another.
This study is achieved by the determination of certain ecological parameters such as:
1) The density: it corresponds to individuals by unit area (m² or ha) or of volume (Dajoz, 1978).
2) The periodicity: it expresses the qualitative and quantitative modifications of the vegetation during the seasons (Lacoste & Solanon, 1969).
3) The Sociology: the type of distribution depends on the mode of distribution of the species (scattering, vegetative multiplication) Lacoste & Salanon, 1969.
4) The Constance: defined the percentage of presence of the species (Dajoz, 1978).
5) The Abundance: It is not always easy to evaluate exactly the abundance of the species; often, the ecologists are content with establishing categories according to more or less precise estimations and adopt five classes of abundance similar to those of the botanists: 0 = absentee; 1 = rare and scattered; 2 = not rare; 3 = abundant and 4 = very abundant.
2. Results and Discussion
2.1. Area of Distribution
2.2.1. In the World
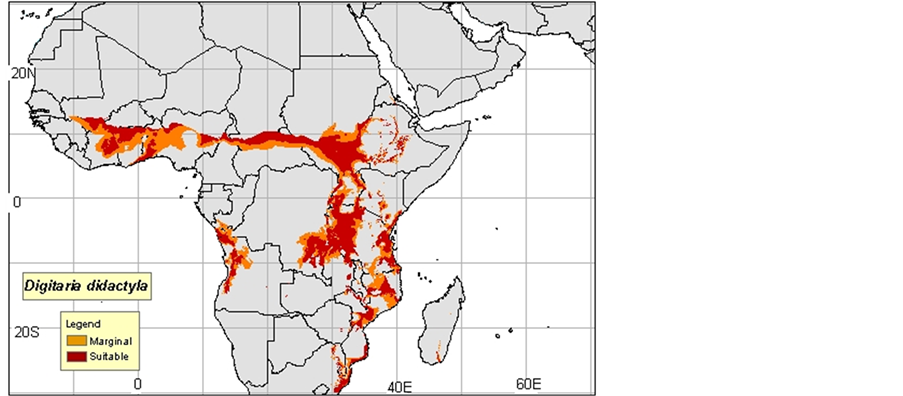
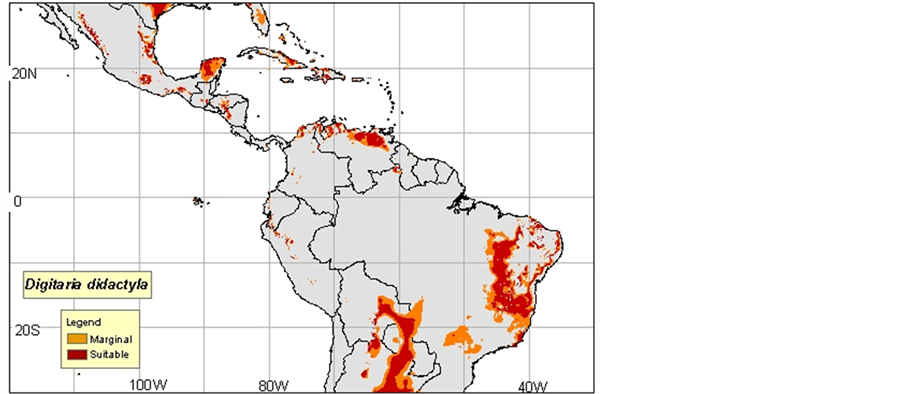
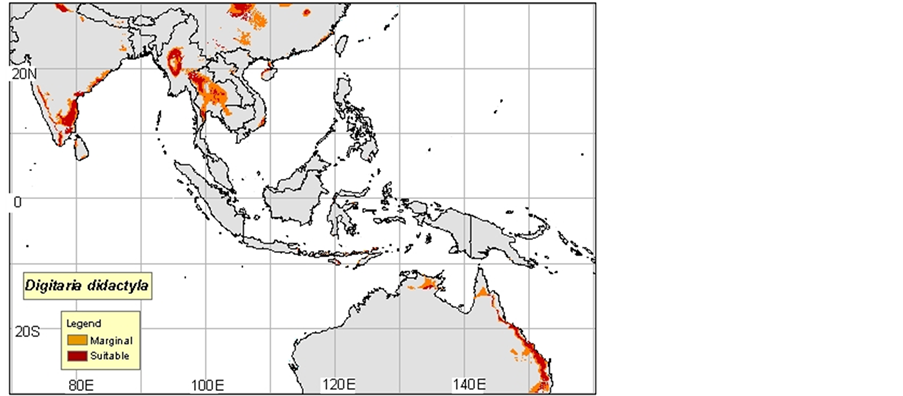
1) Digitaria commutata: Guenod, 1954 mentions that this species is in North Africa and the Canary Islands, while Ozenda, 1977 in Neffati, 1994, and consider it as Saharo sindienne. According to Mayor, 1982, D. commutata appears at the Islands of the Cape Verde in the Canary Islands in Ethiopia in Somalia in Arabia in Iran and in India. Le Houerou, 1969 indicates that D. commutata colonizes the Center and the South of Tunisia, in the Algerian and Moroccan South, in Egypt in Arabia, in Iran, in Afghanistan, in Pakistan and in India (Maps 1(a)-(c)).
