Open Journal of Psychiatry
Vol. 2 No. 1 (2012) , Article ID: 16565 , 9 pages DOI:10.4236/ojpsych.2012.21007
Chronic fatigue syndrome: An update for psychiatrists
![]()
1Department of Psychiatry, Assistance Publique-Hôpitaux de Paris, Bicêtre University Hospital, Le Kremlin Bicêtre, France
2INSERM U669, Le Kremlin Bicêtre, France
Email: emmanuelle.corruble@bct.aphp.fr
Received 24 November 2011; revised 27 December 2011; accepted 8 January 2012
Keywords: Fatigue; Chronic Fatigue Syndrome; Diagnosis; Physiopathology; Treatment
ABSTRACT
Chronic fatigue syndrome (CFS) is a poorly understood condition primarily characterized by debilitateing, persistent or recurrent fatigue, increased physical and mental fatigability, cognitive impairment and widespread musculoskeletal pain. During the past two decades, there have been heated debates about CFS among researchers, practitioners and patients. The existence of the disorder has been questioned, its underlying pathophysiology debated and an effective treatment opposed (such as antidepressants, stimulants or antibiotics). A lot of multidisciplinary literature is found about CFS, but to date, many psychiatrists seem to unknown the existence of this illness or think that it is a purely psychological disorder. However, CFS is sitting on the border between medicine and psychiatry. The aim of this review is to make psychiatrists aware of the existence of CFS and that they will, one day, be confronted with the management of this illness. Thus, this update allows understanding what is CFS, the diversity of physiopathology underlined and its management.
1. BACKGROUND
Fatigue is a physiological phenomenon, induced by physical or intellectual activity. “Fatigue” has been a core symptom in medicine ever since Hippocrates described “the disease of the Scythians”, who spent the day on horseback and had a state of persistent fatigue. The symptom “fatigue” is also a core feature in psychiatry since Esquirol and Galen linked fatigue to melancholia and neurasthenia was described by the neurologist Beard and the psychiatrist Van Deusen [1]. Thus, fatigue is a symptom of many major somatic diseases and some psychiatric disorders.
The process leading to the clinical description of Chronic Fatigue Syndrome (CFS) was slow. Indeed, sporadic CFS-like cases and epidemics were first described in the 19th and 20th centuries [2]. In the 1950s, Myalgic Encephalomyelitis (ME) was described after an outbreak of neurological symptoms at the Royal Free Hospital in London, UK [2]. Concomitantly, Yuppie Flu syndrome (“yuppie” standing for young upwardly mobile professional, as a consequence of it being viewed as affecting only dynamic urban people), a milder Anglo-Saxon version of the ME [3], that appeared on the East Coast of United States [3,4]. On this basis, ME was renamed first “post-viral fatigue syndrome”, following the occurrence of an abnormally persistent fatigue in patients with a serological profile suggestive of Epstein Barr virus infection and finally renamed “Chronic fatigue syndrome” in 1988. Chronic Fatigue Syndrome (CFS) was described as a condition mainly characterized by debilitating, persistent or recurrent fatigue over six months and associated with other related symptoms, and inducing significant distress or impairment in social, occupational or other important areas of functioning. The existence of CFS is controversial and numerous papers have been recently published on this topic.
2. DEFINITION
In 1994, a consensus was reached on a case definition from the US Center for Disease Control and Prevention (CDC) [1,2,5]. It is the most wide-spread definition in research as well as in clinical practice [1]. It comprises main, additional and exclusion criteria (Table 1).
Other definitions are also available such as the Oxford definition, the Australian definition and the Canadian definition. None of them strongly deviate from the CDCdefinition, but some specificities do exist. The OxfordDefinition requires the presence of “mental fatigue” and includes symptoms that might indicate a psychiatric disorder. The Australian definition does not require a new or definite onset of fatigue. And the Canadian definition excludes patients with any symptoms of mental illness (excluding fatigue) [1].
There are currently no objective criteria for the diagnosis of CFS, such as pathognomonic objective signs, abnormalities in laboratory or imaging tests. Thus, CFS remains a diagnosis of exclusion [6].
