Open Journal of Clinical Diagnostics
Vol.3 No.4(2013), Article ID:40280,5 pages DOI:10.4236/ojcd.2013.34032
De novo cavernous aneurysms development after contralateral parent artery occlusion—Two cases report
![]()
1Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
2Department of Interventional Neuroradiology, Beijing Neurosurgical Institute, Beijing, China
Email: *liuaihuadoctor@163.com
Copyright © 2013 Tangming Peng et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 25 September 2013; revised 25 October 2013; accepted 1 November 2013
Keywords: De novo Aneurysm; Parent Artery Occlusion; Endovascular Treatment; Hemodynamic; Risk Factors
ABSTRACT
A 53-year-old woman developed a de novo aneurysm after contralateral internal carotid artery occlusion, and another 42-year-old woman developed a de novo aneurysm after contralateral vertebral artery occlusion. Both patients experienced a rapid development of de novo aneurysm formation, 6 and 9 months, respectively. The development of de novo aneurysm on the contralateral artery after parent artery occlusion showed that female and medium age may be contributory factors. In addition, the hemodynamic changes may be associated with the development of de novo aneurysm after contralateral parent artery occlusion.
1. INTRODUCTION
Parent artery occlusion (PAO) is regarded as a safe and effective method for treatment of the intracranial aneurysm which cannot be dealt with microsurgical clipping or unsuitable for “traditional coiling”, “balloon remodeling” or “stent-assisted coiling” treatment [1,2]. The longterm complications after parent artery occlusion include delayed ischemia, aneurysm regrowth, development of multiple aneurysms, development of de novo intracranial aneurysm, and delayed intracranial hemorrhage due to a de novo or initial aneurysm [3,4]. The incidence of de novo aneurysm is rare and the etiology is unclear. Reported in previous published data with de novo aneurysms, the risk factors include age, female, hypertension, smoking, alcohol consumption, subarachnoid hemorrhage and multiply aneurysms [5-7]. However, few literatures reported the risk factors that induced the occurrence of de novo aneurysm in detail. In the present study, we described the development mechanism of de novo aneurysm formation after PAO in three aspects, including the incidence, the risk factors, and the underlying mechanism.
2. CASES REPORT
Case one: A 53-year-old woman was admitted to the emergency room of our hospital with sudden onset of a frontal and parietal headache in November 2010. Besides, she was presented without any other neurologic significant symptoms and signs. She had been previously healthy without risk factors such as hypertension, cigarettes smoking, alcohol consumption and inherited disease. Initial digital subtraction angiography (DSA) with bilateral carotid artery and vertebral artery showed that a right internal carotid cavernous aneurysm, while there was absent of aneurysm certified in other sites (Figure 1). A furthermore three-dimension rotational reconstruction DSA showed that the aneurysm size was about 3 × 6 mm. This aneurysm was not suitable for clipping and coil embolization, and balloon occlusion test showed sufficient countercurrent flow to the target artery through the posterior communicating artery and anterior communicating artery. So, the proximal of parent artery was occluded by detachable coils via right femoral access, and the distal of parent artery was occluded by detachable coils via left femoral access. Immediate angiography was performed with subtotal occlusion of the aneurysm (Figure 1). The treatment was uneventful. The woman had no further neurological deterioration and was discharged 3 days after the procedure.
Six months later, the patient returned to our clinic for a conventional angiography follow up without any indisposition. Delightfully, the right aneurysm was well cured without regrowth. However, there was a de novo aneurysm formation at the left cavernous segment with a size
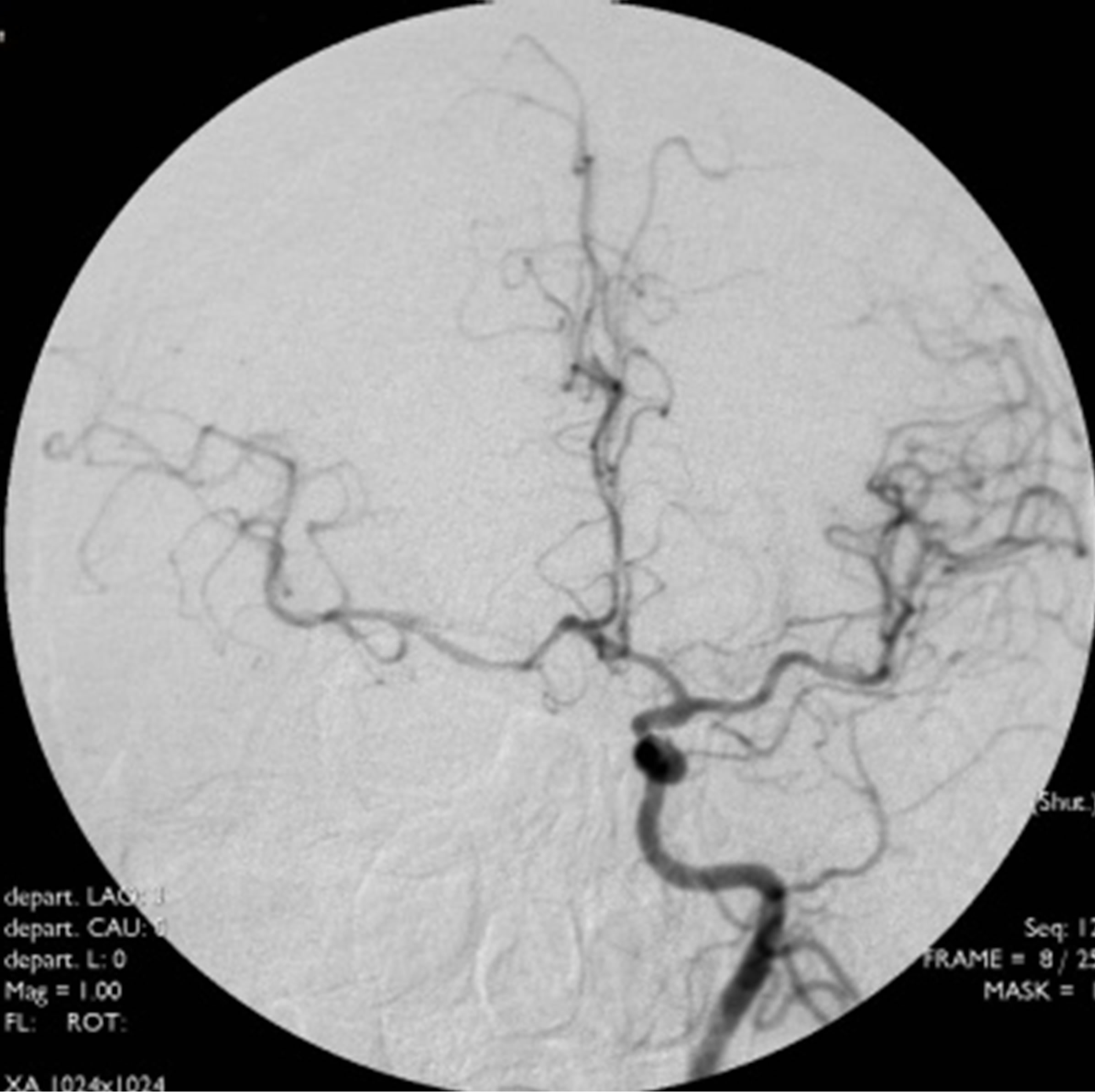