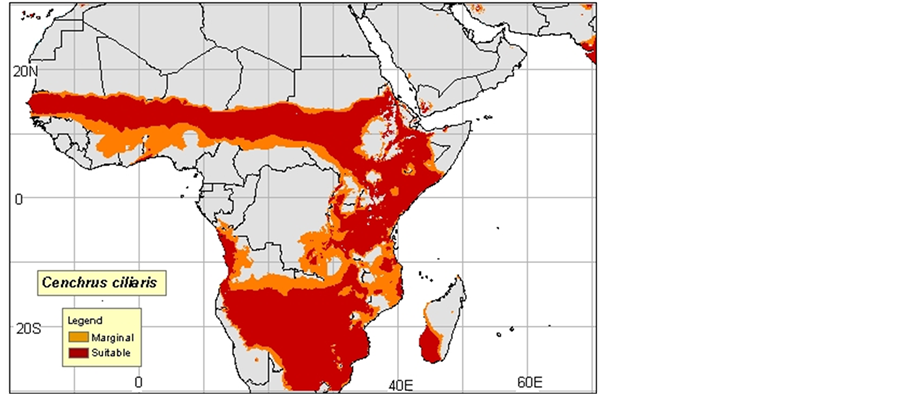
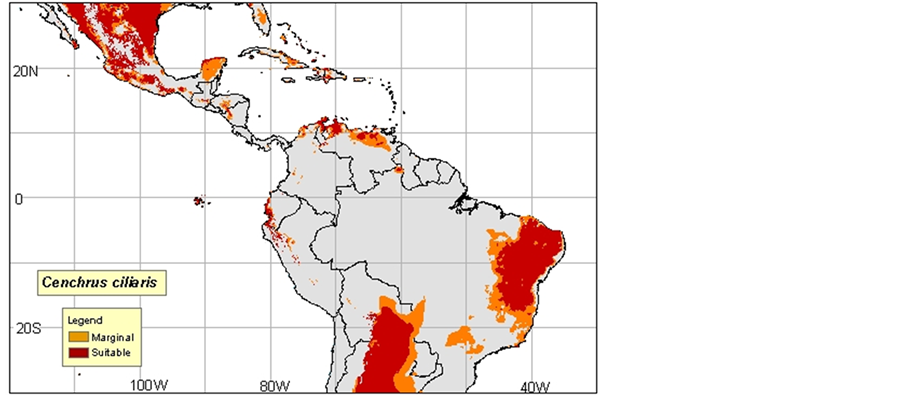
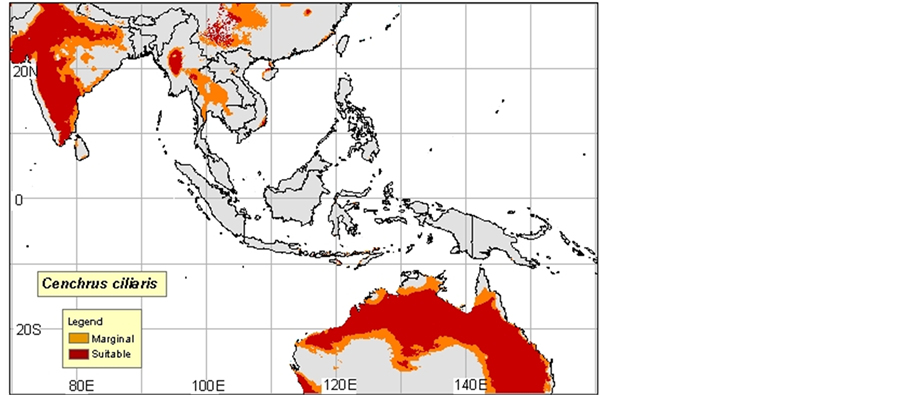
2) Cenchrus ciliaris: Native, dry and sandy regions are everywhere in Africa, Arabia, Canary Islands, Malaga he, Indonesia, North India, and Pakistan. Introduced into many tropical regions and subtropical of the world.
Probably introduced into Western Australia approximately 1870-1880 in Afghan camel harnesses. Adventitious in North America (Texas, Mexico), South America, the Antilles, Hawaii, and Virgin Islands (Neffati, 2008).
(a)
(b)
(c)
Map 1. (a)-(c): http://www.tropicalforages.info/key/Forages/Media/Html/images/_A.jpg
3) Cenchrus ciliaris was often naturalized and often became an intrusive species in Australia, in the southwest of the United States, in the Hawaiian Islands, in Mexico, in Central America, in South America and in Macaronesie. In the desert of Sonora, Cenchrus ciliaris was introduced to fight against the erosion. In the Mexican part of this desert, hit is always cultivated and irrigated to graze the cattle. It propagates very quickly and often kills local plants such as Parkinsonia aculeata by depriving it of water. This plant ignites very easily, even in period of growth. Its fast flammability and its fast regrowth allow it to compete successfully almost with all the vegetation in this region, (Maps 2(a)-(c)).
