Open Journal of Pediatrics
Vol.3 No.4(2013), Article ID:40109,7 pages DOI:10.4236/ojped.2013.34058
Alimentary tract duplications in infancy and childhood. A 25-year experience with focus on rare types of the disease
![]()
13rd Department of Surgery, ATTIKO University Hospital, Athens, Greece
2Pediatric Surgery Department, Penteli General Children’s Hospital, Athens, Greece
3Radiology Department, Penteli General Children’s Hospital, Athens, Greece
Email: nikzav2000@yahoo.com
Copyright © 2013 Nick Zavras et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 29 September 2013; revised 25 October 2013; accepted 2 November 2013
Keywords: Alimentary Tract; Duplication; Infants; Children
ABSTRACT
Background: Alimentary tract duplications (ATDs) are rare congenital anomalies of the gut tube, seen mainly in neonates and infants. Their presentations are often mimicking other conditions, thus posing a diagnostic challenge. Surgical treatment is required in all cases. Objective: The aim of this study is to present our experience in the diagnosis and management of this condition. Subjects and Method: We, retrospectively, reviewed 7 duplications in 7 patients and analyzed sex, age, clinical presentation, location, complications, diagnostic work-up, surgical methods and post-operative course. Encountered diagnostic and surgical difficulties were also reviewed in two extremely rare cases. Results: Patients’ age varied between 2 months and 10 years. All duplications were single. Six of them were intra-abdominal and one thoracoabdominal Three ADTs were asymptomatic and discovered during routine X-ray imaging. One ADT involving the cecum was mimicking appendicitis and complicated by recurrent intussusceptions. The thoracoabdominal one proved a surgical challenge as it was a completely isolated ATD. All patients underwent surgery without postoperative complications. We conclude that despite their rarity, ATDs require a high level of clinical suspicion, especially if they are presented as thoracic masses. Appropriate diagnostic investigation of the pediatric patients is always necessary to avoid delay in diagnosis.
1. INTRODUCTION
Alimentary tract duplications (ATDs) are rare congenital anomalies discovered mainly in children and less often in adults [1-6]. They can be found anywhere from the mouth to the anus [3]. Their shape may be cystic or tubular, located in or adjacent to the mesenteric side of the native AT, and lined by alimentary tract mucosa, not necessarily the same as that of adjacent portion of the alimentary tract [7]. They usually share a common smooth muscle wall and blood supply with the adjacent part of the bowel, with which they may or may not communicate [3]. Clinical diagnosis can represent a challenge as the symptoms of ATDs are diverse and dependent mainly on the type, location of the duplication and presence of heterotopic gastric mucosa [4,8]. Often asymptomatic, ATDs may be identified incidentally during surgery [9], or possibly mimic other conditions such as chronic constipation [4], appendicitis [2], Meckel’s diverticulum [9], pancreatitis [10], or intussusception [2].
The objective of this retrospective study is to present our experience of ATDs, focusing on rare forms of the lesion.
2. SUBJECTS AND METHODS
We retrospectively reviewed ATDs in seven children managed over a 25-year period, from 1987 to 2012. Data were extracted from the patients’ medical records and included information concerning sex, age, clinical presentation, location, diagnostic work-up, treatment, and the postoperative course. The diagnostic and surgical difficulties that were encountered were also reviewed in selected cases. To date, no patients have been lost to follow-up, and they are in satisfactory condition.
3. RESULTS
The characteristics of the seven patients are shown in Table 1.
3.1. Age and Sex
The age of the patients ranged from 2 months up to 10 years, with a median age at diagnosis of 4.2 years. Five of the patients were females and two males: the overall male to female ratio was 1:2.5.
3.2. Localization
All seven duplications were single and their locations varied: two were gastric, three ileal, one cecal, and one thoracoabdominal.
3.3. Clinical Presentation, Diagnosis and Treatment
The clinical presentation of the patients was dependent on the location. Gastric duplications (cases 1 and 2) presented incidentally in one case and with epigastric pain and non-bilious vomiting in the other. Ileal duplications manifested incidentally in two cases (cases 4 and 5) and with abdominal pain and blood in the stool in the third (case 6). All these duplications were diagnosed preoperatively by ultrasonography (U/S). Open surgical laparotomy was performed in all cases. Located in the greater curvature of the stomach, both gastric duplications were cystic and were removed by enucleation, without opening the gastric lumen. Histology showed the presence of gastric tissue. Two of the ileal duplications were tubular (cases 4 and 6), and one cystic (case 5). At laparotomy, a right hemicolectomy was performed in two cases (4 and 6) and resection of the duplication with the adjacent ileal segment in the third (case 5). The wall in case 6 was perforated; it contained ectopic gastric tissue and communicated with the adjacent ileal segment. The lining epithelium of cases 4 and 5 was similar to that of the native bowel.