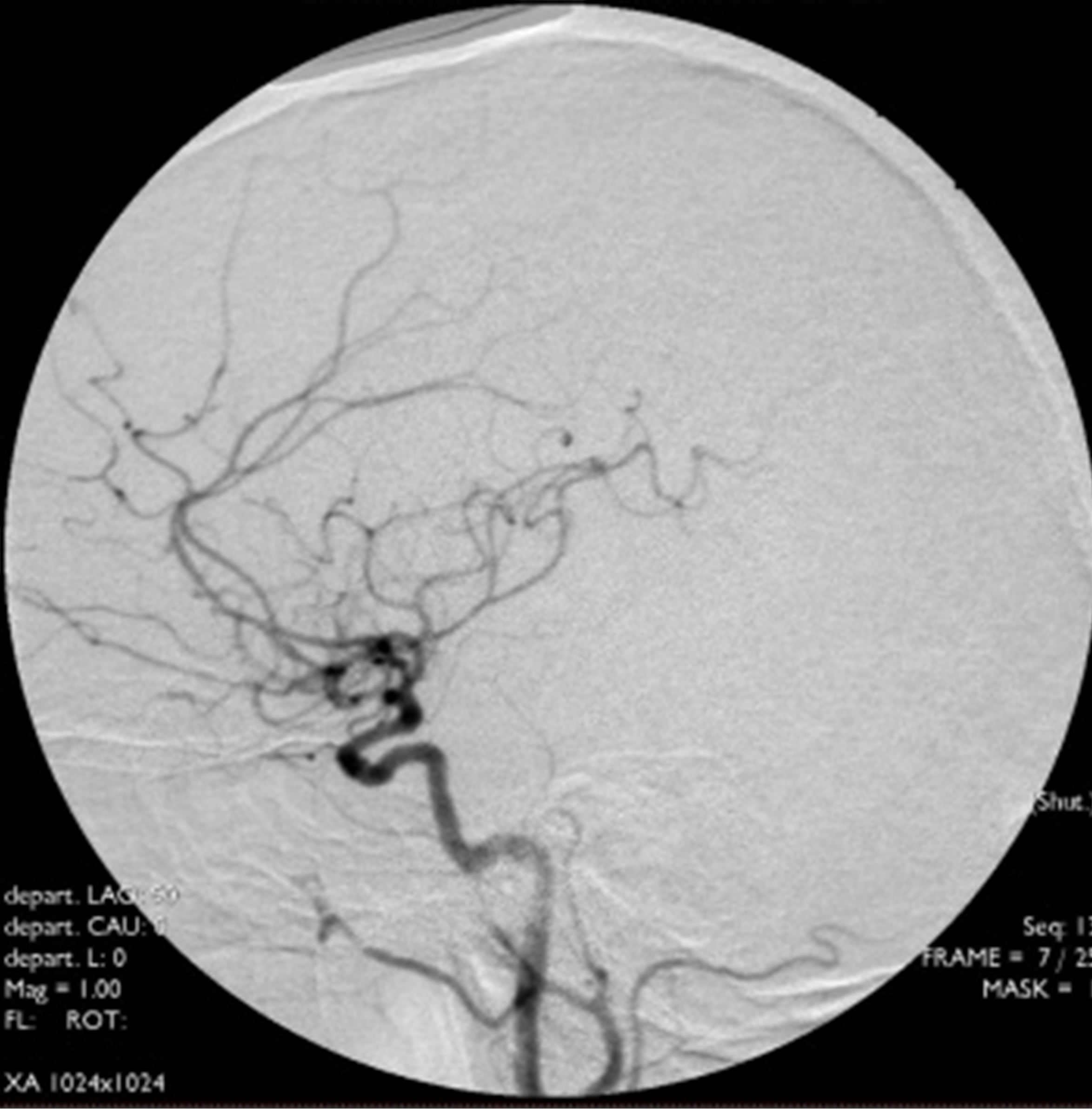
Figure 1. Initial cerebral angiograms. Right carotid angiogram showing a large aneurysm at cavernous segment (L). Left carotid anterior-posterior view and lateral view angiogram showing no aneurysmal shadow (M, R).
of 2 × 3 mm. Even presence of the de novo aneurysm, the patient demanded conservative treatment due to heavy family burden. Another 17-month angiography follow up showed that there was no recurrence with the right cavernous aneurysm, while the novo aneurysm enlarged to 3 × 3 mm. Indication for treatment was decided in multidisciplinary meetings and received the approval of the patient, Enterprise stent-assisted coiling (4.5 × 22 mm) was performed with the help of four coils, postprocedure angiography showed subtotal occlusion of the aneurysm (Figure 2). The patient tolerated the procedure well without any complication and was discharged home.
Case two: A 42-year-old woman underwent the worst tolerated headache all her life without consciousness deficits. She was admitted to the emergency room awake with full consciousness in July 2011. She was also presented without any other neurologic significant symptoms and signs. She had some contributory medical history such as hypotension, cigarette smoking, alcohol consumption. Brian computer tomography was taken and a mild subarachnoid hemorrhage was demonstrated. Cerebral DSA showed a right vertebral artery dissecting aneurysm with a size of 6 × 10 mm (Figure 3). The right vertebral dissecting aneurysm was performed with PAO after a successful balloon occlusion test for carotid obliteration. Immediate angiography showed that the parent artery was occluded and the dissecting aneurysm was also complete occluded without agent filling. The postoperative course was uneventful and the patient was discharged with no neurological deficits. The patient returned to our department for a conventional angiography examination 9-month later without any symptoms and complications. DSA showed a left vertebral dissecting aneurysm which had not been seen in the previous angiography images and films (Figure 4). The de novo aneurysm was followed up conservatively.
3. DISCUSSION
De novo aneurysm formation after therapeutic cerebral artery occlusion is rare. In a review of the literature published from 1962 to 2012, 37 cases with a de novo aneurysm after parent artery occlusion were proved in cerebral angiography, the incidence was reported from 0.7% to 4.3% in 8 large series (including more than 30 patients with parent artery occlusion), whereas, de Gast et al. [3], reported that there was no de novo aneurysm formation in his 52 patients with parent artery balloon occlusion for cerebral aneurysms at a mean of 50.3 months follow-up. Actually, only 5 cases developed a de novo aneurysm after vertebral occlusion in previous reports [8-11]. So, the cumulative incidence may be no more than 2% in calculating all the de novo aneurysms after parent artery occlusion, though those with parent artery occlusion had not been studied systematically [3,12]. Interesting, there was no report about de novo aneurysm formation at the cavernous segment.
In our cohort, there were 243 patients undergone therapeutic cerebral artery occlusion with at least 6 months angiography follow-up, of these, two cases (0.8%) experienced a de novo aneurysm, one at the cavernous segment without any precognition and one experienced a de novo aneurysm after contralateral vertebral artery occlusion. The possibility existed that some individuals with de novo aneurysms were lost to follow-up, died without diagnosis or autopsy. Therefore, the accurate incidence of de novo aneurysms was difficult to judge.
Several events were regarded as risk factors with the development of de novo aneurysm, such as middle age, female, hypotension, alcohol consumption and subarachnoid hemorrhage [13-15]. Although the incidence of de novo aneurysm formation after PAO may be no higher than those patients with clipped aneurysms, some authors considered that PAO itself may contribute to the development of de novo aneurysms.