2.2. In Tunisia
1) Cenchrus ciliaris: Guenod, 1954 mentions that this species is very wide-spread in all the territories of the dry hillsides of the North up to the extreme South. While according to Boukhris and Chaieb, 1998 at present this specie is frequent only in Limestone Mountains and their piedmont as he considers it in the process of regression because of the strong animal pressure.
According to Bogdan, 1977 in Neffati, 1994 this species develops from the sea level until 2000 m of height. Neffati, 1994 considers that C. ciliaris is adapted to even rocky and slightly sandy soil and tolerates the flooded soils where the drainage is bad (Map 3).
2) Digitaria commutata: According to Neffati, 1994, this species is located in the rocky pastures of the dry and hot regions and exists only in Ain Cherichira in Tunisia. Chaieb, 1989 mentions that this species appears in a new station localized on the south hillside of Jebel Kbar belonging to the governorate of Sidi Bouzid characterized by a semi dry bioclimatic under first floor with a variant in moderate winter (Map 3).
2.3. Characteristic Ecological of Specie
1) Digitaria commutata: It possesses a restricted distribution with an important density on rocky and wet soil colonizing slopes to receive more the water of streaming. In fact, it is accessory and appears in bundle. The sexual multiplication gives rates of seeding of the order of 10%, after soaking in distilled water during five hours what explains the presence of hydro soluble inhibitive substances; so the continuation in the time for the same treatment can improve strongly the germinal capacity of this species (61%). However, the denudation of seeds gives only a rate of the order of 19.5% (Dhib, 2007).
In the natural conditions, this species shows an important evolution, from autumn to spring, in height and in diameter, explained by the physiological activity which takes place at the beginning of winter and continues in spring. While in the semi-controlled conditions the obtained results translate a strong sensibility in the wintry cold shown by the death of the young seedlings (Dhib, 2007) (Figure 1).
2) Cenchrus ciliaris: It is for wide distribution and of important abundance on rocky and sandy ground and is at the level of the sea and in low heights up to 2000 meters in height. This bushy species is classified to be constant, called also pleotropicalThe seeding of seeds is weakly favored only after soaking in boiling water (4%) while soaking distilled in water during five hours engenders a higher rate of seeding (47%), thus the inhibition can be due to the presence of inhibitive substance at the level of integuments (Dhib, 2007).
Both studied species have a starting up of the vegetative phase connected to the hydric contributions. So the paleotropicales species present under a Mediterranean climate possess a life cycle characterized by two periods of growth an autumnal and the other one is spring, and two wintry and summer rest periods.
however, these species possess a sensibility in the low temperature, which leads to death especially for the young plantations of the year, but in spring and in the presence of hydric reserve even weak in the ground C. ciliaris and D. commutata mark an important vegetative activity (Dhib, 2007).
With the exhaustion of the hydric reserves at the end of the spring and of beginning summer, we register the drying of these two species entering for a period of summer quiescence, what explains their naming by aridopassive species (Figure 2). This it is demonstrated by Chaieb, 1989 of these two to species as well as Plantago albicans.
Table 1 shows the different characteristics of study sites:
1) The height of the plant: The results show that the height of plants studied by Cenchrus ciliaris is variable from one site to another, for the population Matmata, Bni khdach and Jerba, we register the maximal values
(a)
(b)
(b)
Map 2. (a)-(c): http://www.tropicalforages.info/key/Forages/Media/Html/images/_A.jpg
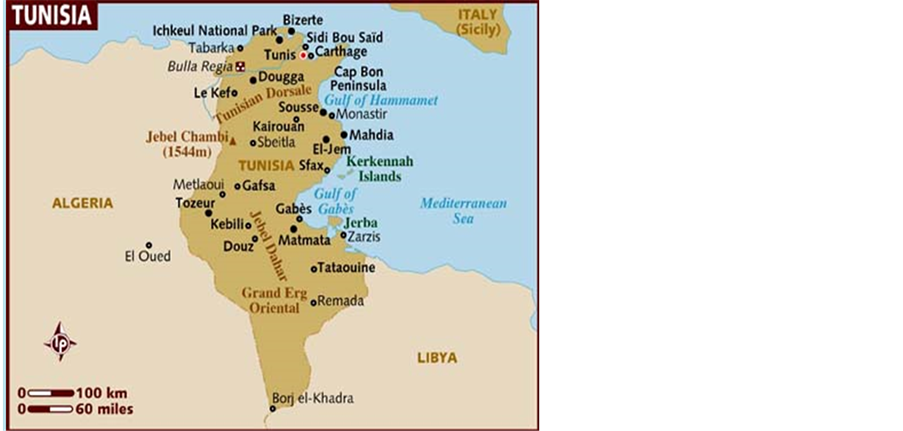
Map 3. Species distribution in Tunisia.