3.4. Selected Cases
3.4.1. Case 3
This 10-year old female was admitted complaining of recurrent episodes of coughing and dyspnea. Radiography revealed an ill-defined, spherical, subdiaphragmatic lesion. An ultrasound scan showed a retroperitoneal cyst measuring 7.5 × 6.5 cm with defined, framed borders and hyperechoic, homogeneous content. Magnetic resonance imaging (MRI) disclosed a cystic mass located in the right upper abdomen below the diaphragm, protruding into the right hemithorax, with concomitant scoliosis of the thoracic segment of the spine (Figure 1). Surgery was performed via a thoracotomy through the sixth intercostal space on the right, dissection of the diaphragm and excision of the cyst from the retroperitoneal space (Figure 2). The cyst did not communicate with the adjacent structures. Blood supply of the cyst was performed by small peripheral vessels. Histology revealed a single-spaced cystic duplication with a median diameter of 8 cm, wall width of 1 - 3 cm and a mucosal lining of columnar cells with patches that contained crypts and glands. A diagnosis of a completely isolated thoracoabdominal duplication was concluded from these findings. The postoperative course of the patient was uneventful and without any further complications to date.
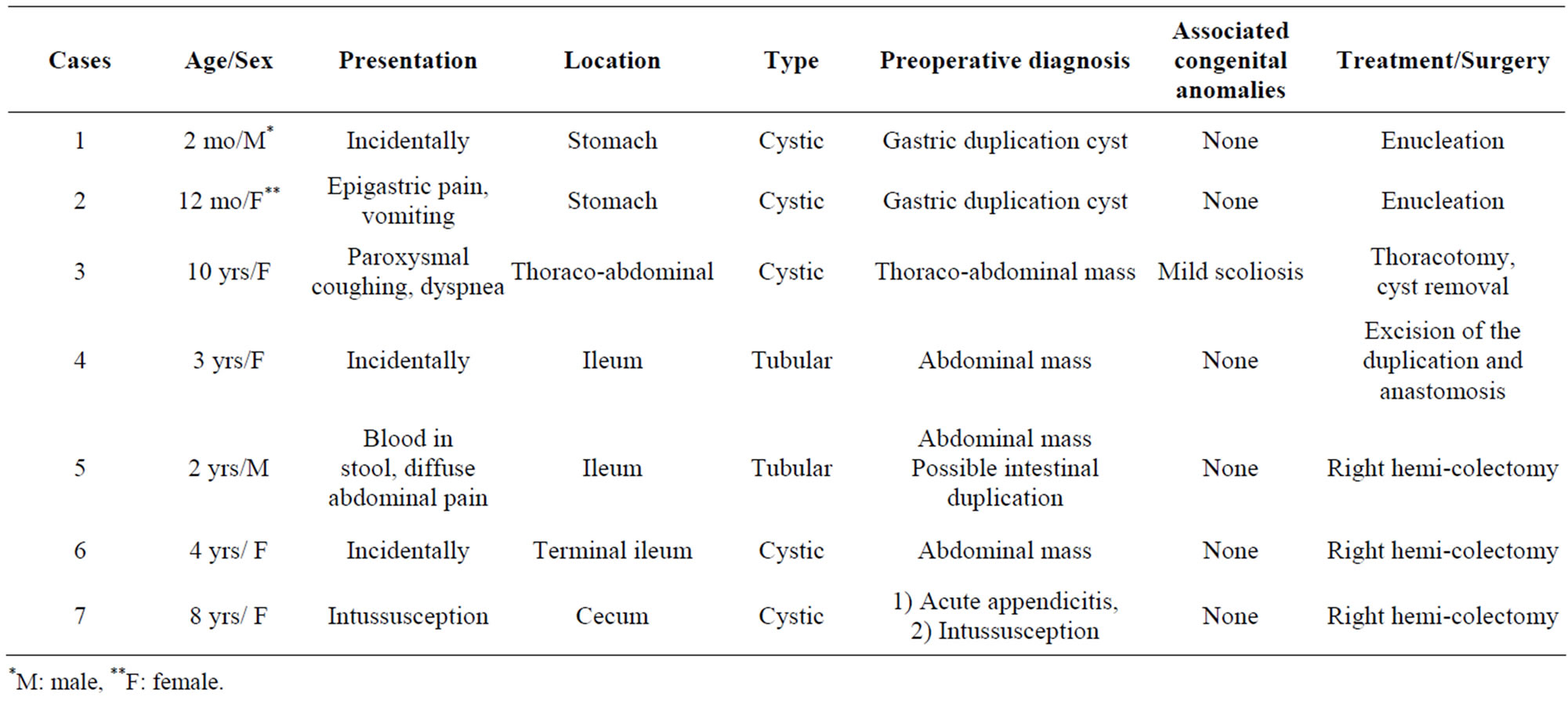
Table 1. Characteristics of children with ATDs.
