Open Journal of Clinical Diagnostics
Vol.3 No.3(2013), Article ID:37231,7 pages DOI:10.4236/ojcd.2013.33020
Comparative studies of serum-free media and detection techniques for in vitro drug sensitivity assessment of Plasmodium falciparum
![]()
1Department of Animal Biology and Conservation Science, Faculty of Science, University of Ghana, Accra, Ghana
2Department of Parasitology, National Institute of Infectious Diseases, Tokyo, Japan
3Department of Environmental Parasitology, Tokyo Medical and Dental University, Tokyo, Japan
4Department of Immune Regulation, Tokyo Medical and Dental University, Tokyo, Japan
Email: *asahih@nih.go.jp
Copyright © 2013 Bethel Kwansa-Bentum et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 8 July 2013; revised 8 August 2013; accepted 15 August 2013
Keywords: Plasmodium falciparum; Chemically Defined Medium; Drug Sensitivity Test; Flow Cytometry
ABSTRACT
Malaria continues to be a devastating disease. In a previous study, we formulated a chemically defined culture medium that is able to sustain the complete intraerythrocytic growth of Plasmodium falciparum. We tested the feasibility of using the medium (CDRPMI) as well as human serum-free media enriched with commercially available human-serum substitutes (GFSRPMI and ALBRPMI) to assess the drug sensitivity of P. falciparum, using chloroquine diphosphate (CQ) and dihydroartemisinin (DHART) as conventional antimalarial drugs. Growth inhibition was measured by four different methods: flow cytometry with SYBR Green I (FCM), microscopy (Giemsa method), enzymatic estimation of parasite lactate dehydrogenase (pLDH), and histidine-rich protein 2 (HRPII) determination. In drug sensitivity tests on asynchronous parasites cultured for 96 h in the presence of drugs, the dose-response curves were similar and differences in the 50% growth inhibition concentrations for the drugs, which were estimated by the four methods, were not statistically significant for the three culture media. The effect of the drugs on the growth of synchronous parasites at the ring stage was also assessed in micro-volume tests by three different methods of FCM: tracking fluorescent erythrocytes, schizont test, and merozoite test. Dose-response curves for the drugs were similar, and differences in the 50% growth inhibition concentrations were not statistically significant for CDRPMI and GFSRPMI. Thus CDRPMI as well as GFSRPMI and ALBRPMI can be similarly useful media for drug sensitivity testing of P. falciparum. The FCM, pLDH and HRPII estimations were fast and reliable detection methods, with FCM allowing schizont and merozoite tests to be performed with shorter periods of culture.
1. INTRODUCTION
Malaria continues to be a devastating disease, particularly in the tropics, with an estimated annual incidence worldwide of 216 million clinical cases. The annual mortality from malaria, which is caused largely by the protozoan Plasmodium falciparum, is estimated to be 0.66 million worldwide [1]. A better understanding of antimalarial treatments is thus needed to allow the development of new medications to combat resistance to conventional antimalarial drugs [2].
The development of P. falciparum requires human serum in the original culture medium [3]. To avoid the obstacles associated with the dependence on human serum, including unpredictable risks with infectious agents and difficulties in securing normal human sera, particularly in malaria endemic areas, and in obtaining a particular blood group, human serum substitutes such as a growth-promoting fraction derived from adult bovine plasma (GFS; Wako Pure Chemical Industries Ltd., Japan) and lipid-enriched bovine albumin (AlbuMAX I; Life Technologies, Japan) have been exploited successfully [4,5], and have been employed widely for in vitro cultivation of P. falciparum. However, sufficient information on the constituents of the serum substitutes is still lacking.
A chemically defined medium is essential for the design of reproducible biochemical, physiological, and genetic studies of microorganisms and various cell types [6,7]. We formulated previously a chemically defined medium that sustained the complete intraerythrocytic growth of P. falciparum, based on the results of characterization of the growth-promoting factors in GFS [8]. All stages of the parasite cultured in the chemically defined medium have been comparable to or better than those grown in human serum or GFS-containing medium. This finding implies that the chemically defined medium formulated may be usefully applied in diverse aspects of malaria research, in a similar way to media containing human serum and human serum substitutes.
The effects of drugs on P. falciparum can be assessed both quantitatively and qualitatively by direct examination of erythrocyte (RBC) smears from blood or cultures with a microscope, although this method is tedious and subjective. Numerous assays have been introduced that are more objective, more sensitive, faster and designed to be easier to handle. The most common include isotopic, enzymatic, and enzyme-linked immunosorbent assays, such as the incorporation of 3H-hypoxathine into the DNA of the parasite, the estimation of parasite lactate dehydrogenase (pLDH), and quantifying biomolecules (pLDH and histidine-rich protein 2 (HRPII)) by doublesite sandwich enzyme-linked immunosorbent assays [9- 13]. These methods are relatively reliable and objective, and are well suited for screening large numbers of drugs; however, some of them are associated with unavoidable weaknesses, including insufficient sensitivity and the use of hazardous radioactive material.