Figure 1. Phenological cycle of Digitaria commutata.

Figure 2. Phenological cycle of Cenchrus ciliaris.
Table 1. Geographic characteristics of station.
Note: But the various detailed study of the morphological parameters during the year show a considerable remarkable difference of these species of one site of study to another.
during December of the order (39, 16; 53, 5; 39, 66 cm) while for that of Bouhedma (57 cm) and Zarzis (20, 33 cm) their maximum is registered during January. A decrease of the height is obtained in spring with a 100% mortality rate of the bundles for the population of Zarzis, Figure 3.
2) Number of green leaves: The results show that the number of green leaves of plants studied by Cenchrus ciliaris is variable from on site to the other, we register the maximal values during December of the order of, 33, 33; 42, 75; 39 respectively for the population of Matmata, Bni khdach and Jerba, while for that of Bouhedma (77) and Zarzis (35, 5) their maximum is registered during January. The number of green leaves decreases for all the populations during the month of Mars, Figure 4.
3) The length of green leaves: The results show that the green length of the leaves of Cenchrus ciliaris is variable from a one site to another, all the populations register maximal values during December of the order of 22 cm for the population of Bouhedma, 25 cm for that of Matmata, 12.5 cm for that of Bni khdach and 5.66 cm for that of Jerba, with the exception of that of Zarzis (7 cm their maximum is registered during January. A drying of the older green leaves is registered since the January followed by a total drying marked at the end of the spring, Figure 5.
4) The number of seed: The results show that the number of seeds to plants studied by Cenchrus ciliaris is variable from one site to another, for the populations of Bouhedma, Matmata, and Jerba, we register the maximal values during December respectively are 66, 62 and 31, 66, while for that of Bni khdach (65) and Zarzis (9, 66) their maximum is registered during January. A decrease in the number of seed by ear is obtained in spring with complete disappearance of seeds since the beginning of the month in May to all the populations, Figure 6.
5) The number of ear: We obtained a greater number of ear during the month of January to the populations BC and KC of the order of 15, 33, 13, while for the other populations the number of the highest ear is registered during the month of December, this number decreases and is reduced to naught at the population KC, ZC, JC during the month of February, Figure 7.
6) The length of inflorescence: The results show that the value of the length of inflorescence is variable from one population to another, the maximal value of which is registered at the population of BKC, BC and MC (6.5, 6.17, and 6 cm) while we obtained a no value for KC, JC, and ZC during the third month of measure, Figure 8.
7) The number of knot: The number of knot varies from one population to another whose maximal values are registered at the population BD and ZC (16, 17, 13, 67) during the month of February for BD and the month of January for the population ZC. The number of knot decreases at KC (1, 5) JC (3, 17) and ZC (1, 83), Figure 9.
8) Distance between knot: We obtained variable value of the distance between knot with a significant difference enter BD and various other populations of Cenchrus ciliaris the distance of which is of the order of 14, 29 cm (in December) and decreases during the month of January and February, 5, 33 and 6, 42 cm respectively for the populations of Cenchrus ciliaris the distance between knot is different, it varies 4, 3 at BC dur ing the month of Mars in 1.27 at ZC during the same month, Figure 10.
9) Variation of the parameters phenology at Dgitaria commutata: The results show that for Digitaria comutata species with localization restricted to Bouhedma, the maximal length of plants is registered during January as most part of the populations of Cenchrus ciliaris, also for the number of green leaves as well as the length of inflorescence. While for the number of ear and the number of seed by ear, the values most brought up are respectively obtained during December are 9, 4 and 59, 4 to this species, we notice onsets of the scattering of
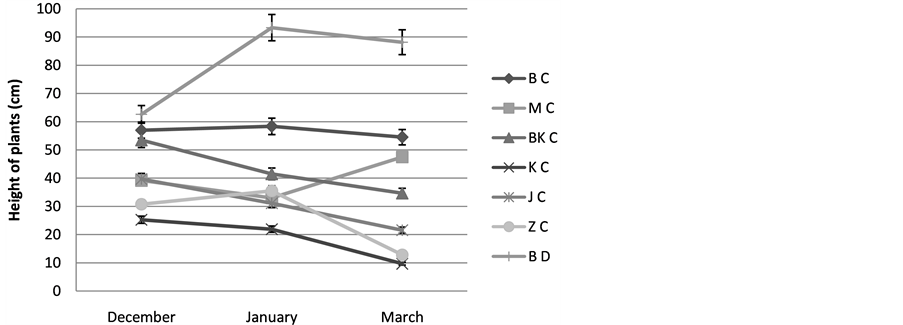
Figure 3. Varying the height of plants; BC: Cenchrus ciliaris of Bouhedma, MC: Cenchrus ciliaris of Matmata, BK C: Cenchrus ciliaris of Bni Khdach, KC: Cenchrus ciliaris of Khanguit aicha, JC: Cenchrus ciliaris of Jerba, ZC: Cenchrus ciliaris of Zarzis.