*M: male, **F: female.
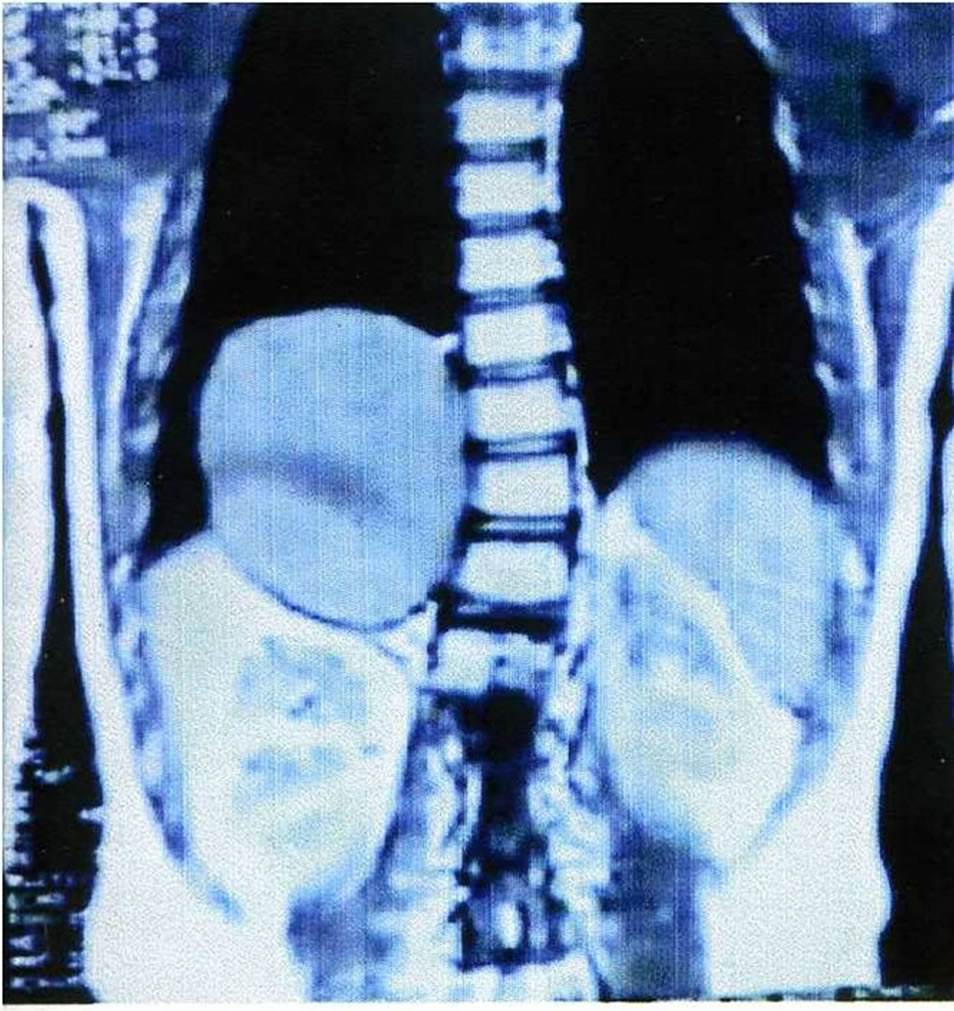
Figure 1. MRI T2 sequence: cystic lesion measuring 7 cm × 6 cm, and located between the liver and the right hemidiaphragm. Note the mild scoliosis of the thoracic segment of the spine.