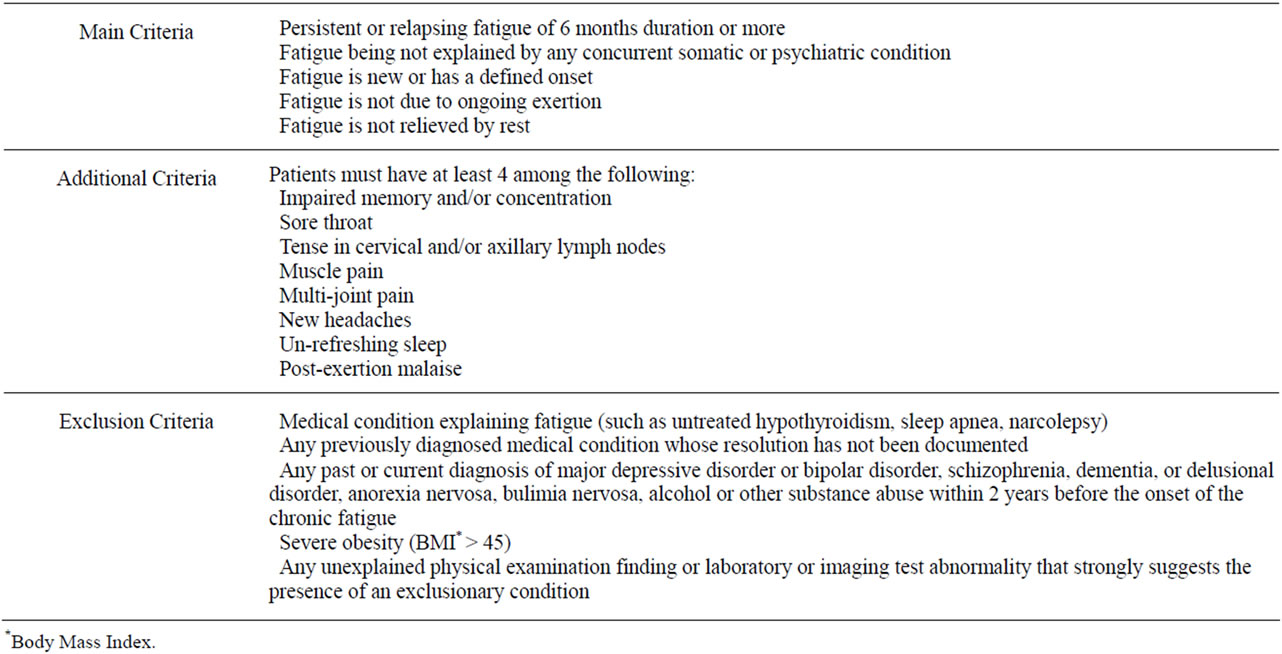
Table 1. Definition and clinical assessment of chronic fatigue syndrome.
3. CFS AND INTERNATIONAL CLASSIFICATIONS
The International Classification of Diseases, 10th revision (ICD-10), classifies CFS in disorders of the nervous system. Thus, it allows for both neurological and psychiatric coding of CFS [7]. Neurasthenia is also classified in ICD-10, Chapter V, F48.0.
In ICD-11, postviral fatigue syndrome and benign myalgic encephalomyelitis are classified in Chapter VI: Diseases of the nervous system; other disorders of the nervous system.
(G90-99); other disorders of brain (G93); postviral fatigue syndrom (or Benign myalgic encephalomyelitis) (G93.3).
Importantly, the DSM-IV does not include descriptions of neurasthenia and CFS. And the DSM-V task force has rejected proposals to classify CFS as a psychiatric condition.
4. EPIDEMIOLOGY
Epidemiological data on CFS depend on the case-definitions used [1].
According to the CDC definition, the prevalence of CFS varies from 0.4% to more than 2% [8]. The sex ratio for CFS is 3/1 with a higher prevalence in women, particularly in the 40-49-year-old age range. For example, about 5% of women between 40 and 60 years of age in metropolitan, urban and rural population in Central Georgia describe symptoms of CFS and almost 3% of women in this age range meet strict clinical criteria for CFS [9].
The mean age at onset of CFS is between 29 years and 35 years. CFS is relatively rare in children younger than 10 years [1]. The mean illness duration ranges from 3 years to 9 years [2].
The prevalence of CFS varies depending on ethnic groups. And CFS prevalence seems to be higher in welldeveloped countries. Physical inactivity, social strain, anxiety symptoms, depressive symptoms and negative aspects of social support are risk factors for CFS [10].
A quarter of CFS subjects are unemployed or receive disability benefits [11].
However, despite the high prevalence of this syndrome, only about 50% of patients with CFS have been referred to a physician, of whom 50% were diagnosed [11,12].
5. RELATIONSHIP WITH DEPRESSIVE AND ANXIETY DISORDERS
It should be noted that CFS and depression are distinct entities. While some symptoms of CFS are also symptoms of major depression, many others (such as sore throat, adenopathies, arthralgies and postexertional fatigue) are not typical manifestations of major depression. Conversely, sleep abnormalities normally found as part of major depression are usually not present in CFS [13].
The prevalence of panic disorder [1,14] and generalized anxiety disorders seems to be higher among CFS patients than in the general population both for adults and teenagers. Literature points to an overlap between CFS and anxiety, as exemplified by a decreased cerebral blood flow [13]. Further investigations are needed.
6. COGNITIVE FUNCTION
Cognitive tests of CFS patients have revealed impairments in memory, attention and information, even in patients without any psychiatric comorbidity [1,15,16].
Constant et al. (2011) assessed cognitive performances of patients with CFS without any psychiatric comorbidity, in comparison with a control group of healthy volunteers and a group of patients with major depressive disorder. Standardized tests of attention, working memory, verbal and visual episodic memory were performed. They confirmed the presence of an objective impairment in attention and memory in patients with CFS [15]. In another study, Schrijvers et al. [16] compared cognitive and motor performance in major depressive disorder and CFS, performing line and figure computerized copying tasks. Both patient groups showed similar cognitive impairments.