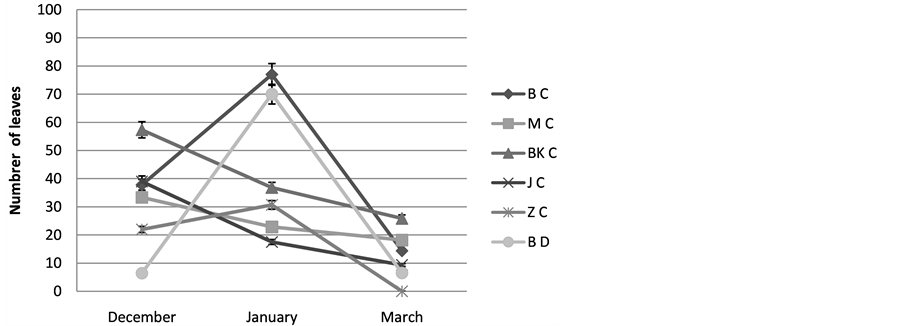
Figure 4. Varying the number of leaves; BC: Cenchrus ciliaris of Bouhedma, MC: Cenchrus ciliaris of Matmata, BK C: Cenchrus ciliaris of Bni Khdach, KC: Cenchrus ciliaris of Khanguit aicha, JC: Cenchrus ciliaris of Jerba, ZC: Cenchrus ciliaris of Zarzis.
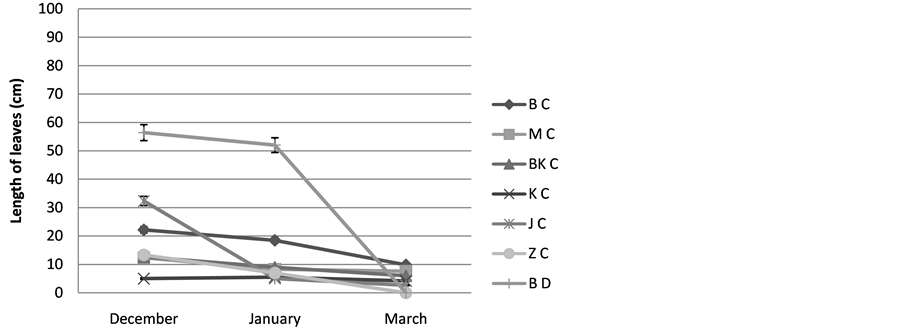
Figure 5. Varying the length of leaves; BC: Cenchrus ciliaris of Bouhedma, MC: Cenchrus ciliaris of Matmata, BK C: Cenchrus ciliaris of Bni Khdach, KC: Cenchrus ciliaris of Khanguit aicha, JC: Cenchrus ciliaris of Jerba, ZC: Cenchrus ciliaris of Zarzis.
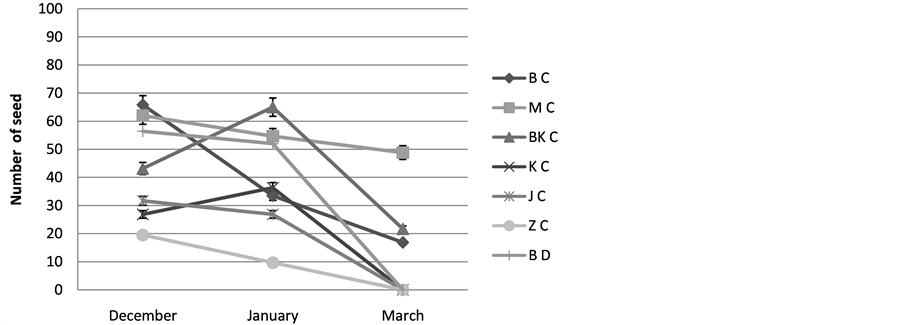
Figure 6. Varying the number of seed; BC: Cenchrus ciliaris of Bouhedma, MC: Cenchrus ciliaris of Matmata, BK C: Cenchrus ciliaris of Bni Khdach, KC: Cenchrus ciliaris of Khanguit aicha, JC: Cenchrus ciliaris of Jerba, ZC: Cenchrus ciliaris of Zarzis.

Figure 7. Varying the number of ear; BC: Cenchrus ciliaris of Bouhedma, MC: Cenchrus ciliaris of Matmata, BK C: Cenchrus ciliaris of Bni Khdach, KC: Cenchrus ciliaris of Khanguit aicha, JC: Cenchrus ciliaris of Jerba, ZC: Cenchrus ciliaris of Zarzis.

Figure 8. Varying the Length of inflorescence; BC: Cenchrus ciliaris of Bouhedma, MC: Cenchrus ciliaris of Matmata, BK C: Cenchrus ciliaris of Bni Khdach, KC: Cenchrus ciliaris of Khanguit aicha, JC: Cenchrus ciliaris of Jerba, ZC: Cenchrus ciliaris of Zarzis.