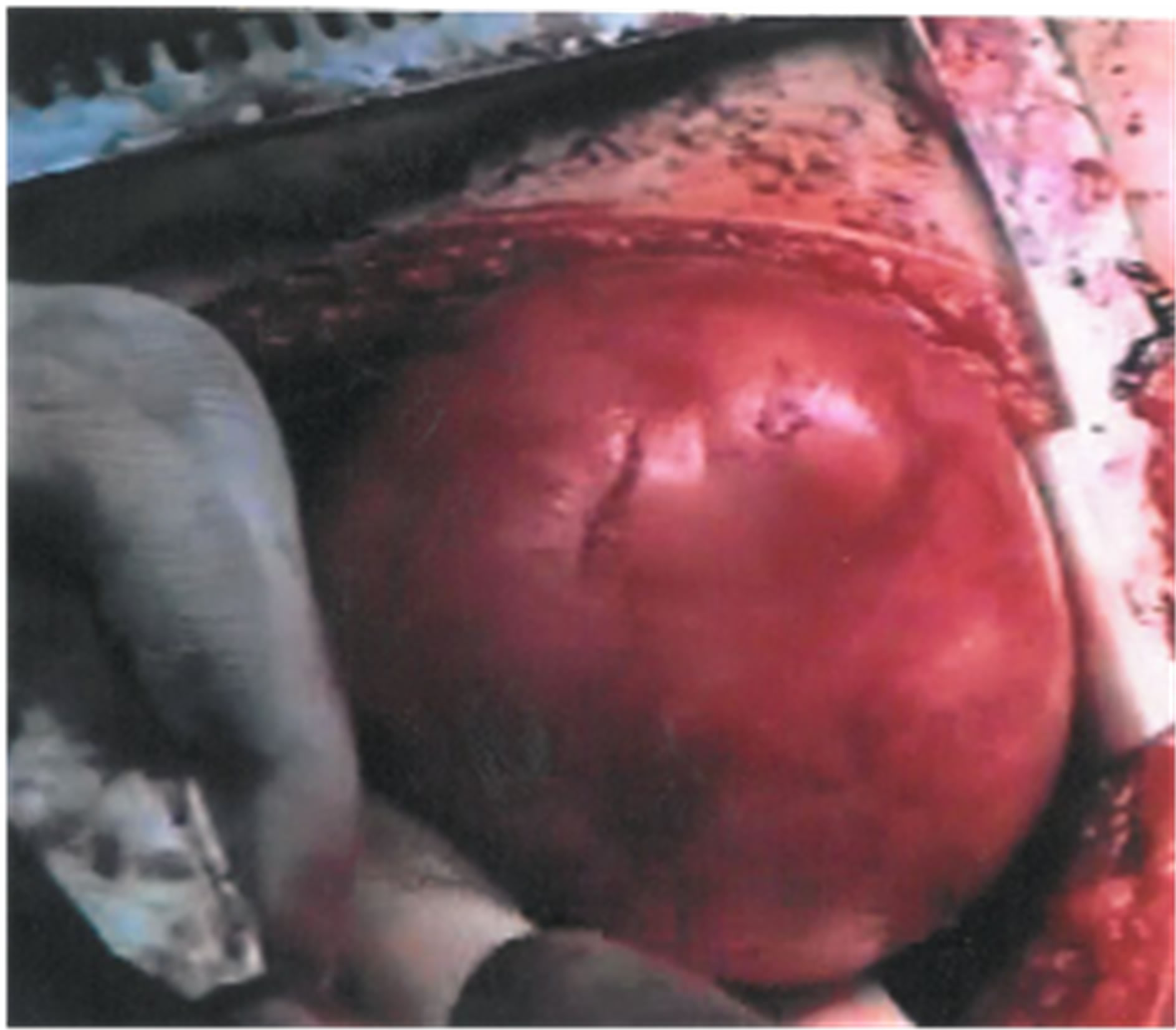
Figure 2. The excised specimen of isolated cystic mass.