The pathophysiology and etiology of de novo aneurysm formation remain unclear. However, hemodynamic changes were regarded as an important contributory factor. Hemodynamic changes were characterized as high
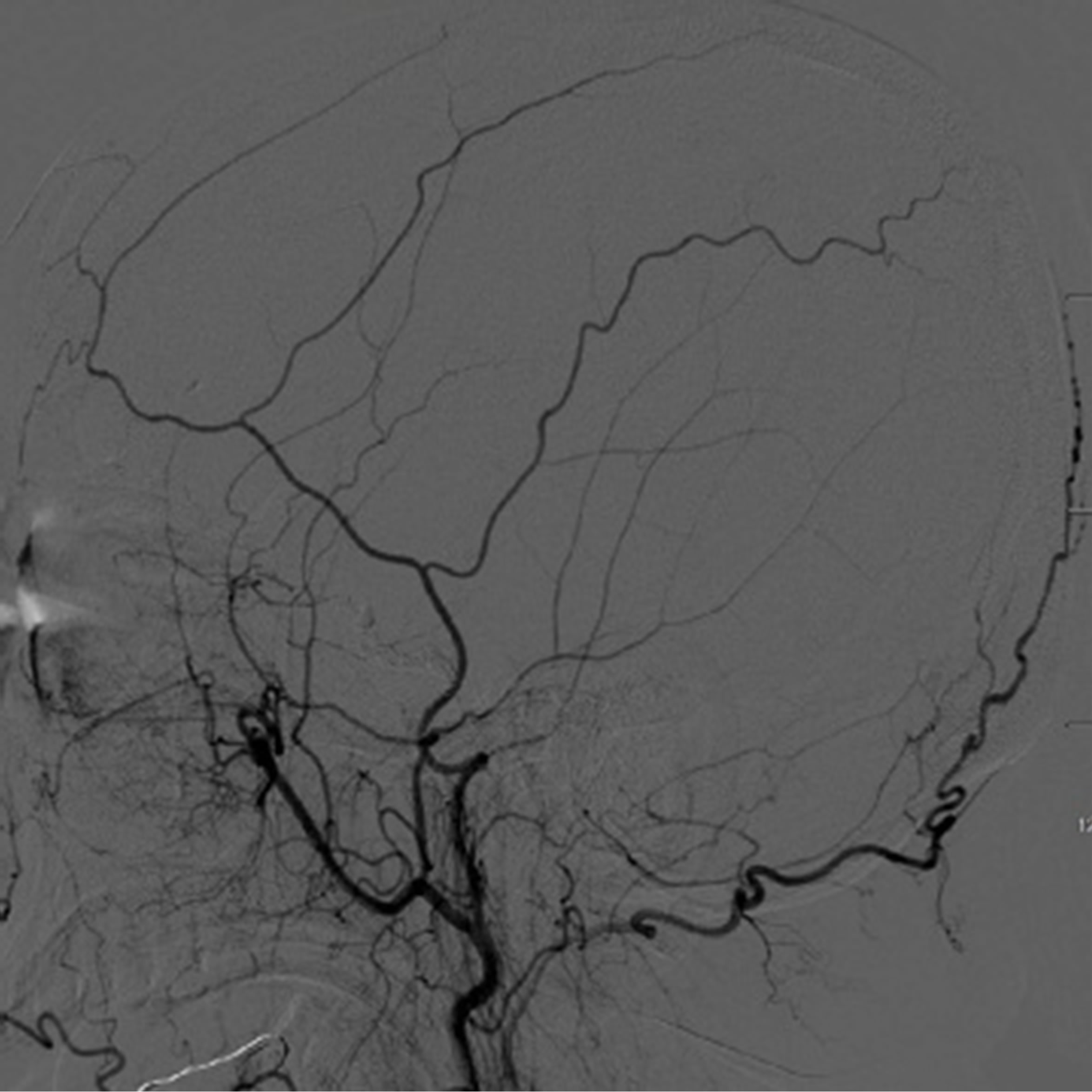
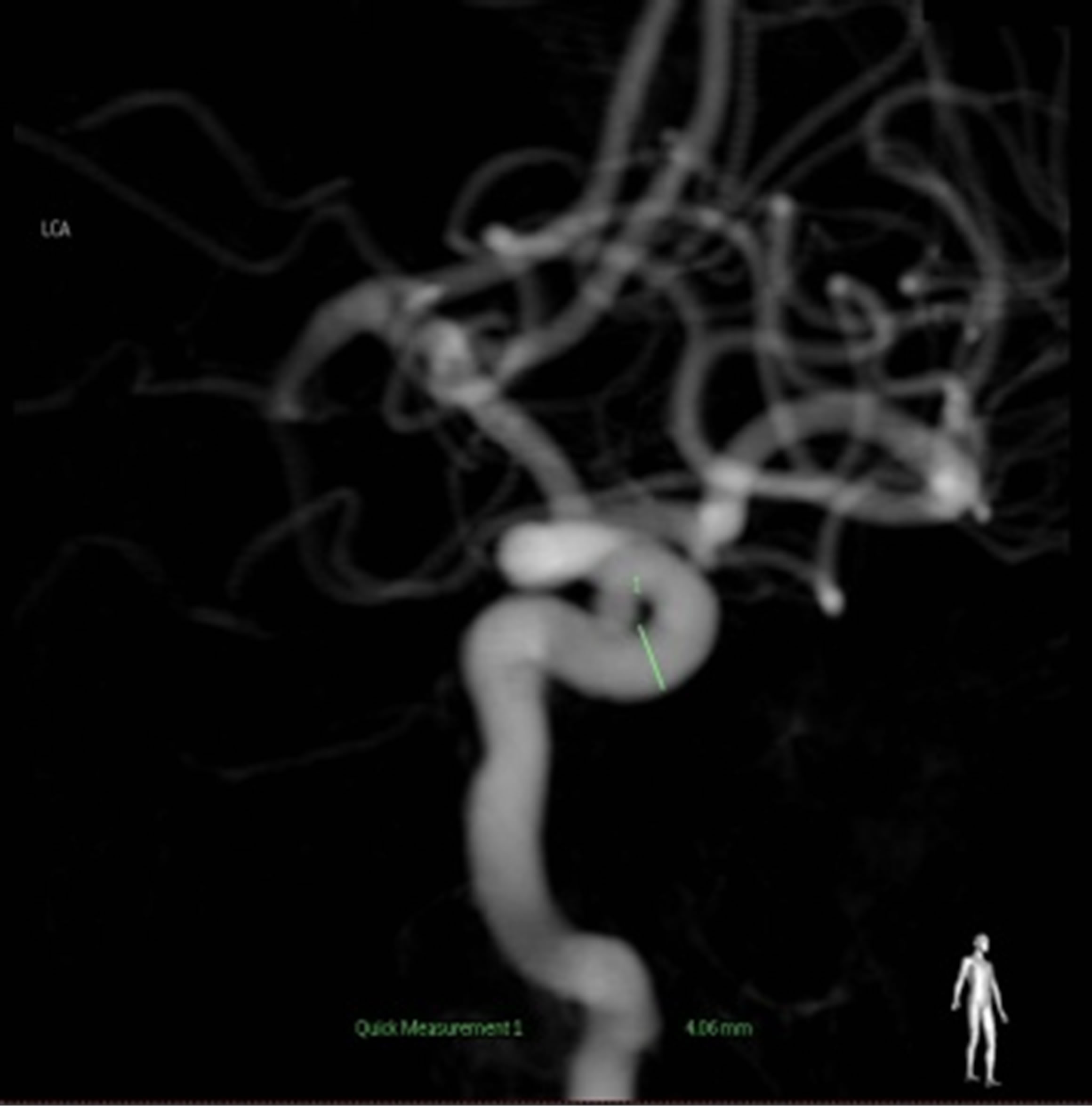
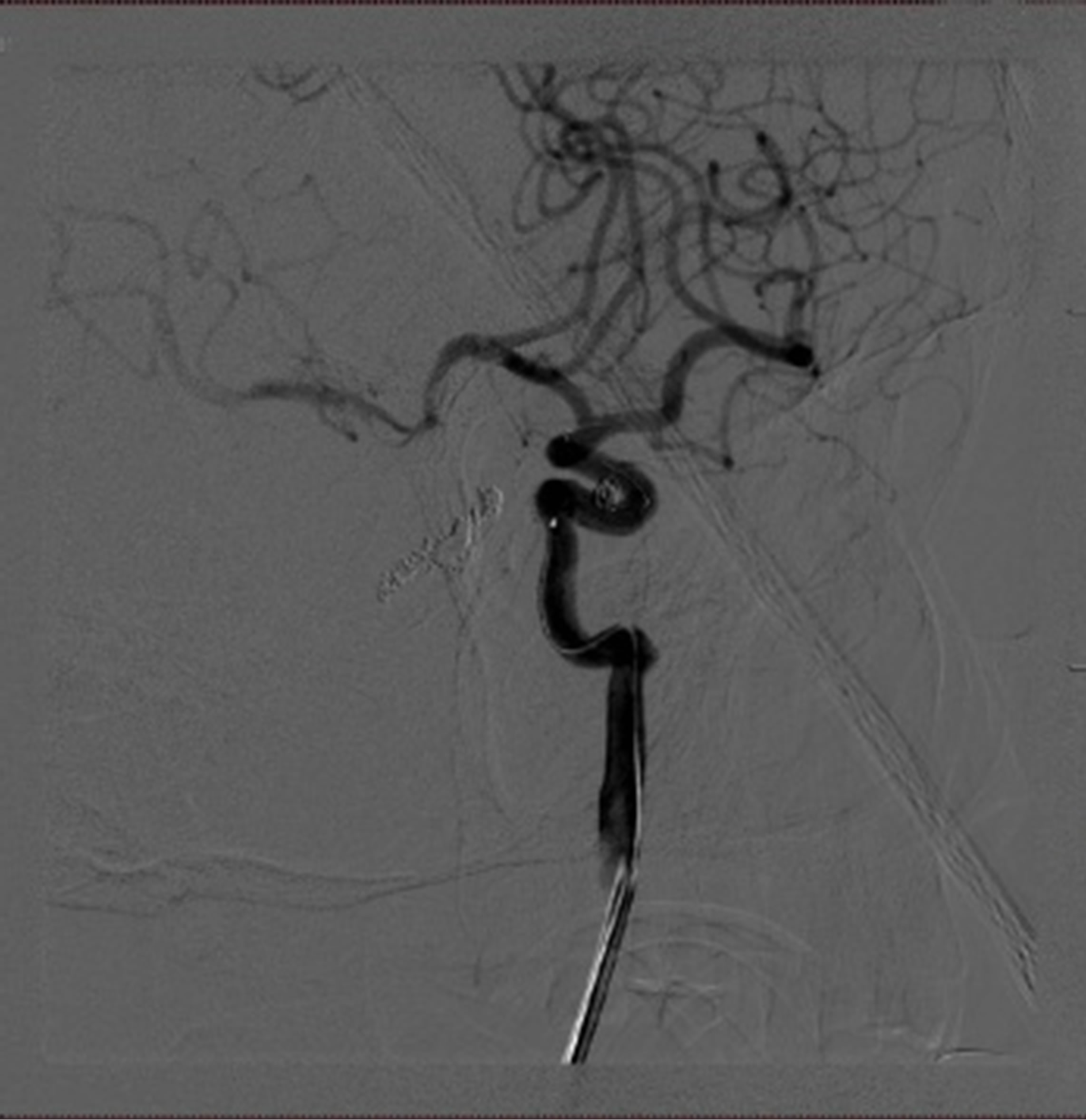