Analysis by flow cytometry (FCM) using different fluorescent dyes has also proven useful for analyzing the blood stage of malaria parasites. In particular, FCM using SYBR Green I as a fluorescent dye has been useful in the assessment of the growth of P. falciparum and P. berghei [14-16]. This system allows visualization of parasitized RBCs (PRBCs) with high accuracy, and can be used to follow the development of P. falciparum [14] and merozoite invasion into new RBCs [15; Asahi, unpublished].
In the present study, we tested the feasibility of using a chemically defined medium for in vitro drug sensitivity assessment of P. falciparum, in comparison with two other commercially available non-human serum media, to obtain a more detailed understanding of this principle of the assessment of antimalarial efficacy. We used chloroquine diphosphate (CQ) and dihydroartemisinin (DHART) as conventional antimalarial drugs, and inhibition of parasite growth was assessed by FCM using SYBR Green I in comparison with three other assays: the Giemsa method, pLDH estimation, and HRPII quantification.
2. MATERIALS AND METHODS
2.1. Culture Media
The basal medium (CRPMI) consisted of RPMI 1640 containing 2 mM glutamine, 25 mM 4-(2-hydroxylethyl)- piperazine ethansulfonic acid, and 24 mM NaHCO3 (Invitrogen Ltd., USA), with 25 µg/ml gentamicin (SigmaAldrich Corp., USA), and 150 µM hypoxanthine (SigmaAldrich). The complete culture medium termed GFSRPMI comprised CRPMI with 10% GFS (Daigo’s GF21), as reported previously [4,8,12]. The complete medium termed ALBRPMI consisted of CRPMI supplemented with AlbuMAX I at a final concentration of 3 mg/ml. The chemically defined medium termed CDRPMI consisted of CRPMI supplemented with bovine serum albumin free of non-esterified fatty acids at a final concentration of 3 mg/ml, two non-esterified fatty acids (100 µM cis-9-octadecenoic acid and 60 µM hexadecanoic acid), and four phospholipids (15 µM 1,2-dioleoyl phosphatidic acid sodium salt, 130 µM 1,2-dioleoyl-sn-glycerol-3-phosphocholine, 25 µM 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, and 15 μM 1,2-dioleoyl-sn-glycero-3-phosphoserine, sodium salt), as described previously [8]. All the compounds were obtained from Sigma-Aldrich. Dried lipid precipitates were prepared, added to the culture media, and sterilized to reconstitute the lipids, as described previously [8,12].
2.2. Parasite Culture and Synchronization
Cultures of the FCR/FMG (ATCC Catalogue No. 30932, Gambia) strain of P. falciparum were used in the experiments. The parasites were maintained routinely by in vitro culture techniques using GFSRPMI. The RBCs were preserved in Alsever’s solution [4] for 3 - 30 days, washed, and then dispensed in a 24-well culture plate at a hematocrit of 2% (1 ml suspension per well), and cultured under a humidified atmosphere of 5% CO2, 5% O2, and 90% N2 at 37˚C. Parasitemia was adjusted to 0.1%, except where specified otherwise, by adding uninfected RBCs, and the hematocrit was adjusted to 2% by adding the appropriate volume of culture medium. Cultures were synchronized at the ring stage by three successive exposures to 5% (w/v) D-sorbitol (Sigma-Aldrich) at 41- and 46-h intervals [17]. After the third treatment with sorbitol, residual schizonts and cell debris were removed by isopycnic density centrifugation on 63% Percoll (GE Healthcare Bio-Science Corp., USA).
2.3. Assessment of Parasite Growth Inhibition
The GFSRPMI was replaced by other culture media containing graded concentrations of CQ (Sigma) or DHART (Fluka-Sigma-Aldrich, Japan) to test for growth inhibition. Stock solutions (10 mM) were dissolved initially in either 50% ethanol (for DHART) or distilled water (for CQ), diluted, and dispensed at optimal volumes. Asynchronous PRBCs were dispensed in a 48-well culture plate at a hematocrit of 2% (500 μl suspension per well), and cultured for 96 h (2 cycles).
For micro-volume tests, synchronized PRBCs were dispensed in a 96-well culture plate containing graded amounts of CQ or DHART, at a hematocrit of 2% (100 µl suspension per well), and cultured for 25 or 45 h.
The drug concentrations required to inhibit growth of the parasite and schizont/merozoite formation by 50% in comparison with drug-free controls (GIC50 and EC50, respectively) were extrapolated from the concentration-response curves.
The culture wells were run in triplicate or quadruplicate in all experiments, and each experiment was repeated two to four times.
2.4. Techniques for Measuring Antimalarial Activity
2.4.1. Growth Rate (Giemsa Method)
Thin smears were prepared on microscope glass slides, which were stained with Giemsa. Parasitemia was determined microscopically based on the examination of more than 10,000 PRBC/RBCs. The growth rate was estimated by dividing the parasitemia of the test sample, after incubation for the indicated times, by the initial parasitemia.