3.4.2. Case 7
This 8-year-old female presented with episodes of acute abdominal pain in the lower right quadrant. The initial diagnosis was that of acute appendicitis and thus an appendectomy was performed in an otherwise non-inflamed appendix. Six months later, the patient was readmitted with pain in the lower right quadrant. An ileocolic intussusception was diagnosed which was managed successfully with barium enema. One month later, another episode of intussusception was diagnosed, which persisted despite appropriate management. Abdominal U/S showed an hourglass cystic mass (Figure 3) because of an incipient intussusception, and computed tomogramphy (CT) revealed the presence of a right lower quadrant mass (Figure 4). During surgery, a 10-centimeter ileocolic intussusception was identified. After unsuccessful efforts of manual reduction, the patient underwent a right hemicolectomy with subsequent end-to-end ileocolic anastomosis. The surgical specimen was opened, revealing an intracecal cystic mass, about 4 cm in size, at the mesenteric border of the cecum, measuring about 4 cm, was identified (Figure 5). Histological examination showed that the mesenteric portion of the wall was composed of smooth muscles in continuity with the terminal ileum, while the intraluminal portion consisted of large bowel tissue. No ectopic mucosa was seen.
4. DISCUSSION
W E Ladd [11] first coined the term “alimentary tract duplication” in 1937 to describe congenital malformations that involve the mesenteric side of the adjacent alimentary tract and share a common blood supply with the native bowel. Up to then, ATDs had been described as enterogenous cysts, ileal, jejunal or colon duplications, giant diverticulae, or unusual Meckel’s diverticulum [1].
The incidence of ADTs is reported to be 1/5000 live newborns [12]. Although in previous studies [2,3,13-15] ATDs were seen to be commoner in boys, the present study noted a female predilection. No familial or racial association has been reported [16].
The etiology of ATDs is a topic of speculation. Several embryological theories have been discussed in the literature [17-21]; however, none explain the entirety of the types of duplications. These theories can be summarized as follows: 1) a split notochord syndrome in which the primary defect is abnormal adhesions between the ectoderm and endoderm that results in herniation of the yolk sac through the two halves of vertebra, leading to the subsequent duplication of the gut [17], possibly explaining the thoracic duplications associated with spinal and central nervous malformations deformities, but not the intraabdominal duplications in which no spinal deformities are observed [11]; 2) abnormalities of recanalization of the solid stage in which an embryologic error leads to failure of recanalization [18]; 3) the abortive twinning