Figure 9. Varying the number of knot; BC: Cenchrus ciliaris of Bouhedma, MC: Cenchrus ciliaris of Matmata, BK C: Cenchrus ciliaris of Bni Khdach, KC: Cenchrus ciliaris of Khanguit aicha, JC: Cenchrus ciliaris of Jerba, ZC: Cenchrus ciliaris of Zarzis.
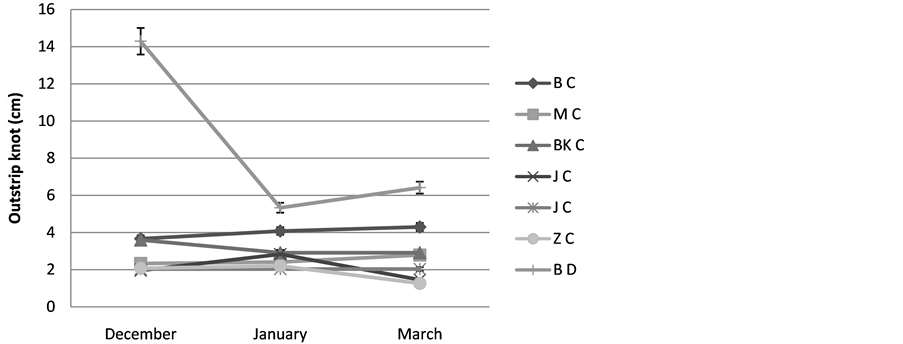
Figure 10. Varying the outstrip knot; BC: Cenchrus ciliaris of Bouhedma, MC: Cenchrus ciliaris of Matmata, BK C: Cenchrus ciliaris of Bni Khdach, KC: Cenchrus ciliaris of Khanguit aicha, JC: Cenchrus ciliaris of Jerba, ZC: Cenchrus ciliaris of Zarzis.
seeds since the month of March which becomes more marked at about May Figure 11.
3. Statistics
Since the first dates of measure, the comparison inter popular of the averages of length of the plant shows the presence of four different groups with significant differences between (ZC), (KC), (MC; JC), (BKC; BC) and (BD).
Concerning the number of green leaves, the forth group distinguishes itself with a significant difference between (ZC; KC), (BD; MC; BKC), (BC) and (JC).
Four significantly different groups for the length of leaves were registered, (KC, ZC); (BD, MC, and BK C); (BC); (JC). For the number of ear we note four different group, (KC, MC); (ZC, BC, JC); (BD); (BK C). We obtained four so significantly different groups (ZC) (KC, JC); (BD, MC, BC) for the NBG.
For the length of inflorescence, we note four groups so significantly different (KC, JC, ZC); (ZC, BC); (BC, BK C, MC); (BD).
As regards the number of knot so significantly different, four groups were registered (recorded) (ZC); (BD, KC, BC); (KC, BC, JC); (JC, MC, BK C).
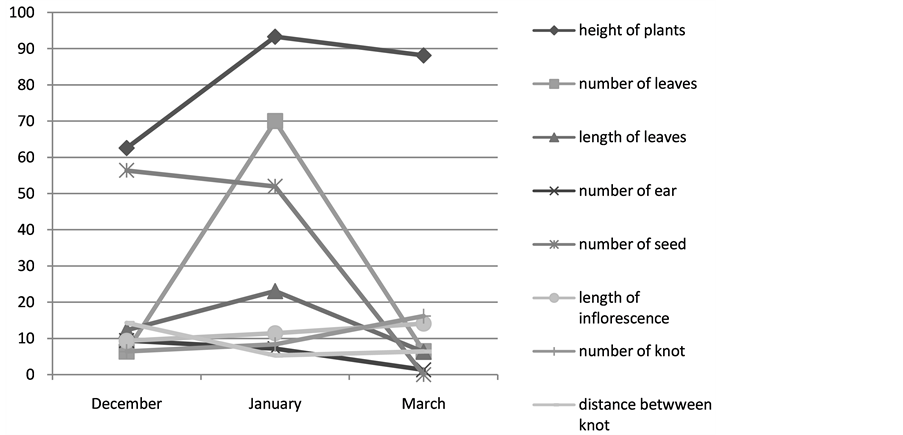
Figure 11. Varying of characteristic morphological of Digitaria commutata of Bouhedma.
Two group for the criterion outstrips between knot (ZC, JC, KC, MC, BK C, BC) (BD) and four group for the color of ear (KC, MC, BK C); (BK C, BD); (BD, BC, JC); (ZC). This variation inter and intra site is revealed also during the second date of measure (I of January)
For the length of plants the analysis shows an important variability which defined four different groups (KC, JC, MC); (JC, MC, ZC, BK C); ( BC); (BD), while the criterion number of green leaves only defined only two different groups (JC, KC MC, ZC, BK C) (BD, BC). Four significantly different groups for the character length of leaves (JC, KC ZC, MC) (BK C) (ZC) (BD) and two significantly different groups for the number of ear (ZC, MC, KC, JC, BK C, BD) (BC).