2.4.2. Tracking Fluorescent PRBCs (FPRBCs) and Released Merozoites
After fixation by the addition of 1% paraformaldehyde combined with Alsever’s solution, PRBCs (8 × 105 cells in a 16 µl aliquot of 0.5% PRBC/RBC suspension) were stained by mixing with 0.5 - 1 ml SYBR Green I (×1 dilution, Invitrogen) prepared in Tris-saline solution consisting of 20 mM Tris (hydroxylmethyl) aminomethane hydrochloride at pH 8.8 and 138 mM NaCl. Parasitemia and each developmental stage were determined by examining more than 10,000 PRBC/RBCs. The numbers of PRBCs and each developmental stage of the parasite were measured by FCM using either a PAS flow cytometer (PAS, Partec Co. Ltd., Germany) or a FACSCalibur (Becton Dickinson Immunocytometry Systems, USA), as described previously [14]. Analysis was performed using FCS express software (De Novo Software Inc., Canada).
In the schizont test, the number of high-fluorescent FPRBCs (schizonts) was measured by FCM after rings of the parasite had been cultured for 25 h [14].
In the merozoite test, merozoites released from mature schizonts into the surrounding medium were counted by FCM after rings of the parasite had been cultured for 45 h [14]. The number of merozoites was counted in more than 5000 PRBCs.
2.4.3. pLDH Enzymatic Assay
The pLDH enzymatic assay was performed according to the methods described by Makler and Hinrichs [9] and Asahi et al. [12]. Briefly, at the indicated incubation times, PRBC/RBCs in culture were hemolyzed by three freeze-thaw cycles, and 15 µl aliquots were transferred to each well of a 96-well microtiter plate. Subsequently, 100 μl of Malstat reagent (Flow Inc., USA), 10 µl of 1 mg/ml nitroblue tetrazolium (Wako), and 10 µl of 1 mg/ml diaphorase (Wako) were added to each well. The plated contents were allowed to stand for 40 min at 37˚C, and the reaction was stopped by adding 50 μl of 5% (v/v) acetic acid. The absorbance at 650 nm (OD650) was read on a plate reader. The initial OD650 value, measured when the assay reagents were first added, was subtracted from the endpoint reading.
2.4.4. HRPII Assay
The HRPII assay was performed according to the manufacturer’s instructions for the Malaria Ag CELISA (Cellabs Pty Ltd., Australia), with some modifications. Briefly, 5 µl aliquots of thawed samples from a 2% hematocrit were transferred to each well of the CELISA plate. These were topped up with 95 µl of phosphate-buffered saline, and then allowed to stand for 1 h at room temperature. The plate was washed five times, 100 µl of the diluted conjugate was added, and the plate was incubated again for 1 h at room temperature. After five washes, 100 µl of the substrate was added and incubated in the dark at room temperature for 15 min. The reaction was stopped by adding 50 µl of stopping solution. The absorbance at 450 nm (OD450) was read on a plate reader.
2.5. Statistical Analysis
The significance of the differences between means was evaluated using multifactorial analysis of variance. All calculations were performed using GraphPad PRISM 5 (GraphPad Software, USA). The P value for significance was 0.05, and all pairwise comparisons were made post hoc with Bonferroni’s test. Error bars were added to the y-axes on the graphs to indicate the standard deviation for each point.
3. RESULTS
3.1. Drug Sensitivity of Asynchronous Parasite Culture
The effects of CQ and DHART on the growth of parasites cultured asynchronously in CDRPMI, GFSRPMI and ALBRPMI for 96 h were assessed by four methods: FCM, the Giemsa method, and estimations of pLDH and HRPII. The dose-response curves obtained for all culture medium-estimation method combinations were similar at all concentrations of CQ and DHART (Figure 1). Also, differences in GIC50 values that were estimated by the four methods were not statistically significant among the three culture media (Table 1). However, slightly higher values of near total parasite killing concentrations of CQ and DHART were detected by the HRPII estimation method (Figure 1).