Figure 2. Cerebral angiograms on follow-up. The right cavernous segment was well occluded and no regrowth of previously treated aneurysms (L). carotid angiogram showed a de novo aneurysm at the cavernous segment (arrow) (M). Successful coil embolization of the aneurysm(arrow) (R).
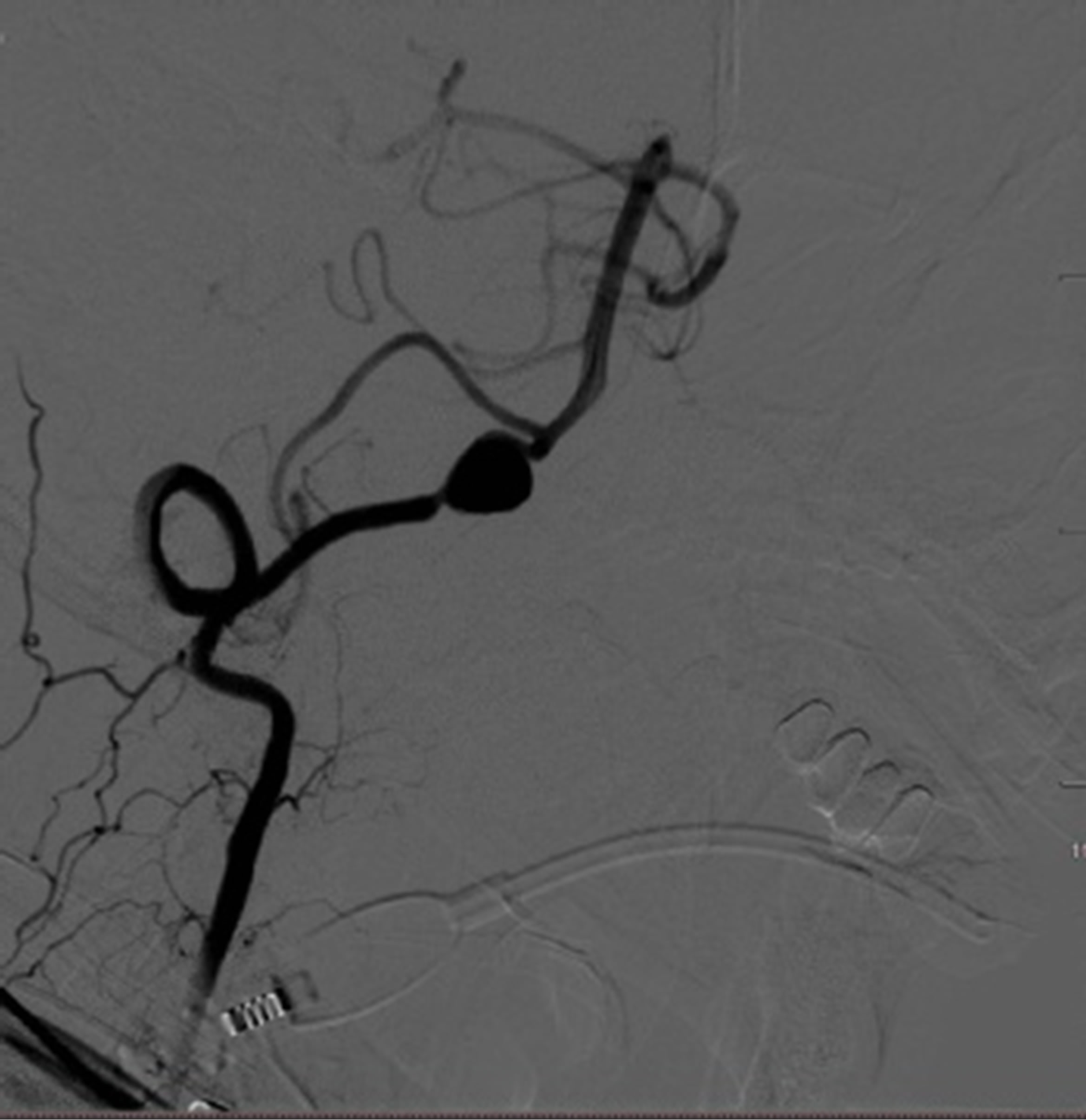