7. PERSONALITY
CFS is associated with an increased prevalence of maladaptive personality features and personality disorders [1,17]. It is not however known whether personality dispositions are a risk factor or whether they are a conesquence of the chronicity and severity of CFS [11]. Among personality features, neuroticism and introversion have been reported as risk factors for CFS [2]. Moreover, the duration of the CFS may be related to specific patterns of coping styles. Indeed, Brown et al. (2010) showed a higher use of active coping, planning, acceptance and a lower use of behavioral disengagement in the long illness duration group as compared to the short illness duration group [18].
8. COMORBIDITIES WITH SOMATIC DISORDERS
Patients with CFS, fibromyalgia and temporo-mandibular disorder (acute or chronic inflammation of the temporo-mandibular joint) share many clinical illness features such as myalgia, fatigue, sleep disturbances and impairment in ability to perform activities of daily living as a consequence of these symptoms. A growing body of literature suggests that a variety of comorbid illnesses may also coexist in these patients, including irritable bowel syndrome (IBS), chronic tension-type headache and interstitial cystitis. Aaron et al. (2000) found that patients with CFS, fibromyalgia and temporo-mandibular disorder share symptoms including generalized pain sensitivity, sleep and concentration difficulties and bowel complaints [19]. In some studies, the tension points of fibromyalgia are found in 50% - 70% of patients [4].
Comorbidity between CFS and IBS (abdominal pain and discomfort) has been identified in a number of studies and a high degree of overlapping symptoms has been reported [20].
Future research is required to evaluate physical impairment, potential mechanisms and psychiatric comorbidities among patients with overlapping conditions.
9. CORRELATES AND PHYSIOPATHOLOGY
The etiology and pathogenesis of CFS are generally believed to be multifactorial.
Distinction between categories of predisposing, precipitating and perpetuating factors is useful for understanding this complex disorder [2]. A predisposing factor is a factor that increases the risk of disease [21]. A precipitating factor is a factor accelerating the occurrence of the disease. A perpetuating factor is a factor extending the disease in time.
9.1. Genetics
There is some evidence from several twin studies [2,22] that CFS is a moderately heritable condition. Moreover, a significant excess familial clustering and significantly elevated risk for CFS has been shown among first, second and third degree relatives of CFS cases. These results support a genetic predisposition to CFS [23].
Chronic fatigue is related to polymorphisms of genes involved in the monoaminergic system [22]. A significant increase of longer allelic variants of the 5-HTTLPR (serotonin transporter gene) was found in CFS patients compared to controls [24]. The monoamine oxidase (MAO) and catechol-O-methyltransferase genes (COMT) seem also to be variably implicated [1].
A variable cluster of nine genes whose mRNA expressions are significantly different in some patients with CFS compared with ageand sex-matched controls has been identified: genes encoding granzyme in activated T or natural killer cells (GZMA), energy regulators (ATP5J2, COX5B, and DBI), proteasome subunits (PSMA3 and PSMA4), putative protein kinase c inhibitor (HINT), GTPase (ARHC), and signal transducers and activators of transcription 5A (STAT5A) [25]. As suggested by the authors, these genes might be useful to detect differential diagnoses such as mood disorders, somatoform disorders, major depression, personality disorders, adjustment disorder, unexplained fatigue, diabetes mellitus and sleep apnea.
9.2. Neuroimaging
Neuroimaging studies have revealed some structural and functional alterations of the CNS among patients with CFS. Two separate cohorts of CFS patients showed a marked decline in grey matter volume, compared with matched healthy controls, this reduction being associated with objectively measured decline in physical activity [26]. Information-processing dysfunction among CFS patients was linked to an increased neural resource allocation shown by functional resonance imaging [27]. Others MRI studies have detected significantly more abnormalities in the subcortical white matter of CFS subjects, compared to controls [28,29].
Moreover, lower levels of regional cerebral blood flow throughout the brain were reported [13], particularly through the insula cortex which controls visceral functions and integrates autonomic information and in younger patients, reduced blood flow in the left temporal lobe which controls access to language. Magnetic resonance spectroscopy identified increased levels of free choline in the brain, which is consistent with a response to an infection resulting in increased breakdown of cell membranes that would cause loss of function [30]. Glucose hypometabolism in the frontal cortex and brain stem, decreased number and/or affinity for the receptor protein 5-HT1A in the hippocampus and the serotonin transporter proteins in the cingulate gyrus have also been evidenced [1].
9.3. Neurobiological Abnormalities
A low circulating cortisol and a central down-regulation is reported in CFS patients [1,13], that might be due to an increased sensitivity or number of glucocorticoid receptors in the brain [1]. Conversely, severe major depression is associated with up-regulation of the hypothalamic-pituitary-adrenal (HPA) axis resulting in mild hypercortisolism. In addition, abnormalities of central nervous system serotonin functioning have also been shown as indicated. Administration of serotonin agonists significantly increases the prolactin levels of CFS patients, as compared to depressed and healthy comparison subjects. These data suggest a central nervous system up-regulation of the serotoninergic system [13].
9.4. Infections
CFS often has an acute onset with symptoms strongly resembling an infection [1]. Many microorganisms have been implicated such as Cytomegalovirus, Parvovirus B19, Brucella species, Toxoplasma gondii, Coxiella burnetii, Mycoplasma species and Chlamydia pneumoniae, Herpesvirus, Borna Virus, Candida albican [1,4,31,32] but their pathogenic relationship with the syndrome has not been demonstrated yet [12,31,32].