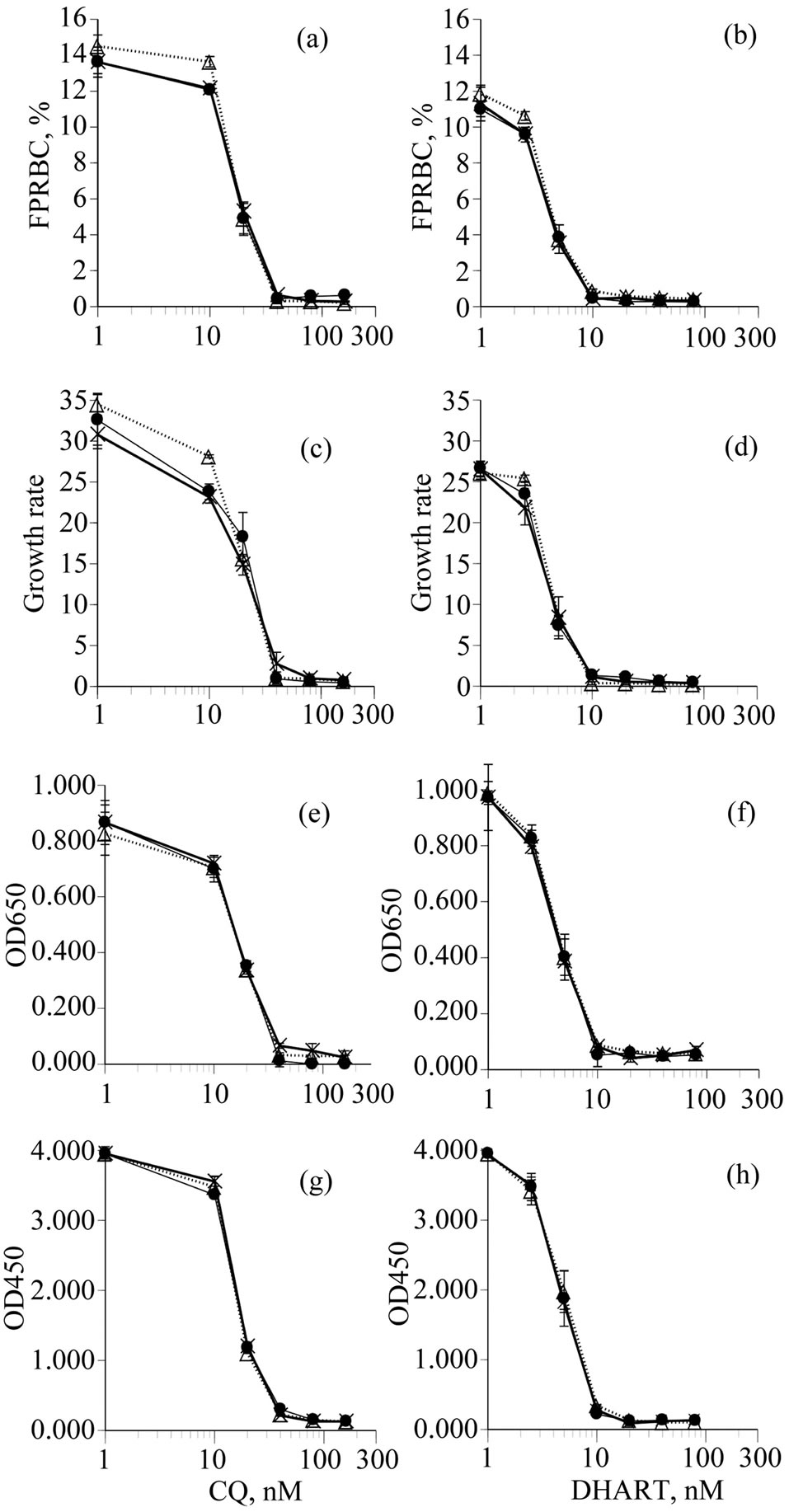
Figure 1. Growth inhibition curves of P. falciparum parasites cultured asynchronously in CDRPMI (––●––), GFSRPMI (––X––) and ALBRPMI (…Δ…) in the presence of CQ and DHART. Parasite growth was assessed by FCM (a, b), Giemsa method (c, d), pLDH levels (e, f), and HRPII assay (g, h). The initial parasitemia was adjusted to 0.3%.
3.2. Microtitration Analyses on Synchronized Parasite Cultures
The effects of CQ and DHART on the growth of ring forms of the parasite cultured in CDRPMI and GFSRPMI for 25 or 45 h were assessed by three different types of FCM: (1) tracking FPRBCs for parasitemia; (2) tracking high-fluorescent FPRBCs (schizont test); and (3) counting released merozoites (merozoite test) in a 96- well microplate containing graded concentrations of the antimalarial drugs, because clinical blood samples from individuals infected with P. falciparum contain predominantly ring forms. The dose-response curves obtained for all culture medium-estimation method combinations were similar at all concentrations of CQ and DHART (Figure 2). In addition, the GIC50 (parasitemia) and EC50 (schizont test and merozoite test) values for the two drugs in the two culture media were not statistically different (Table 2). Further, only the schizont and merozoite tests showed near total parasite killing: at 40 nM for CQ and 10 nM for DHART (Figure 2).
4. DISCUSSION
The advent of in vitro culture with the use of human serum has led to unprecedented advances in diverse aspects of malaria research, including studies on drug resistance, vaccine development, genetics and parasite biochemistry [3]. Although the potential complexity of human serum supplementation has posed an enormous challenge, the successful exploitation of human serum-free culture media using alternatives to human serum, such as GFS and AlbuMAX I, has avoided the disadvantages of human serum. Sufficient information on the constituents of the serum alternatives is, however, still lacking. Culture of malarial parasites in chemically defined media may not only avoid any unpredictable effects that can arise when human serum and substitutes are used, but also secure reproducible study designs and thus increase the opportunities for interdisciplinary research on malaria.