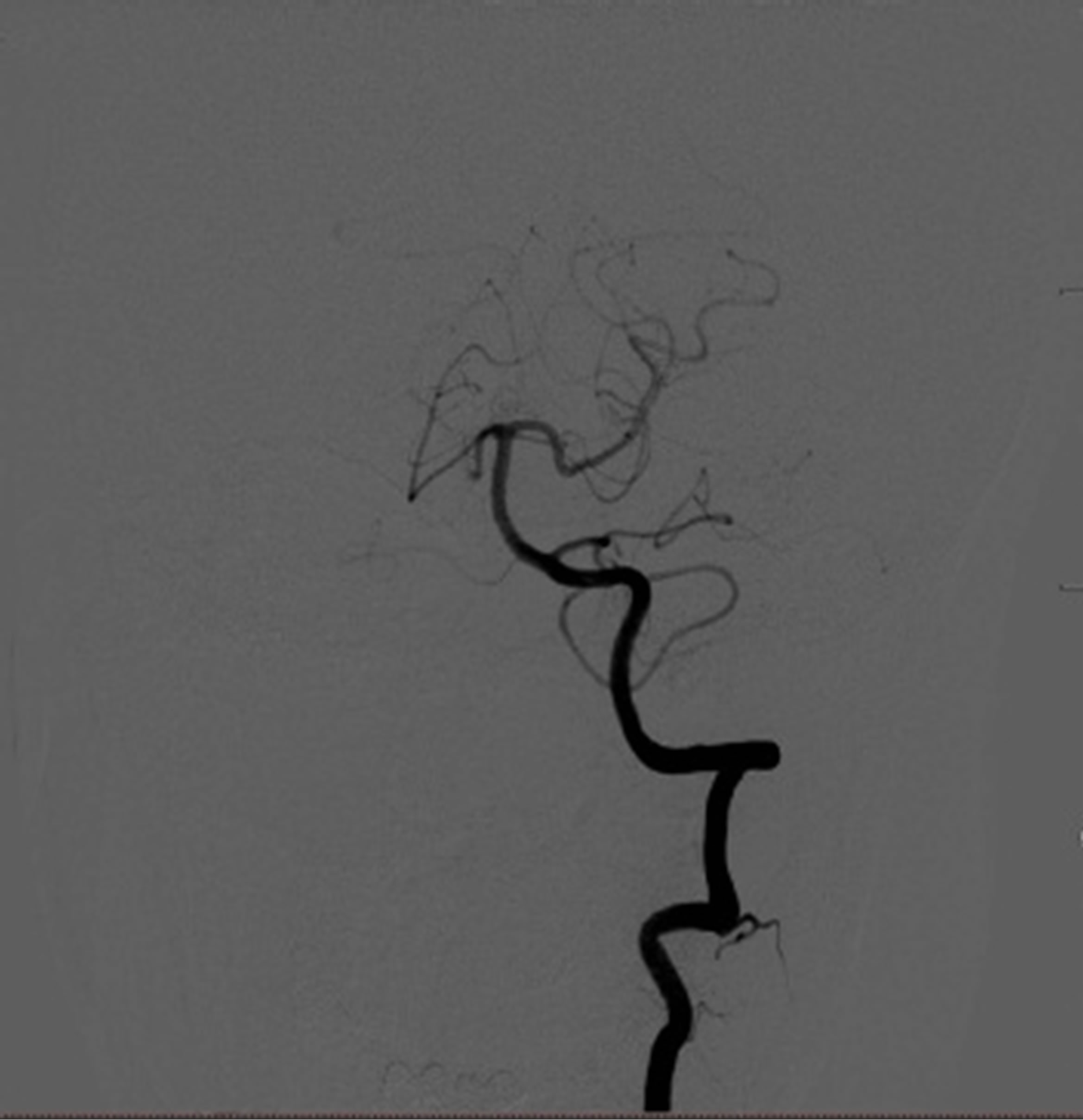
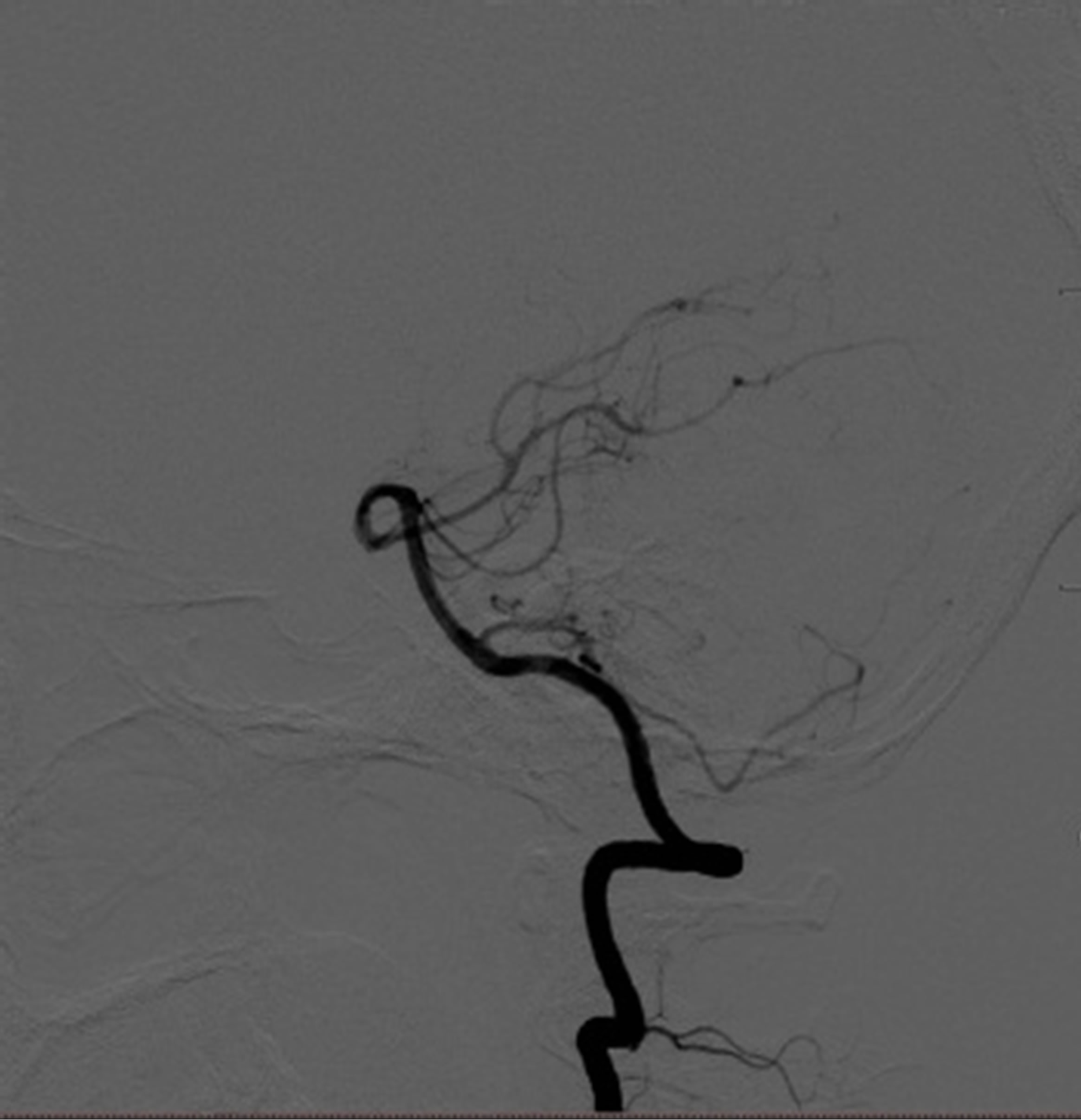
Figure 3. Initial cerebral angiograms. The right vertebral angiogram showing a dissecting aneurysm (arrow) (L). The left vertebral anteroposterior view angiogram showing no abnormal findings (M). The left vertebral lateral view angiogram showing no abnormal findings (R).