More recently, the presence of the retrovirus xenotropic murine leukaemia virus-related virus (XMRV) has been reported in peripheral blood molecular cells of patients with chronic fatigue syndrome [33].
Several recent studies have failed to confirm the association between XMRV and CFS [34-38]. With regards to viral implication, no conclusive data are proposed to find an infectious etiologic agent of CFS with viral detection potentially being more casual than causal [31].
Nevertheless, Lo et al. (2010) found a murine leukemia virus-like virus (MLV-like virus) “gag” gene sequences in 32 of 37 CFS patients (86.5%), compared with only 3 of 44 (6.8%) healthy volunteer blood donors. Seven of 8 gag-positive patients tested again positive in a sample obtained nearly 15 years later. Moreover, they identified a genetically diverse group of MLV-related viruses. The “gag” and “env” sequences from CFS patients were more closely related to those of polytropic mouse endogenous retroviruses than to those of XMRVs and were even less closely related to those of ecotropic MLVs. Now, further studies are needed to determine whether the same strong association with MLV-related viruses is found in other groups of patients with CFS and whether these viruses play a causative role in the development of CFS [39].
9.5. Immune System Disturbances
Different immune abnormalities have been shown in CFS patients such as reduced level of the cytokine TGFB1 which contributes to myalgia [22], increased level of IL-6, reduced natural killer (NK) cell activity [14], increased expression of activation markers on the cell surface of T lymphocytes, especially increased number of CD8+ cytotoxic cells that bear certain antigenic markers [13], decrease in certain subclasses of immunoglobulins, presence of various antibodies and circulating immune complexes, increased levels of TNFa, TNFb, IL1a, TNFa and TNFb [4]. Abnormalities in CD26 and in NK cell function could have a particular relevance for the possible role of infections in the initiation and/or persistence of CFS [40].
Another argument supporting the involvement of the immune system in the pathogenesis of CFS is the communication between the HPA axis and immune system via glucocorticoids. They can modulate immune responses by inhibiting the production of pro-inflammatory cytokines, therefore promoting the switch from Th1-type towards Th2-type responses. Several studies investigated immune responses during and after stress, as it is well known that stress and exercise can substantially exacerbate fatigue in CFS patients. Increased doses of dexamethasone in CFS patients undergoing a psychosocial stress enhanced sensitivity of circulating leucocytes. Moreover, it was found that lipopolysaccharide-stimulated cytokine production decreases shortly after stress in CFS subjects while it increases in controls [1,4,14]. However, there is no scientific evidence that these immune abnormalities have a causal role in CFS [14].
Furthermore, the intestinal microflora in CFS patients has also been shown to be altered with low levels of Escherichia coli and Bifidobacterium species and with a significant increased numbers of enterococci compared to healthy controls. Moreover, there is evidence suggesting that the intestinal microflora of patients with intestinal bowel syndrome differs from that of healthy individuals. Indeed, comorbidity between CFS and irritable bowel syndrome (abdominal pain and discomfort) has been identified in a number of studies and a high degree of overlapping symptoms has been reported [41].
An elevated number of Candida albicans species in the faecal microflora of CFS patients during the acute phase of the illness has recently been reported [20].
9.6. Skeletal Muscle Function
Recent reports indicate that skeletal muscle function is weaker in patients than sedentary controls [1]. Neurophysiological experiments suggest that one probable explanation for this phenomenon is the altered activation of cortical motor areas in the central nervous system of CFS patients [1].
9.7. Circulatory Homeostasis
Several abnormalities have been found: sympathetic predominance in the modulation of heart rate and total peripheral resistance during rest and orthostatic challenge, orthostatic hypotension, and alterations in blood flow after dynamic exercise due to sympathetically induced vasoconstriction [1].
9.8. Gynecological Factors
A higher frequency of gynecological conditions has been reported in CFS as compared to healthy controls. Indeed, women with CFS were more likely than control to have reported a history of menstrual problems, galactorrhea, endometriosis, uterine fibroids, polycystic ovary syndrome, sexually transmitted diseases and cervical problems [9]. Moreover the frequency of hysterectomies is higher in CFS patients than in matched controls [42].
Some authors [9] suggest that frequent anovulatory cycles associated with irregular menses and decreased progesterone, suggested by the high prevalence of polycystic ovary syndrome, contributed to an elevated estrogen/progesterone ratio among women with CFS and that this caused chronic immune activation. These authors highlight the importance of evaluating gynecological health in patients with CFS because of the higher prevalence of gynecological conditions and gynecological surgeries in these women. There is a need to clarify the chronologic and the pathophysiological relationships between these conditions and CFS.
10. PHARMACOLOGICAL TREATMENTS
No pharmacological treatment has yet demonstrated clear benefits in CFS.
Indeed, immunological, antiviral, and antibiotic treatments have been investigated in high quality controlled trials involving large patient numbers to allow sufficient statistical power. All of them have failed to provide consistent evidence for their efficacy in the treatment of CFS [8]. Probiotics, such as the lactic acid producing bacteria, as regulators of the microflora, have recently been suggested as therapeutic agents in CFS [20]. Their potential benefits might be related to their impact on the cytokine balance and their antioxidant properties [20]. No benefit was found in CFS patients from treatments with antihypertensive agents such as clonidine, steroid drugs, anticholinergics or growth hormone (6.7 microg/kg/day for 12 weeks) [9,43].