For the number of seed five significantly different groups (BC, JC) (JC, BC, KC) (BC, KC, BD), (KC, BD), (BK C, MC, BD).
Also for the length of inflorescence we have five significantly different groups (ZC) (JC KC) (KC MC) (BC, BK C) (BD)
While for the number of knot we obtained two significantly different groups (KC, MC, JC, BD, BK C, BC) (ZC) and three significantly different groups for the distance between knots (ZC, KC, MC, BK C) (JC, BK C, BC) (BD) and for the color of ear we obtained 3 groups ( BC) (BC, BD, JC, KC, MC) (KC, MC, BK C).
For the third date of measure that is last phase of the phenologic cycle of both species, we obtained a difference which is not significant for the criterion length of the green leaves as well as for the number of knot.
The analysis shows five different groups (KC, ZC) (ZC, JC) (BK C) (MC, BC) (BD) for the criterion length of plants.
For the number of green leaves, four significantly different groups are obtained (KC, ZC, BD, JC) (BD, JC, BC) (BC, MC) (MC, BK C)
The significant difference for the number of ear of which we obtained three groups (KC, BK C, BD, MC) (BK C, BD, MC ABC, ZC) (MC ABC, ZC, JC).
For the number of green seeds, the difference is significant and it defines three groups (JC, ZC, KC, BD) (BK C, BC) ( MC), for the length of inflorescence we obtained four groups (KC) (BK C, JC, ZC, BC) (MC) (BD) for the distance between knots four significantly different groups are obtained (ZC, JC, KC) (KC, MC, BK C) (BC) (BD) and for the color of ear, three groups are defined significantly different (JC) (MC, BK C, KC, BD, BC) (ZC), Table2
BC: Cenchrus ciliaris of BouhedmaMC: Cenchrus ciliaris of MatmataBK C: Cenchrus ciliaris of Bni KhdachKC: Cenchrus ciliaris of Khanguit aichaJC: Cenchrus ciliaris of JerbaZC: Cenchrus ciliaris of ZarzisBD: Digitaria commutate of Bouhedma.
4. Discussion
The distribution of these species shows their intense need in water. The location of all the sites of studies well justifies this remark: At the level of the national park of Bouhedma C. ciliaris and D. commutata colonize the
ground in the energy tour(tower, ballot) of the water lowering from Ain Cherchara along the valley the presence of the species become rare especially for D. commutata (Chaieb, 1996).
For the sites of study of Matmata, Khanguit aicha and Bni khdach it is about the foothill from which they receive enough water, while the sites of studies of Jerba and Errissifet (Zarzis) is at the level of a quotation of sea.
All the results obtained reveal that these two endangered grasses are dependent basically on the hydric state of the ground. In fact since the first drops of rain in December, even with very low quantities, we notice a starting up of the vegetative activity which becomes more marked with the increase of the quantities of water in the ground (important rain quantity) marked during January and February. Although this study met the work of Chaieb, 1993 who demonstrated the fact of these two species ), we have a different point of view in that he indicated that there is a certain gap of time between both species as regards the initiation and the full vegetative activity, while I see that both species begin together their vegetative cycle.
Cenchrus ciliaris and Digitaria commutata show a particular reproductive growth rate:
1) They are very relying on the hydric state of the ground 2) They can present two cycles of reproductions:
The first cycle begins after the first autumnal rains, continues during winter showing a slow vegetative growth rate, because of the sensibility indicated by these species towards the cold winter. The phase of earing begins at the end of February followed by the fruiting during March, continues till the end of May where the phase of scattering begins during June.
The second cycle which concerns only Cenchrus ciliaris and begins after the spring rains, but it is necessary to indicate that in certain site of study there is absence of this second cycle such as the populations of Zarzis and Jerba that can be unexplained by the absence of haste in these sites of study. Besides, the important temperature stresses the evapotranspiration and accelerates the hydric exhaustion of the reserves of the ground what obliges plants to end their vegetative cycle since May.
All these results defined the strategy adopted by these two plants to face the major constraint which is the hydric deficit and thus the intense vegetable dehydration of tissues. However, these two species show the strategy of escape the drought called also strategy of dodge where plants manage to close their cycle before the installation of the constraint (Hamza, 1996).
According to Ozenda (1977), C. ciliaris and D. commutata belong to all the species called géophytes that resist the constraint by subterranean organs as the fleshy rhizome, bulb, after the dry period in the state of slow life ephemeroids.