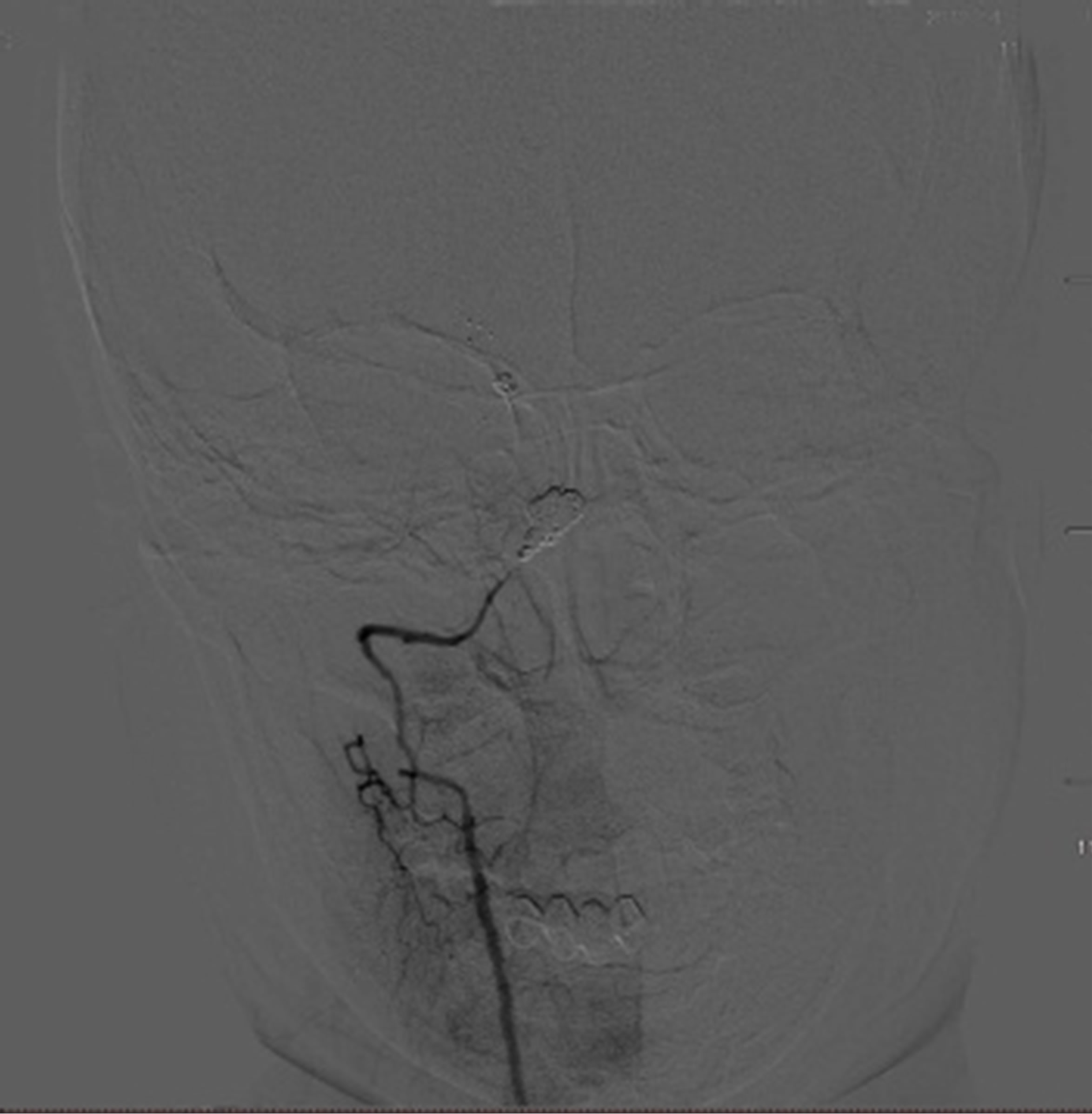
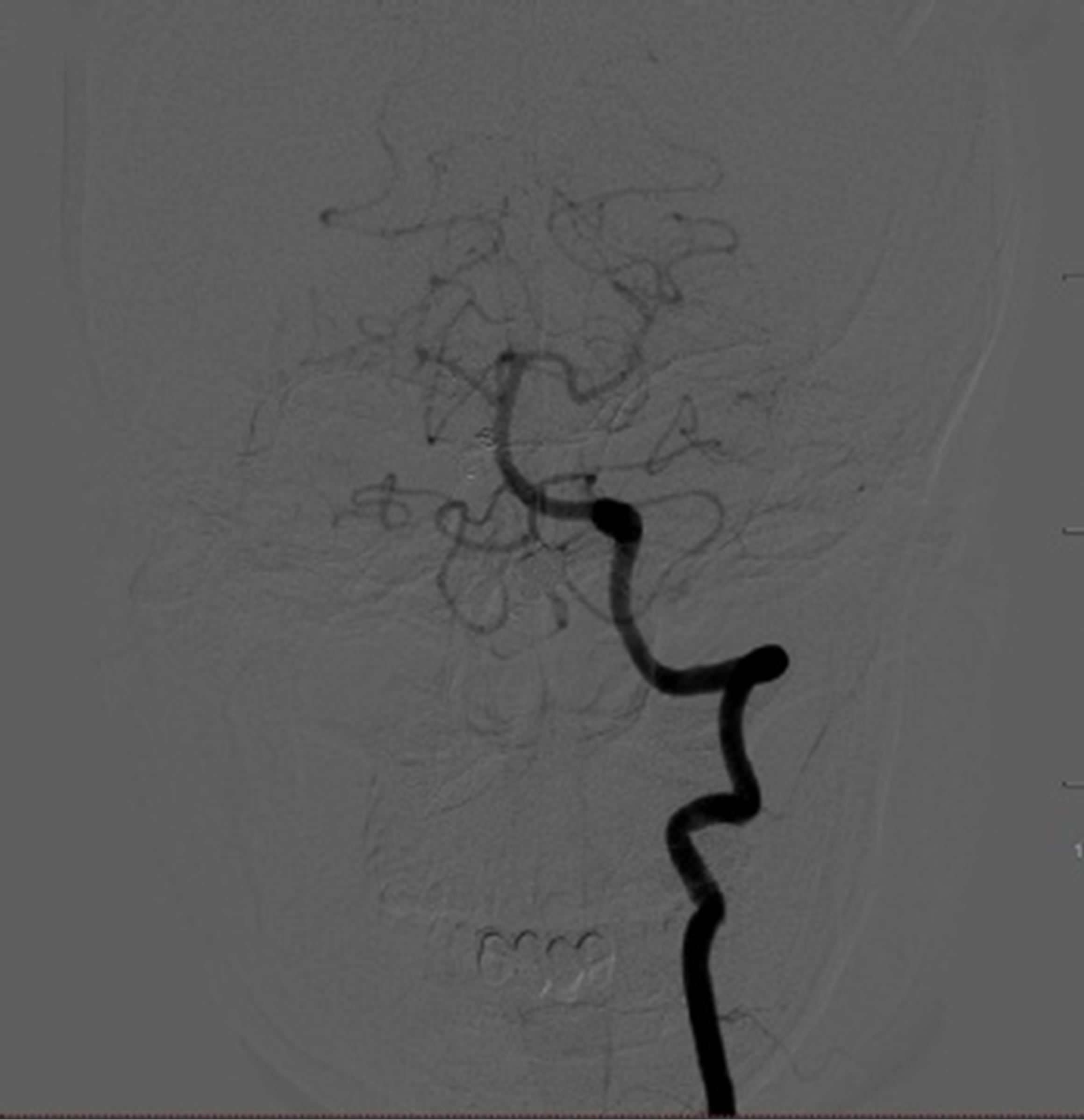
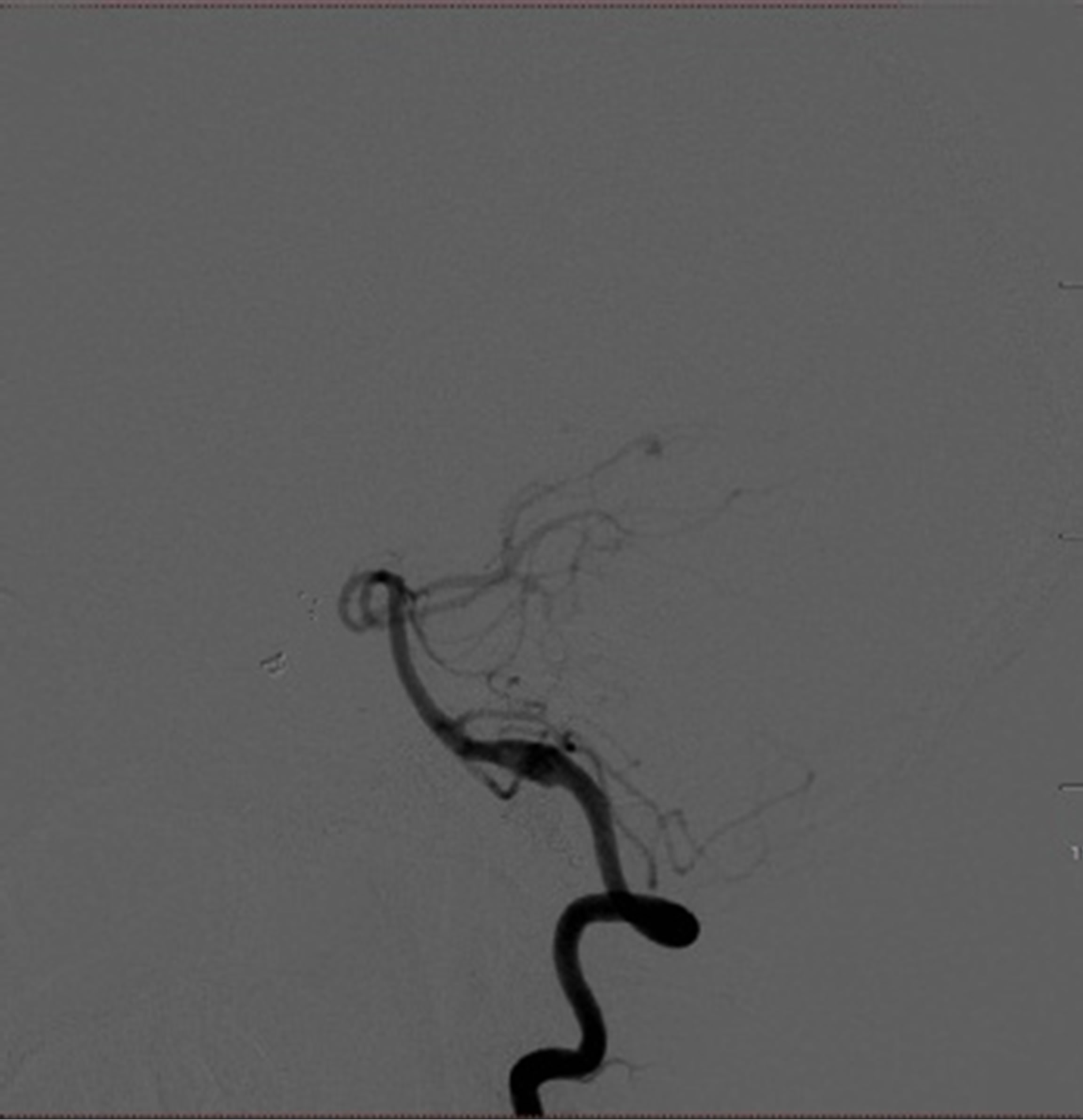
Figure 4. Cerebral angiograms on admission. No regrowth of previously treated aneurysms was observed (L). Left vertebral angiogram showing a de novo fusiform aneurysm (arrow) (M, R).
wall shear stress (WSS) and high wall shear stress gradient (WSSG) [2,13,14,16-18]. To supply the ipsilateral circulation following PAO, contralateral blood flow was subject to increase, which leaded to high wall shear stress and high wall shear stress gradient mainly in contralateral carotid artery. Robert et al. [19] showed that mean WSS was substantially higher in the carotid artery than in the brachial artery and femoral artery in humans. Cho et al. [20] reported that high WSS and a high WSSG are critical parameters for determining cerebral vessel flow in vitro study. The combination of a high WSS and a high WSSG may induce the changes of endothelium cells and smooth muscular cells in contralateral vessel wall, especially at those sites with inherent weakness, which will give rise to degeneration of the endothelial basement membranes, subendothelial connective tissue and the increase of middle membrane damage [21]. Therefore, a de novo aneurysm formation can be triggered following local maladaptive vascular remodeling. Thus, the formation and location of de novo aneurysm were likely determined by multifactors in the vessel wall as well as the changes in hemodynamic.