We formulated previously a chemically defined medium (CDRPMI) that sustained complete intraerythrocytic growth of P. falciparum [8]. The CDRPMI consisted of paired non-esterified fatty acids, phospholipids with specific fatty acid moieties, and specific proteins dissolved in the basal medium RPMI-1640, supplemented with hypoxanthine [8,18]. All stages of the parasite cultured in the CDRPMI have been comparable to those grown in GFSRPMI. Also, on genome-wide transcriptome profiling, only slight differences in transcript levels have been detected between the parasites cultured in CDRPMI and GFSRPMI [19]. In the current study, the feasibility of using CDRPMI, as well as GFSRPMI and ALBRPMI, for drug sensitivity tests on P. falciparum was investigated. The growth curves were similar for all three media after
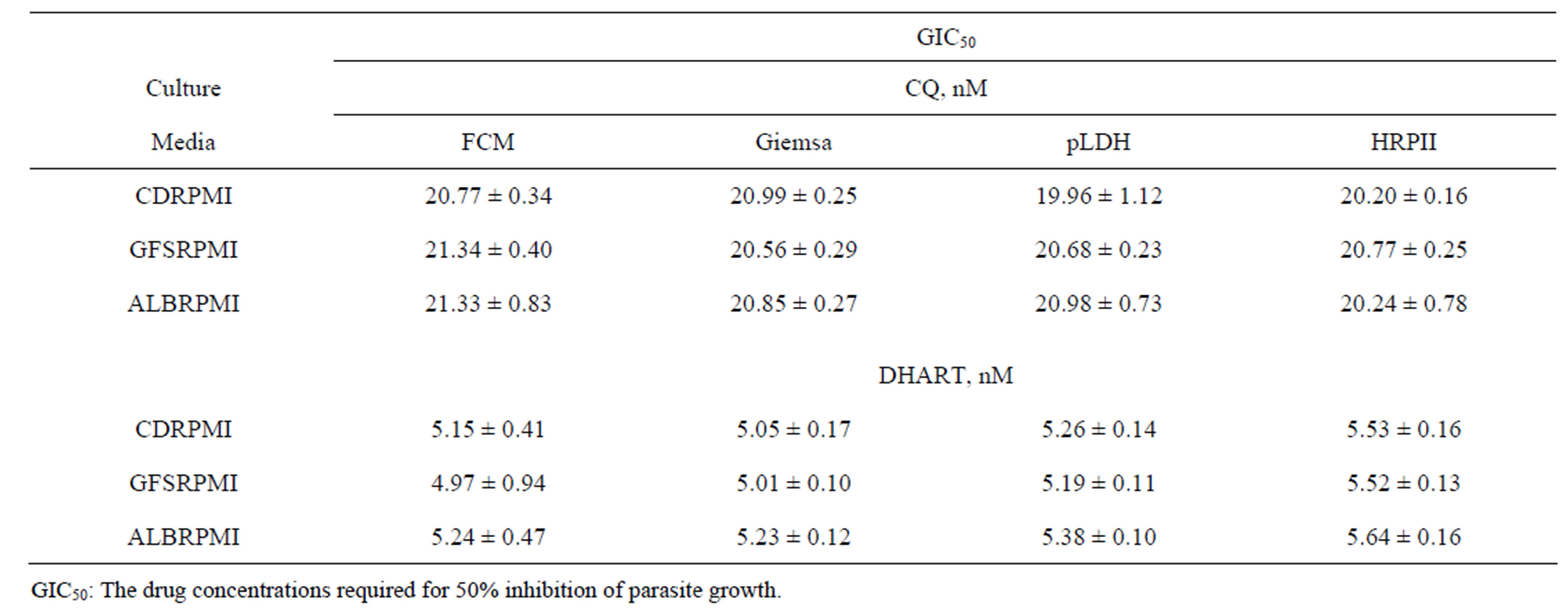
Table 1. Comparison of GIC50 determined by FCM, Giemsa method, pLDH levels, and HRPII levels. P. falciparum was cultured in CDRPMI, GFSRPMI, or ALBRPMI in the presence of graded concentrations of CQ or DHART.
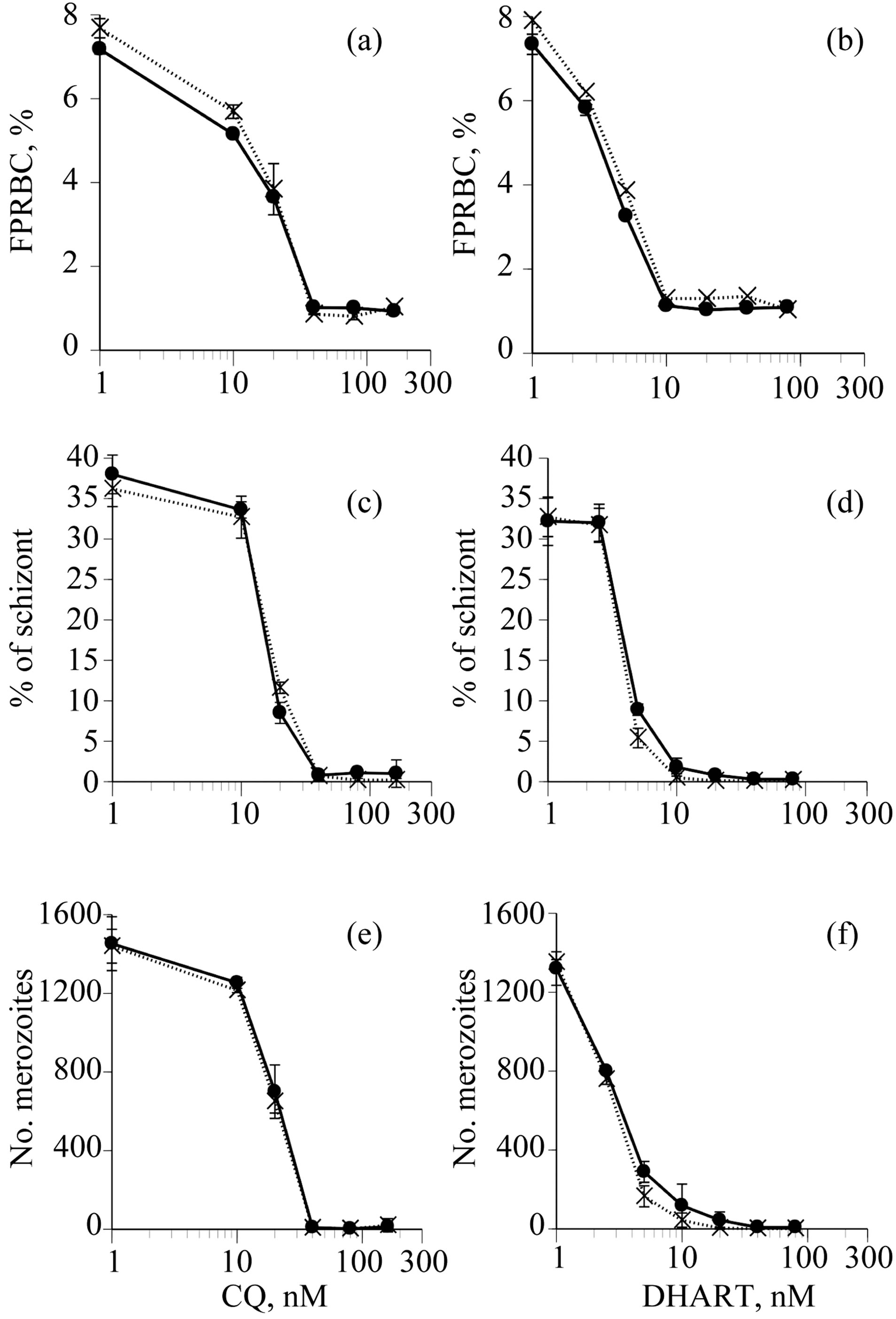
Figure 2. Growth inhibition curves of synchronized P. falciparum cultured in CDRPMI (––●––) or GFSRPMI (…X…) in the presence of CQ and DHART. Parasite growth was assessed bymicro-volume tests using three types of FCM: tracking FPRBC after 45 h culture (a, b), tracking schizonts after 25 h culture (c, d), and counting the number of merozoites released after 45 h culture (e, f). The initial parasitemia (rings) was adjusted to 1%.