Therapeutic doses of antidepressants have not been overwhelmingly effective in treating CFS [13]. In particular, during a randomized, double-blind, placebo-controlled study of fluoxetine (20 mg/day for 8 weeks), no effect was observed on any characteristic of CFS [44].
Regarding stimulants, modafinil (200 - 400 mg/day), a non-amphetamine CNS-stimulant, has no significant effect on self-rating of fatigue, quality of life or mood, whereas mixed results were shown on cognitive tests. Significant improvements were found with dexamphetamine but reduced food consumption was a prominent side effect. A clinical significant effect on fatigue was also found with methylphenidate (20 mg/day), an amphetamine derivative, but no effect was observed on bodily pain, mental health, depression and anxiety. All these stimulants could be beneficial for some CFS patients in the short-term but their long-term effects are uncertain.
Mixed results were obtained with nicotinamide adenine dinucleotides (NADH), which trigger energy production through ATP generation [8]. The results of a randomized double-blind, placebo-controlled, crossover study with NADH (10 mg/day for 4 weeks) indicate that NADH may be a valuable adjunctive therapy in the management of the CFS [45]. Another study showed that oral NADH (20 mg/day) was associated with a decrease in anxiety and maximum heart rate after a stress test in patients with CFS, without any change in other clinical variables and global functional performance [46].
A recent study showed that high cocoa polyphenol rich chocolate (45 g/day for 8 weeks) may improve fatigue and residual function in subjects with CFS, chocolate being able to increase neurotransmitters like phenylethylamine (an alkaloid monoamine), serotonin and anandamide [47].
11. NON PHARMACOLOGICAL TREATMENTS
Cognitive behavior therapy (CBT) [48-50] has been shown to improve CFS outcome. Recent well-controlled trials found that more than 70% of patients who received 13 - 16 sessions of CBT improved their physical and global functioning, with sustained improvement over 6 - 14 months of follow-up [13]. Central CBT components for CFS include explanation of the etiological model, motivation for CBT, challenging and changing fatigue-related cognitions, achievement and maintenance of a basic amount of physical activity, gradual increase of physical activity and planning work rehabilitation or rehabilitation in other personal activities [2]. The hypothesis regarding the mechanism of action of CBT is that it plays a role in perpetuating the symptoms of CFS [13]. Indeed, behaviorally focused interventions are some of the most effective ways of reducing fatigue, even when there is a clear underlying cause, such as rheumatoid arthritis, multiple sclerosis or cancer [6].
Graded exercise therapy (GET) [48,49] has also been shown to improve CFS outcome with sustained improvement over 6-14 months of follow-up [13].
So, CBT and GET can be used to increase activity and teach effective coping strategies. These two strategies may also be combined together [13,51].
Adaptive pacing therapy (APT) might also be useful. The aim of this therapy is to achieve optimum adaptation to the illness. This adaptation is achieved by helping the participant to plan their activities in order to reduce or avoid fatigue and provide the best conditions for natural recovery. However, the adjunction of APT to CBT or GET is not effective in the treatment of CFS [48].
12. HEALTHCARE MANAGEMENT
After the initial diagnostic suspicion, it is recommended to refer patients to a second level of specialized care for confirmation of the diagnosis and treatment guidance [30]. General management strategies are helpful for individuals with CFS. They should be maintained after referral to CFS specialists [51]. Since several medical specialties are involved in care and treatment of CFS patients (rheumatology, internal medicine, psychiatry, etc.), such specialists should also receive adequate training. Cooperation and coordination between primary and specialized care is central for the correct management of CFS [14]. Moreover, the intervention of the physiotherapist or psychologist in the treatment is strongly recommended and adequate training should also be offered to these healthcare professionals.
For people with moderate or severe CFS, providing or recommending specific equipments and adaptations (such as wheelchairs, blue badges or stairlifts) should be considered as part of an overall management plan, taking into account the risks and benefits for each patient. This may help them to maintain their autonomy and improve their quality of life. Healthcare professionals should proactively advise about fitness for work and education, and recommend flexible adjustments or adaptations to work. This may include education providers or support services (such as school or occupational health services).
For people with CFS who are able to continue or return to education or employment, healthcare professionals should help employers, occupational health or education institutions to have sufficient information about the condition and the agreed management plan.
If possible, and with the informed consent of the individual with CFS/ME, healthcare professionals should discuss employment issues with occupational health professionals, who will communicate with the person’s manager or human resources representatives. If there is no access to occupational health services, the responsible clinician should liaise with the employer directly.
Healthcare professionals should work closely with social care and education services to ensure a common understanding of the goals of the person with CFS/ME. The use of a flexible approach should be favored, including home tuition and use of equipments that allow a gradual reintegration into education [51].
13. PROGNOSIS
Recovery of CFS has been defined as the disappearance of symptoms and functional impairment, ability to return to work and to undertake other activities, no longer interpreting everyday bodily signs as indicating CFS and letting go of the label “CFS patient” [2].