In our cases, both patients experienced rapid development of de novo aneurysm (i.e, 6 months and 9 months, respectively) with similar risk factors, such as female and middle age. In addition, the younger woman had a history of had a history of hypotension, cigarette smoking and alcohol consumption. Consequently, the conventional angiographic follow-up should be scheduled at 6 months, 12 months and 24 months after procedure to monitor the initial and de novo aneurysm.
4. CONCLUSION
De novo aneurysm formation followed therapeutic PAO is rare. However, a strict long-term follow-up is necessary for those patients with a history of smoking, alcohol consumption and hypertension, especially for females ranging from 40 - 60 years.
5. ACKNOWLEDGEMENTS
This article was supported by the National Natural Science Foundation of China (No. 30901557), The High Level Health Technique Talent Training Plan of Beijing Health System (No. 2011-3-036), and the Nova Plan of Beijing Municipal Science and Technology (2007A043).
REFERENCES
- Itoyama, Y., Fujioka, S., Takaki, S., Morioka, M., Hide, T. and Ushio, Y. (1994) Occlusion of internal carotid artery and formation of anterior communicating artery aneurysm in cervicocephalic fibromuscular dysplasia— Follow-Up Case Report. Neurologia Medico-Chirurgica (Tokyo), 34, 547-550. http://dx.doi.org/10.2176/nmc.34.547
- Jin, S.C., Choi, C.G. and Kwon, D.H. (2009) Development of “de novo” aneurysm after therapeutic carotid occlusion. Journal of Korean Neurosurgical Society, 45, 236-239. http://dx.doi.org/10.3340/jkns.2009.45.4.236
- de Gast, A.N., Sprengers, M.E., van Rooij, W.J., Lavini, C., Sluzewski, M. and Majoie, C.B. (2007) Long-term 3T MR angiography follow-up after therapeutic occlusion of the internal carotid artery to detect possible de novo aneurysm formation. American Journal of Neuroradiology, 28, 508-510.
- Wolf, R.L., Imbesi, S.G., Galetta, S.L., Hurst, R.W., Sinson, G.P. and Grossman, R.I. (2002) Development of a posterior cerebral artery aneurysm subsequent to occlusion of the contralateral internal carotid artery for giant cavernous aneurysm. Neuroradiology, 44, 443-446. http://dx.doi.org/10.1007/s00234-001-0723-5
- Arnaout, O.M., Rahme, R.J., Aoun, S.G., Daou, M.R., Batjer, H.H. and Bendok, B.R. (2012) De novo large fusiform posterior circulation intracranial aneurysm presenting with subarachnoid hemorrhage 7 years after therapeutic internal carotid artery occlusion: Case report and review of the literature. Neurosurgery, 71, E764-E771. http://dx.doi.org/10.1227/NEU.0b013e31825fd169
- Hashimoto, Y., Takayama, K., Inoue, M., Fujishige, M., Yamamura, A. and Nakagawa, T. (2000) A case of distal posterior cerebral artery aneurysm associated with occlusion of the internal carotid artery. No Shinkei Geka Neurological Surgery, 28, 725-729.
- Perrin, J.M., Turowski, B., Steiger, H.J. and Hanggi, D. (2012) Double-barrel extracranial-intracranial bypass surgery followed by endovascular carotid artery occlusion in a patient with an extracranial giant internal carotid artery aneurysm due to Ehlers-Danlos syndrome. Journal of Neurointerventional Surgery, 5, e40.
- Hecht, S.T., Horton, J.A. and Yonas, H. (1991) Growth of a thrombosed giant vertebral artery aneurysm after parent artery occlusion. American Journal of Neuroradiology, 12, 449-451.
- Inui, Y., Oiwa, Y., Terada, T., Nakakita, K., Kamei, I. and Hayashi, S. (2006) De novo vertebral artery dissecting aneurysm after contralateral vertebral artery occlusion—Two case reports. Neurologia Medico-Chirurgica (Tokyo), 46, 32-36. http://dx.doi.org/10.2176/nmc.46.32
- Kubo, Y., Miura, K., Suzuki, M., Tsuiki, K., Kuwata, N., Kubo, N., Kuroda, K. and Ogawa, A. (1998) Development of a dissecting aneurysm on the vertebral artery immediately after occlusion of the contralateral vertebral artery: A case report. Neurosurgical Review, 21, 177-180. http://dx.doi.org/10.1007/BF02389328
- Oya, S., Tsutsumi, K., Yonekura, I. and Inoue, T. (2001) Infra-posterior inferior cerebellar artery aneurysm arising after occlusion of the ipsilateral vertebral artery—Case report. Neurologia Medico-Chirurgica (Tokyo), 41, 545- 547. http://dx.doi.org/10.2176/nmc.41.545
- Briganti, F., Cirillo, S., Caranci, F., Esposito, F. and Maiuri, F. (2002) Development of “de novo” aneurysms following endovascular procedures. Neuroradiology, 44, 604-609. http://dx.doi.org/10.1007/s00234-001-0732-4
- Arambepola, P.K., McEvoy, S.D. and Bulsara, K.R. (2010) De novo aneurysm formation after carotid artery occlusion for cerebral aneurysms. Skull Base, 20, 405- 408. http://dx.doi.org/10.1055/s-0030-1253578
- Okazaki, T., Nishi, T., Yamashiro, S., Koga, K., Nagahiro, S. and Fujioka, S. (2010) De novo formation and rupture of an intracranial aneurysm 10 months after normal findings on conventional magnetic resonance angiography in a patient with no history of intracranial lesions: Case report. Neurologia Medico-Chirurgica (Tokyo), 50, 309-312. http://dx.doi.org/10.2176/nmc.50.309
- van Gijn, J. and Rinkel, G.J. (2001) Subarachnoid haemorrhage: Diagnosis, causes and management. Brain: A Journal of Neurology, 124, 249-278.
- Fujiwara, S., Fujii, K. and Fukui, M. (1993) De novo aneurysm formation and aneurysm growth following therapeutic carotid occlusion for intracranial internal carotid artery (ICA) aneurysms. Acta Neurochir (Wien), 120, 20-25. http://dx.doi.org/10.1007/BF02001464
- Rahmah, N.N., Horiuchi, T., Kusano, Y., Sasaki, T. and Hongo, K. (2011) De novo aneurysm: Case reports and literature review. Neurosurgery, 69, E761-E766. http://dx.doi.org/10.1227/NEU.0b013e3182196489
- Suzuki, M.T., Aguiar, G.B., Jory, M., Conti, M.L. and Veiga, J.C. (2011) De novo basilar tip aneurysm. Case report and literature review. Neurocirugia (Asturias), 22, 251-254. http://dx.doi.org/10.4321/S1130-14732011000300005
- Reneman, R.S. and Hoeks, A.P.G. (2008) Wall shear stress as measured in vivo: Consequences for the design of the arterial system. Medical & Biological Engineering & Computing, 46, 499-507. http://dx.doi.org/10.1007/s11517-008-0330-2
- Cho, Y.-I. and Cho, D.J. (2011) Hemorheology and microvascular disorders. Korean Circulation Journal, 41, 287. http://dx.doi.org/10.4070/kcj.2011.41.6.287
- Meng, H., Wang, Z., Hoi, Y., Gao, L., Metaxa, E., Swartz, D.D. and Kolega, J. (2007) Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke, 38, 1924-1931. http://dx.doi.org/10.1161/STROKEAHA.106.481234
NOTES
*Corresponding author.