exposing asynchronous parasite culture to CQ and DHART for 96 h. Likewise, microtitration analyses using the schizont test and merozoite test on synchronized parasite culture showed a similar trend in parasite growth inhibition in the presence of graded concentrations of the drugs. These findings support the feasibility of using CDRPMI for drug sensitivity tests, in a similar way to GFSRPMI and ALBRPMI.
Affordable and reliable analytical techniques to test growth-promoting and antimalarial effects on plasmodia are needed to improve tracking of malaria parasites. FCM has a particular advantage in determining populations of dividing stages of the parasite. FCM that uses different intercalating dyes has already been used successfully to test human and murine samples [14,20,21]. However, some of the dyes lack sufficient sensitivity and/or require complicated preparation procedures that make their use problematic. To overcome those limitations we selected SYBR Green I as a DNA intercalating dye to take advantage of the excellent bright signal of SYBR Green I and the simple preparation procedure for FCM [14].
FCM using SYBR Green I has been shown to enable not only visualization of PRBCs with high accuracy, but also to allow development of the parasite to be followed during its life cycle. In the current study, when investigating the effect of antimalarial drugs on synchronized cultures using FCM, the readings of the number of highfluorescent FPRBCs (schizonts) in 25-h cultures gave comparable results to microscopic examination of slides stained with Giemsa. This suggests that readings from a number of high-fluorescent FPRBCs could be used to assess the drug-sensitivity of the parasite, in a similar way to the conventional microscopic method that uses changes in the percentages of schizonts after 25 - 28 h of culture.