theory [19], which may explain duplications of the large bowel, usually associated with abnormalities of the genitourinary tract; 4) the theory of embryologic diverticulae [20] which could justify the higher frequency of ATDs in the terminal ileum, though the presence of heterotopic mucosa, the location in the mesenteric side, and the presence of tubular duplications puts this theory into question; and 5) the concept of environmental factors such as trauma or hypoxia [21] in which
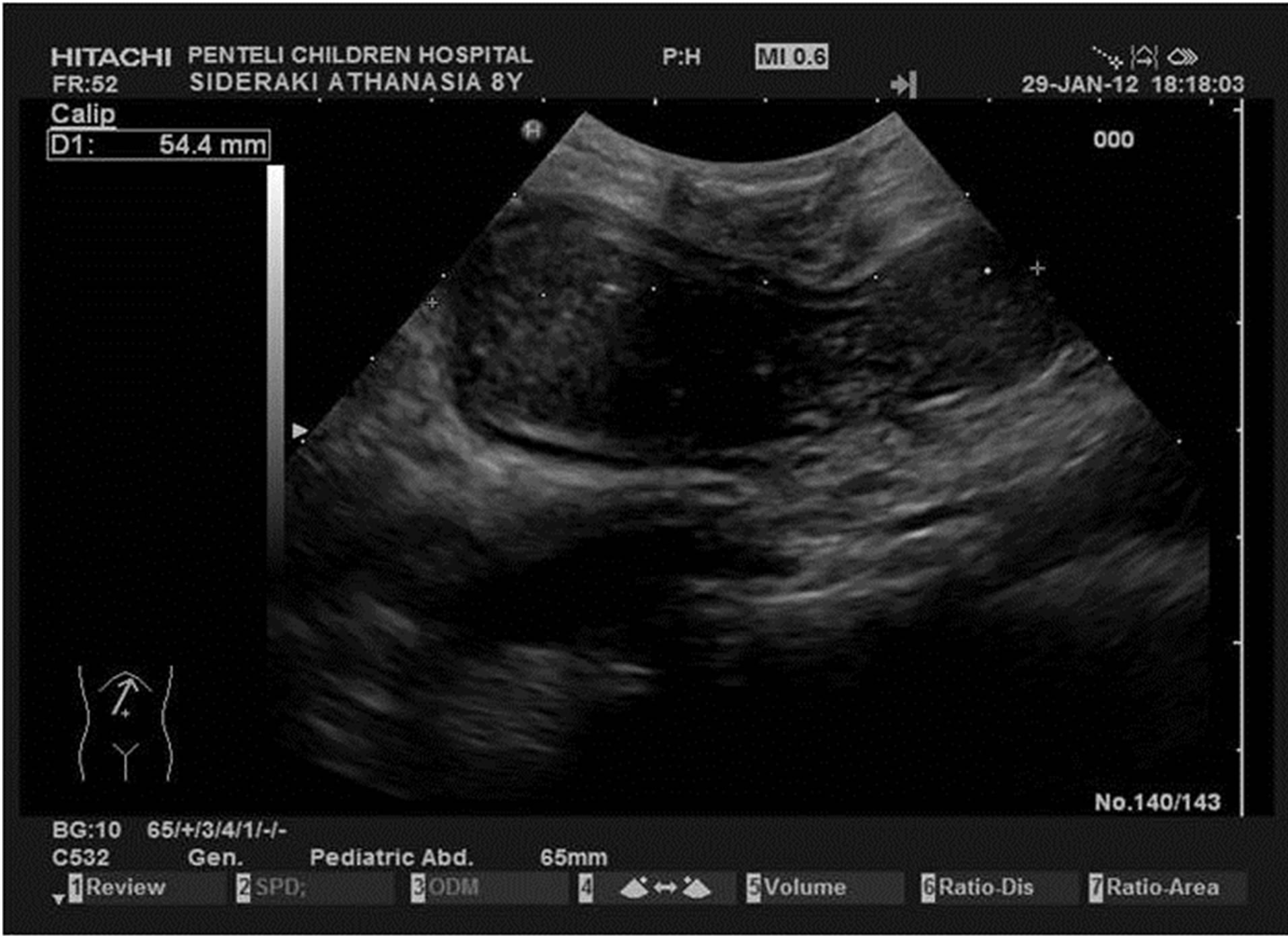
Figure 3. Cecal duplication: duplicating cyst: cystic lesion with peristalsis simultaneously to the neighborhood small intestine. The double wall, hypoechoic at the periphery (1 - 2 mm thickness) which represents the muscularis and the inner hyperechoic ring (1 - 2 mm thickness) which represents the mucosa, is pathognomonic for the diagnosis. The appearance like hourglass is due to incipient intussusception.
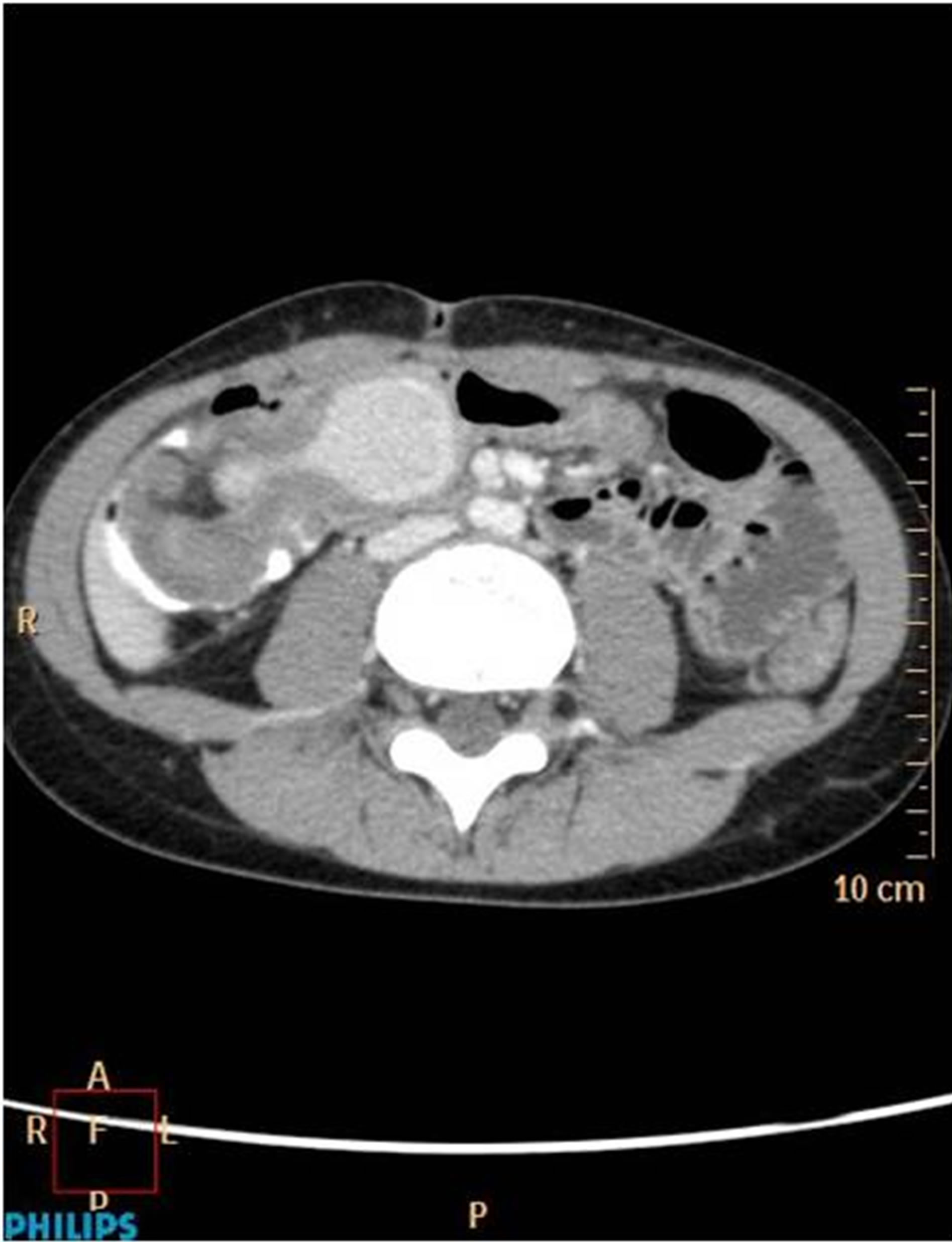
Figure 4. Axial CT image after I.V. and per os contrast administration: cystic lesion at the ileocecal region with hyperdense content and peripheral enhancement.
duplications form part of the spectrum of intestinal atresia.
Most of the reported ATDs are cystic in shape (53% - 85%) [1-3,15]. In our study, there were five cystic du-
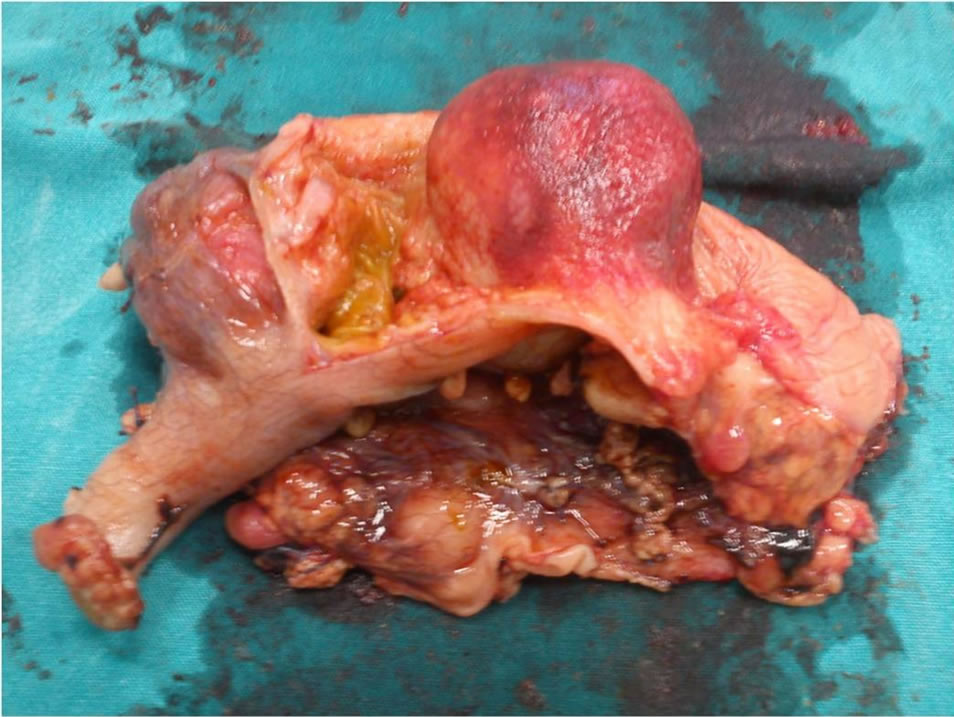
Figure 5. The duplicated cyst is protruding into the lumen of the cecum.