The long-term prognosis of CFS has been poorly studied. However, in adults, a recent review of 28 studies describing the prognosis of chronic fatigue and CFS reported a median of 40% for improvement for the 14 studies of subjects meeting operational criteria for CFS. In children and teenagers, the prognosis of CFS seems to be better, with full or partial recovery in 60% - 80% of patient [1].
Several predictors of poor treatment outcome have been identified: membership of a self-help group, receipt of a sickness benefit, claiming a disability benefit, a low sense of control, a strong focus on symptoms and a pervasively passive activity pattern [2]. No significant correlation has been shown between the presence of comorbid psychiatric disorders and the outcome of CFS [52].
14. HEALTHCARE COST AND LEGAL ASPECTS
The costs imposed by CFS on healthcare have been estimated up to 24 billion dollars per year [40].
Although many people suffering from CFS continue to work despite their illness, based on economic reasons and social prestige, CFS represents an annual global loss of productivity of approximately 15,200 dollars per patient and day. These figures are comparable to the loss caused by other diseases such as digestive system-related conditions or infectious and parasitic diseases. These results suggest that CFS can be included with other chronic processes among the highest healthcare and socioeconomic burdens [14].
CFS is a highly disabling condition in some patients, frequently requiring legal support for managing possible social aids, handicaps or even disabilities. Ancillary personnel or social workers in healthcare centers and city councils should provide information and advice to patients. It will also be necessary for the administrators to adapt the help to each case and the training of their personnel to become familiar with CFS, avoiding excessive litigation when looking for social aids [14].
15. CONCLUSIONS
CFS is still a controversial disorder and it can be diagnosed only after ruling out other causes of fatigue. This chronic process is a social disorder because of the inability to maintain professional, social and family tasks. Much recent multidisciplinary literature is available about its etiology and treatment and there is a need of several different reading levels of the literature. Current etiological assumptions are based on predisposing, precipitating and perpetuating factors but physiopathology is still unclear although the viral origin is now largely discarded. Patients with CFS require multidisciplinary management due to the multiple and different issues affecting them. This multidisciplinary management requires coordination between the different specialists. At present, no curative treatment exists for patients with CFS although new prospects are in sight. Cognitive Behavioral Therapy (CBT) and Graded Exercise Therapy (GET) have currently a scientifically proven beneficial effect. Treatment objecttives must be focused on improving the clinical manifesttations, maintaining the functional capacity and quality of life and developing a tailored program providing each patient with the maximum perception of improvement.
CFS is in many ways an orphan illness, sitting on the border between medicine and psychiatry. Psychiatrists must know the existence of this illness, because CFS patients are sometimes refered to psychiatrists.
The contribution of funds for research is also necessary and the regulated identification and management of patients or the creation of adequate records by the healthcare system would be useful.
REFERENCES
- Wyller, V.B. (2007) The chronic fatigue syndrome—An update. Acta Neurologica Scandinavica, 115, 7-14. doi:10.1111/j.1600-0404.2007.00840.x
- Prins, J.B., Van der Meer, J.W.M. and Bleijenberg G. (2006) Chronic fatigue syndrome. Lancet, 367, 346-355. doi:10.1016/S0140-6736(06)68073-2
- Orth, J.P. (1993) La fatigue au quotidien. Sante Edition Odile Jacob, 103-115.
- Rouillon, F., Delhommeau, G. and Vinceneux, P. (1996) Le syndrome de fatigue chronique, Masson, Paris.
- Fukuda, K., Straus, S.E., Hickie, I., Sharp, M.C., Dobbins, J.C. and Komaroff, A. (1994) The chronic fatigue syndrome: A comprehensive approach to its definition and study. Annals of Internal Medicine, 121, 953-959.
- Harvey, S.B. and Wessely, S. (2009) Chronic fatigue syndrome: Identifying zebras amongst the horses. BMC Medicine, 7, 58. doi:10.1186/1741-7015-7-58
- Wojcik, W., Armstrong, D. and Kanaan, R. (2011) Chronic fatigue syndrome: Labels, meanings and consequences. Journal of Psychosomatic Research, 70, 500-504. doi:10.1016/j.jpsychores.2011.02.002
- Van Houdenhove, B., Pae, C.U. and Luyten, P. (2010) Chronic fatigue syndrome: Is there a role for non-antidepressant pharmacotherapy? Expert Opinion, 11, 215-223. doi:10.1517/14656560903487744
- Boneva, R.S., Maloney, E.M., Lin, J.M., et al. (2011) Gynecological history in chronic fatigue syndrome: A population-based case-control study. Journal of Women’s Health, 20, 1. doi:10.1089/jwh.2009.1900