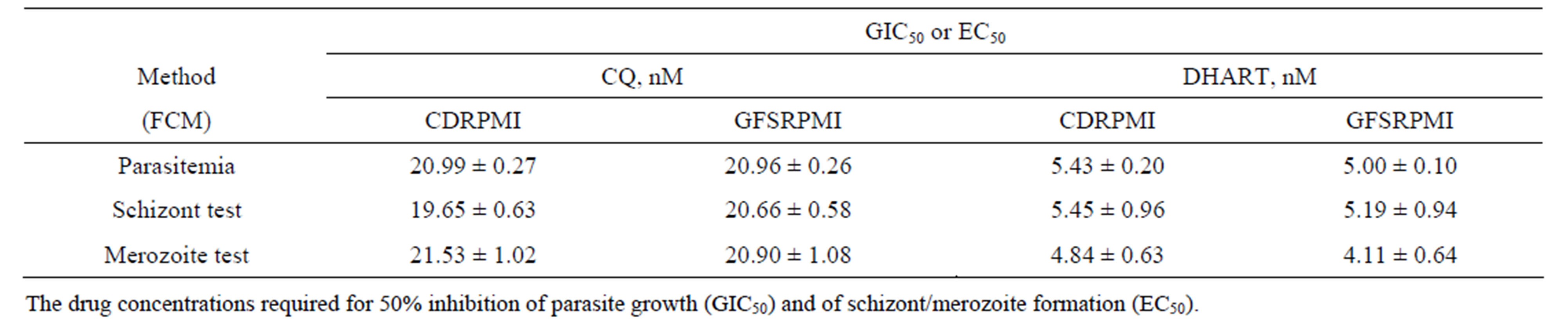
Table 2. Comparison of GIC50 and EC50 determined by microtitration using tracking FPRBCs (parasitemia, 45 h culture), schizont test (25 h culture), and merozoite test (45 h culture). P. falciparum was cultured in CDRPMI or GFSRPMI in the presence of graded concentrations of CQ or DHART.
The merozoite test (counting the number of released merozoites) using FCM has also been described to be a valuable tool for assessment of the effects of antimalarial drugs on synchronous growth of the parasite after 45 h of culture [14]. The schizont and merozoite tests involving FCM described here consistently detected the sensitivity of the parasite to CQ and DHART in shorter culture periods, even in micro-volume tests. However, the merozoite test may have limitations in the assessment of parasite growth in the case of some antimalarial agents, such as antifolates, that begin acting at a later stage in parasite development. Parasites under the influence of these drugs tend to produce relatively high numbers of merozoites, which then stop growing before reaching the next stage of the cycle, including invasion into new RBCs.
The correlations among FCM, the Giemsa method, pLDH estimation, and HRPII assay were excellent. Although the HRPII estimation method is highly sensitive and reliably detects growth inhibition in drug-sensitivity tests, the amounts of HRPII produced have been known to reflect not parasite viability but the presence of the biomolecule. This implies that the HRPII of killed parasites may be measured, and may mean that the near total parasite killing concentrations required in the HRPII assay were somewhat higher than those for other methods. On the other hand, levels of pLDH have been known to correlate well with viable development of P. falciparum [9,11]. Regardless of the estimation of parasite viability, the differences in GIC50 values for the long culture method were not statistically significant among the assays employed here.
Concerns about artemisinin resistance have been reported recently [22], and all malaria-endemic countries have been warned to be more vigilant in monitoring antimalarial drug efficacy, to allow the early detection of artemisinin resistance and aid global malaria control [23- 25]. Furthermore, most rapid diagnostic test kits currently available on the market are based on HRPII and pLDH (as a biomolecule). The finding that the concentrations that show near total killing of the parasite in the HRPII assay are variable should be taken into account in monitoring antimalarial drug efficacy.
5. CONCLUSION
This study confirmed that CDRPMI, as well as the human serum-free media GFSRPMI and ALBRPMI, was useful for assessment of the drug-sensitivity of P. falciparum. FCM, using SYBR Green I as a fluorescent dye, and pLDH and HRPII estimations were fast and reliable detection methods. Furthermore, FCM allowed schizont and merozoite tests to be performed with shorter periods of culture.
6. ACKNOWLEDGEMENTS
We acknowledge the invaluable support of Drs. Mohammed E. M. Tolba and Sumio Sugano of the Department of Medical Genomics, Graduate School of Frontier Sciences, the University of Tokyo, Japan. We thank the Japanese Red Cross Society for providing RBCs. This work was partially supported by a Grant-in-Aid from the Ministry of Health, Labor and Welfare (H20-Shinkou-ippan-020) of Japan.
REFERENCES
- World Health Organization (2012) World malaria report 2012. WHO Press, Geneva. http://www.who.int/malaria/publications/world_malaria_report_2012/wmr2012_factseet.pdf
- Ridley, R.G. (2002) Medical need, scientific opportunity and the drive for antimalarial drugs. Nature, 415, 686-693. doi:10.1038/415686a
- Trager, W. and Jensen, J.B. (1997) Continuous culture of Plasmodium falciparum: Its impact on malaria research. International Journal for Parasitology, 27, 989-1006. doi:10.1016/S0020-7519(97)00080-5
- Asahi, H. and Kanazawa, T. (1994) Continuous cultivation of intraerythrocytic Plasmodium falciparum in a serum-free medium with the use of a growth-promoting factor. Parasitology, 109, 397-401. doi:10.1017/S0031182000080641
- Cranmer, S.L., Magowan, C., Liang, J., Coppel, R.L. and Cooke, B.M. (1997) An alternative to serum for cultivation of Plasmodium falciparum in vitro. Transactions of the Royal Society of Tropical Medicine and Hygiene, 91, 363-365. doi:10.1016/S0035-9203(97)90110-3
- Kim, Y.J., et al. (2012) Development of a chemically defined minimal medium for the exponential growth of Leuconostocmesenteroides ATCC8293. Journal of Microbiology and Biotechnology, 22, 1518-1522. doi:10.4014/jmb.1205.05053
- Mimura, S., et al. (2011) Growth factor-defined culture medium for human mesenchymal stem cells. International Journal of Developmental Biology, 55, 181-187. doi:10.1387/ijdb.103232sm
- Asahi, H. (2009) Plasmodium falciparum: Chemically defined medium for continuous intraerythrocytic growth using lipids and recombinant albumin. Experimental Parasitology, 121, 22-28. doi:10.1016/j.exppara.2008.09.009
- Makler, M.T. and Hinrichs, D.J. (1993) Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. The American Jornal of Tropical Medicine and Hygiene, 48, 205-210.