The hierarchical clustering (AHC) determined by the XLSTAT software shows a dendrogram (Figure 12)
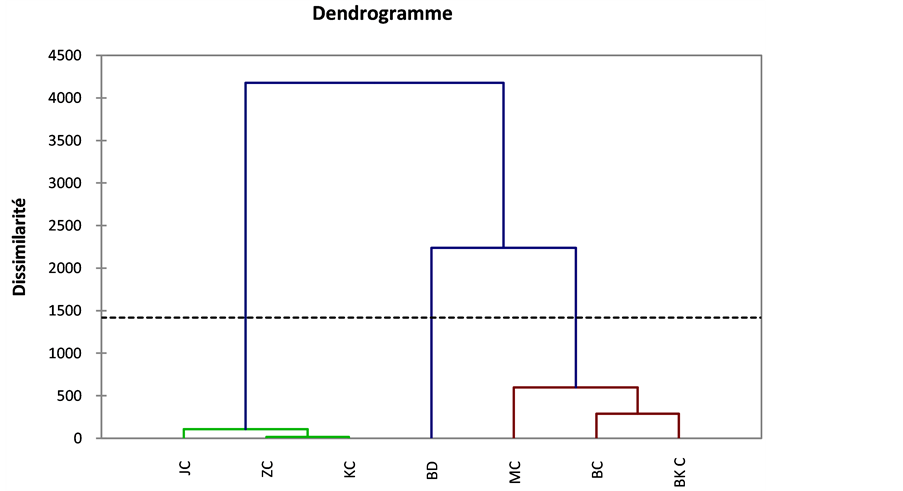
Figure 12. The hierarchical clustering of population.
that groups two pairs of close relatives or (people) in their ecological characteristics: BKC, BC and KC, ZC. MC population and kinship with the first couple and the JC population is closer to the second couple. BD, which is the population of Digitaria commutata Bouhedma, has different ecological characteristics of other populations and therefore a far relationship.
5. Conclusion
We notice that the morphological modifications are translated by a reduced consumption system of water and a considerable development of the food system.
The limitation of loss of water is made by the reduction of transpiring surfaces with a roll-up of leaves to protect stomata and thus to minimize the loss made by evapotranspiration. This is demonstrated by B. Henchi et al. (1986) concerning Plantago albican species not tolerant to the drought but capable of avoiding the dry.
References
- Chaieb, M. (1993). Dynamique actuelle de la végétation naturelle en Tunisie méridionale, sujet complémentaire. Thèse, FST de Sfax, 42.
- Chaieb, M. (1989). Influence des réserves hydriques du sol sur le comportement comparé de quelques espèces végétales de la zone aride tunisienne. Thèse, Université des sciences et techniques de Languedoc, Académie de Montpellier. 292.
- Chaieb, M. (1996). Réponse écophysiologique de trois graminées pérennes soumises à des conditions écologiques contrastes en milieu aride de la Tunisie, étude en condition naturelle, semi controlées et controlées. Thèse de doctorat es sciences. Université de Sfax pour le sud, Faculté des sciences de Sfax, 283.
- Dajoz, R. (1978). Précis d’écologie (p. 534). Paris: Dunod.
- Dhib, I. (2007). Etude des caractéristiques morphologiques, botanique et écophysiologiques de quelques espèces menacées de disparition du parc national de Bouhedma. Mastère écophysiologie végétale, Faculté de science de Tunis, 120.
- Guenod, A. (1954). Flore de la Tunisie (Cryptogame, vasculaire, Gymnospermes et Monocotyledones), Ouvrage imprimé par S.E.F.A.N Tunis, 287.
- Le Houerou, H. N. (1969). La végétation de la Tunisie steppique (1) (Structure, écologie, sociologie, répartition, évolution, utilisation, biomasse, productivité) (avec référence aux végétations analogues d’Algérie, de Libye et du Maroc). Annales de l’Institut National de la Recherche Agronomique de la Tunisie, 42, 622.
- Hamza (1996). Adaptation écophysiologique in “Adaptation des végétaux au milieu aride”. Ouvrage collectif coordonné par Nabli M.A.H. Vol. 8.
- Henchi et P. Louguet et J.B. Viera Di Silva (1986). Evitement ou tolérance dans l’adaptation à l’aridité du plantain blanchatre: Plantago albican ssp albicans L. colloque sur les végétaux en milieu aride Tunisie (Jerba). 8-10 Septembre 1986.
- Khedim, A. H. E., Khelifi, M., Nabi, K., Hadj-Omar, M., Mefti, S., Mouche, D., Bellague, M., M’Hammdi Bouzina, M. Laouar, B. A., Merabet, H., & Bouzezour, A. A. (2004). Etude de comportement de trois graminées fourragères: Dactylis glomerata L., Festuca arundinacea Schreb., Phalaris aquatica Desf., en Algérie.
- Lacoste. A., & Salanon, R. (1969). Eléments de biogéographie et d’écologie (p. 189). Paris: Fernard Nathan.
- Neffati, M. (2008). Domestication des plantes spontanées autochtones à usage multiples en zones arides et désertiques. Guide pratique de collecte, de conditionnement et de germination de leurs semences. Aout 2008.
- Neffati, M. (1994). Caractérisation morpho biologique de quelques espèces végétales Nord Africaines: Implication pour l’amélioration pastorale. PhD, Sci, biolo, uric, Gent, 264.
- Ouled Belgacem, A. (2001). Dry land pasture, Forage and Range Network News. Tunisie: Issue IRA Medenine.
- Ozenda, P. (1977). Flore du Sahara 2ème édit. CNRS, 622.