- Bhui, K.S., Dinos, S., Ashby, D., Nazroo, J., Wessely, S. and White, P.D. (2011) Chronic fatigue syndrome in an ethnically diverse population: The influence of psychosocial adversity and physical inactivity. BMC Medicine, 9, 26. doi:10.1186/1741-7015-9-26
- Nater, U.M., Jones, J.F., Lin, J.M.S, Maloney, E., Reeves, W.C. and Heim, C. (2010) Personality features and personality disorders in chronic fatigue syndrome: A population-based study. Psychotherapy and Psychosomatics, 79, 312-318. doi:10.1159/000319312
- Jason, L.A., Richman, J.A., Rademaker, A.W., et al. (1999) A community-based study of chronic fatigue syndrome. Archives of Internal Medicine, 159, 2129-2137. doi:10.1001/archinte.159.18.2129
- Afari. N. and Buchwald, D. (2003) Chronic fatigue syndrome: A review. American Journal of Psychiatry, 160, 221-236. doi:10.1176/appi.ajp.160.2.221
- Fernandez, A.A., Martin, A.P., Martinez, M.I., et al. (2009) Chronic fatigue syndrome: Aetiology, diagnosis and treatment. BMC psychiatry, 9, S1. doi:10.1186/1471-244X-9-S1-S1
- Constant, E.I., Adam, S., Gillain, B., Lambert, M., Masquelier, E. and Seron, X. (2011) Cognitive deficits in patients with chronic fatigue syndrome compared to those with major depressive disorder and healthy controls. Clinical Neurology Neurosurgery, 113, 295-302. doi:10.1016/j.clineuro.2010.12.002
- Schrijvers, D., Van Den Eede, F., Maas, Y., Cosyns, P., Hulstijn, W. and Sabbe, B.G.C. (2009) Psychomotor functioning in chronic fatigue syndrome and major depressive disorder: A comparative study. Journal of Affective Disorders, 115, 46-53. doi:10.1016/j.jad.2008.08.010
- Goodwin, L., White, P.D., Hotopf, M., Stanfeld, S.A. and Clark, C. (2011) Psychopathology and physical activity as predictors of chronic fatigue syndrome in the 1958 british birth cohort: A replication study of the 1946 and 1970 birth cohorts. Annals of Epidemiology, 21, 343-350. doi:10.1016/j.annepidem.2010.12.003
- Brown, M.M., Brown, A.A. and Jason, L.A. (2010) Illness duration and coping style in chronic fatigue syndrome. Psychological Reports, 106, 383-393. doi:10.2466/pr0.106.2.383-393
- Aaron, L.A., Burke, M.M. and Buchwald, D. (2000) Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia and temporomandibular disorder. Archives of Internal Medicine, 160, 221-227. doi:10.1001/archinte.160.2.221
- Sullivan, A., Nord, C.E. and Evengard, B. (2009) Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutrition Journal, 8, 4. doi:10.1186/1475-2891-8-4
- Kato, K., Sullivan, P.F., Evengard, B. and Pedersen, N.L. (2006) Premorbid predictors of chronic fatigue. Archives of General Psychiatry, 63, 1267-1272.
- Cho, H.J., Skowera, A., Cleare, A. and Wessely, S. (2006) Chronic fatigue syndrome: An update focusing on phenomenology and pathophysiology. Current Opinion in Psychiatry, 19, 67-73. doi:10.1097/01.yco.0000194370.40062.b0
- Albright, F., Light, K., Light, A., Bateman, L. and Cannon-Albright, L.A. (2011) Evidence for a heritable predisposition to chronic fatigue syndrome. BMC Neurology, 11, 62. doi:10.1186/1471-2377-11-62
- Narita, M., Nishigami, N. and Narita, N. (2003) Association between serotonin transporter gene polymorphism and chronic fatigue syndrome. Biochemical and Biophysical Research Communications, 311, 264-266. doi:10.1016/j.bbrc.2003.09.207
- Saiki, T., Kawai, T., Morita, K., et al. (2008) Identification of marker genes for differential diagnosis of chronic fatigue syndrome. Molecular Medicine, 14, 599-607. doi:10.2119/2007-00059.Saiki
- de Lange, F.P., Kalkman, J.S., Bleijenberg, G., et al. (2005) Gray matter volume reduction in the chronic fatigue syndrome. Neuroimage, 26, 777-781. doi:10.1016/j.neuroimage.2005.02.037
- Lange, G., Steffner, T.J. and Cook, D.B., et al. (2005) Objective evidence of cognitive complaints in chronic fatigue syndrome: A blod fMRI study of verbal working memory. Neuroimage, 26, 513-524. doi:10.1016/j.neuroimage.2005.02.011
- Natelson, B.H., Cohen, J.M., Brasloff, I. and Lee, H.J. (1993) A controlled study of brain magnectic resonance imaging in patients with fatiguing illnesses. Journal of the Neurological Sciences, 120, 213-217. doi:10.1016/0022-510X(93)90276-5
- Lange, G., Deluca, J., Maldijian, J.A., Lee, H.J., Tiersky, L.A. and Natelson, B.H. (1999) Brain MRI abnormalities exist in a subset of patients with chronic fatigue syndrome. Journal of the Neurological Sciences, 171, 3-7. doi:10.1016/S0022-510X(99)00243-9
- Hooper, M. (2007) Myalgic encephalomyelitis: A review with emphasis on key findings in biomedical research. Journal of Clinical Pathology, 60, 466-471. doi:10.1136/jcp.2006.042408
- Morinet, F. and Corruble, E. (in press) Chronic fatigue syndrome and viral infections. In: “Chronic Fatigue Syndrome,” InTech, Rijeka, Croatia.
- Cameron, B., Hirschberg, D.L., Rosenberg-Hassan, Y., Ablashi, D. and Lloyd, A. (2010). Serum cytokine levels in postinfective fatigue syndrome. Clinical Infectious Diseases, 50, 278-280.