- Druilhe, P., Moreno, A., Blanc, C., Brasseur, P.H. and Jacquier, P. (2001) A colorimetric in vitro drug sensitivity assay for Plasmodium falciparum based on a highly sensitive double-site lactate dehydrogenase antigen-capture enzyme-linked immunosorbent assay. The American Journal of Tropical Medicine and Hygiene, 64, 233-241.
- Noedl, H., Wongsrichanalai, C. and Wernsdorfer, W.H. (2003) Malaria drug-sensitivity testing: New assays, new perspectives. Trends in Parasitology, 19, 175-181. doi:10.1016/S1471-4922(03)00028-X
- Asahi, H., Kanazawa, T., Hirayama, N. and Kajihara, Y. (2005) Investigating serum factors promoting erythrocytic growth of Plasmodium falciparum. Experimental Parasitology, 109, 7-15. doi:10.1016/j.exppara.2004.10.002
- Piper, R.C., Buchanan, I., Choi, Y.H. and Makler, M.T. (2011) Opportunities for improving pLDH-based malaria diagnostic tests. Malaria journal, 10, 213. doi:10.1186/1475-2875-10-213
- Izumiyama, S., Omura, M., Takasaki, T., Ohmae, H. and Asahi, H. (2009) Plasmodium falciparum: Development and validation of a measure of intraerythrocytic growth using SYBR Green I in a flow cytometer. Experimental Parasitology, 121, 144-150. doi:10.1016/j.exppara.2008.10.008
- Bei, A.K., et al. (2010) A flow cytometry-based assay for measuring invasion of red blood cells by Plasmodium falciparum. American Journal of Hematology, 85, 234- 237. doi:10.1002/ajh.21642
- Somsak, V., Srichairatanakool, S., Yuthavong, Y., Kamchonwongpaisan, S. and Uthaipibull, C. (2012) Flow cytometric enumeration of Plasmodium berghei-infected red blood cells stained with SYBR Green I. Acta tropica, 122, 113-118. doi:10.1016/j.actatropica.2011.12.010
- Asahi, H., Izumiyama, S., Tolba, M.E. and Kwansa-Bentum, B. (2011) Plasmodium falciparum: Differing effects of non-esterified fatty acids and phospholipids on intraerythrocytic growth in serum-free medium. Experimental Parasitology, 127, 708-713. doi:10.1016/j.exppara.2010.11.001
- Asahi, H. (2012) Intraerythrocytic Plasmodium falciparum Growth in Serum-Free Medium with an Emphasis on Growth-Promoting Factors. In: Okwa, O.O., Ed., Malaria Parasites, InTech, Croatia, 73-90. doi:10.5772/33212
- Asahi, H., Tolba, M.E.M., Tanabe, M. and Ohmae, H. (2013) Molecular factors that are associated with early developmental arrest of intraerythrocytic Plasmodium falciparum. Canadian Journal of Microbiology, 59, 485-493. doi:10.1139/cjm-2013-0166
- Grimberg, B.T. (2011) Methodology and application of flow cytometry for investigation of human malaria parasites. Journal of Immunological Methods, 367, 1-16. doi:10.1016/j.jim.2011.01.015
- Bouillon, A., Gorgette, O., Mercereau-Puijalon, O. and Barale, J.C. (2013) Screening and evaluation of inhibitors of Plasmodium falciparum merozoite egress and invasion using cytometry. In: Menard, R., Ed., Malaria: Methods and Protocols, Methods in Molecular Biology, Springer Science + Business Media, 523-534.
- Dondorp, A.M., et al. (2009) Artemisinin resistance in Plasmodium falciparum malaria. The New England Journal of Medicine, 361, 455-467. doi:10.1056/NEJMoa0808859
- World Health Organization (2011) Global plan for artemisinin resistance containment. WHO press, Geneva.
- Kwansa-Bentum, B., et al. (2011) Administrative practices of health professionals and use of artesunate-amodiaquine by community members for treating uncomplicated malaria in southern Ghana: Implications for artemisininbased combination therapy deployment. Tropical Medicine and International Health, 16, 1215-1224. doi:10.1111/j.1365-3156.2011.02833.x
- Kwansa-Bentum, B., et al. (2011) Plasmodium falciparum isolates from southern Ghana exhibit polymorphisms in the SERCA-type PfATPase6 though sensitive to artesunate in vitro. Malaria Journal, 10, 187. http://www.malariajournal.com/content/10/1/187 doi:10.1186/1475-2875-10-187
NOTES
*Corresponding author.