- Lombardi, V.C., Ruschetti, F.W., Das Gupta, J., et al. (2009) Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science, 326, 585-589. doi:10.1126/science.1179052
- Van Kuppeveld, F.J.M., De Jong, A.S., Lanke, K.H., et al. (2010) Prevalence of xenotropic murine leukaemia virus-related virus in patients with chronic fatigue syndrome in the Netherlands: Retrospective analysis of samples from an established cohort. British Medical Journal, 340, c1018. doi:10.1136/bmj.c1018
- Satterfield, B.C., Garcia, R.A., Jia, H., Tang, S., Zheng, H. and Switzer, W.M. (2011) Serologic and PCR testing of persons with chronic fatigue syndrome in the United States shows no association with xenotropic or polytropic murine leukemia virus-related viruses. Retrovirology, 8, 12. doi:10.1186/1742-4690-8-12
- Furuta, R.A., Miyazawa, T., Sugiyama, T., et al. (2011) No association of xenotropic murine leukemia virus-related virus with prostate cancer or chronic fatigue syndrome in Japan. Retrovirology, 8, 20. doi:10.1186/1742-4690-8-20
- Groom, H.C.T., Boucherit, V.C., Makinson, K., et al. (2010) Absence of xenotropic murine leukaemia virus-related virus in UK patients with chronic fatigue syndrome. Retrovirology, 7, 10. doi:10.1186/1742-4690-7-10
- Switzer, W.M., Jia, H., Hohn, O., et al. (2010) Absence of evidence of Xenotropic Murine Leukemia Virus-related virus infection I persons with Chronic Fatigue Syndrome and healthy controls in the United States. Retrovirology, 7, 57. doi:10.1186/1742-4690-7-57
- Lo, S-C., Pripuzova, N., Li, B., et al. (2010) Detection of MLV-related virus gene sequences in blood of patients with chronic fatigue syndrome and healthy blood donors. Proceedings of the National Academy of Sciences, 107, 36. doi:10.1073/pnas.1012780107
- Fletche, M.A., Zeng, X.R., Maher, K., et al. (2010) Biomarkers in chronic fatigue syndrome: Evaluation of natural killer, cell function and dipeptidyl peptidase IV/CD26. Plos One, 5, e10817.
- Withehead, W.E., Palsson, O. and Jones, K.R. (2002) Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology, 122, 1140-1156. doi:10.1053/gast.2002.32392
- Reyes, M., Dobbins, J.G., Mawle, A.C., et al. (1996) Risk factors for CFS: A case control study. Journal of Chronic Fatigue Syndrome, 2, 17-33. doi:10.1300/J092v02n04_03
- Moorkens, G., Wynants, H. and Abs, R. (1998) Effect of growth hormone treatment in patients with chronic fatigue syndrome: A preliminary study. Growth Hormone & IGF Research, 8, 131-133.
- Vercoulen, J.H., Swanink, C.M., Zitman, F.G., et al. (1996) Randomised, double-blind, placebo-controlled study of fluoxetine in chronic fatigue syndrome. Lancet, 374, 858-861. doi:10.1016/S0140-6736(96)91345-8
- Forsyth, L.M. and Preuss, H.G (1999) Therapeutic effects of oral NADH on the symptoms of patients with chronic fatigue syndrome. Annals of Allergy, Asthma, and Immunology, 82, 185-191.
- Alegre. J., Roses, J.M., Javierre, C., Ruiz-Baques. A., Segundo, M.J. and De Sevilla, T.F. (2010) Nicotinamide adenine dinucleotide (NADH) in patients with chronic fatigue syndrome. Revista Clínica Española, 210, 284-288. doi:10.1016/j.rce.2009.09.015
- Sathyapalan, T., Beckett, S., Rigby, A.S., Mellor, D.D. and Atkin, S.L. (2010) High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutrition Journal, 9, 55. doi:10.1186/1475-2891-9-55
- White, P.D., Goldsmith, K.A., Johnson, A.L., et al. (2011) Comparison of adaptive pacing therapy, cognitive behavior therapy, graded exercise therapy and specialist medical care for chronic fatigue syndrome (PACE): A randomized trial. Lancet, 377, 823-836. doi:10.1016/S0140-6736(11)60096-2
- Chambers, D., Bagnal, A.M., Hempel, S. and Forbes, C. (2006) Interventions for the treatment, management and rehabilitation of patients with chronic fatigue syndrome/ myalgic encephalomyelitis: An update systematic review. Journal of the Royal Society of Medicine, 99, 506-520. doi:10.1258/jrsm.99.10.506
- Deale, A., Chalder, T., Marks, I. and Wessely, S. (1997) Cognitive behavior therapy for chronic fatigue syndrome: A randomized controlled trial. American Journal of Psychiatry, 154, 408-414.
- Nye, F. (2007) NICE clinical guideline 53—Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy). http://www.nice.org.uk/nicemedia/live/11824/36193/36193.pdf
- Van Houdenhove, B. and Luyten, P. (2009) Treatment of chronic fatigue syndrome: How to find a “new equilibrium”? Patient Education and Counseling, 77, 153-154. doi:10.1016/j.pec.2009.09.001