plications (71%) and two tubular (29%). In general, tubular duplications tend to be larger in size, rendering their excision more difficult. They also generally communicate with the adjacent gut tube segment in contrast to cystic duplications that do not [4]. The age at the onset of symptoms in our series ranged from 2 months to 8 years; five patients were younger than 5 years of age and two older. It has been reported that the majority of symptomatic duplications present within the first year of life [1-4], and should therefore be considered a condition of early infancy.
The most common location reported in the literature is the jejunum and mainly the ileum and ileocecal region [1-4,22,23]. Stern et al. [24] reported an incidence of 30% in the ileum, 30% in the ileocecal calve, 8% in the stomach, 10% in the duodenum, 8% in the jejunum, 7% in the colon and 5% in the rectum. Synchronous ADTs found in more than one part of the alimentary tract have also been reported [25]. Although the number of patients in this study was small, the results are virtually in line with previous reports since the majority of ADTs (43%) were located in the ileum and ileocecal valve.
This study presents two rare cases of ADT, a thoracoabdominal completely isolated duplication cyst (CIDP), and a duplication cyst of the cecum. Thoracoabdominal duplications are very rare, accounting for about 2% of patients with ATDs [25]. Representing a variety of tubular duplications that arise in the abdomen and pass through the diaphragm, these duplications can produce respiratory symptoms secondary to pressure in the posterior mediastinum [1,7,26]. The majority (60%) communicate with the duodenum, jejunum or ileum [7], and the remainder end blindly in the abdomen. They are associated with vertebral anomalies [2], while 29% contain ectopic gastric mucosa [7]. On the other hand, CIDCs are extremely rare with only few reports in the pediatric literature [27-33]. A CIDC is defined as having its own blood supply, and it does not communicate or share a common wall with the adjacent normal intestine [27,28]. The majority have been reported in the mesentery of the ileum, and near the ileocecal valve [27-32]; just one has been documented in the stomach [33]. The exact etiology has not yet been established. Steiner et al. [30] hypothesized that duplication is the result of torsion or a vascular accident at the proximal end of a diverticulum, leading to detachment from the intestinal wall resulting in a CIDC. As in other types of thoracoabdominal duplications, the CIDC in our case presented with respiratory problems. To our knowledge, a thoracoabdominal CICD has never been reported.
Duplications located in the cecum are rare. In summarizing a review of 362 patients with 400 duplications, Oudshoorn et al. [34] reported that only 16 (0.4%) were found located in the cecum. On the other hand, in a study [35] of 140 children with intussusceptions, a duplication cyst was a leading point in only two (1.4%) cases. The clinical presentation of cecal duplications may be that of intussusception [36], intestinal obstruction [37], or mass [38]. Our series is unique as the duplications emerged from the inner wall of the cecum, mimicking appendicitis before being recognized as intussusception. The incomeplete partial abortion theory [19] could explain this rare variety.
The natural history of ATDs is quite variable. Thoracic duplications usually present with mild or severe respiratory distress and coughing [2]. However, some thoracic duplications can remain asymptomatic until they are discovered by a routine X-ray [1,2]. Esophageal duplications may also present with dysphagia [2] or even hematemesis and anemia due to bleeding of ectopic gastric mucosa [22]. Gastric duplications almost invariably present with vomiting and abdominal discomfort as seen in the present study. A duodenal duplication may be the cause of pancreatitis due to obstructive phenomena at the pancreatic duct [10]. Patients with small bowel duplications may suffer from recurrent pain, obstruction, volvulus, melena, or may be asymptomatic presenting a palpable abdominal mass [4]. Clinical symptoms of duplications of the colon can include bowel obstruction and hemorrhage [7], or may mimic other conditions such as intussusception as shown in this study. Finally, a rectal duplication may present with constipation, hematochezia, perirectal abscess or urinary problems [7,39].
Various congenital anomalies have been associated with ATDs, most notably vertebral and genitourinary. Although no such defects were found in our patients, save for a mild scoliosis of the thoracic spine, it is well documented that thoracic and abdominal duplications coexist with a large spectrum of associated anomalies such as hemivertebra of the cervical and thoracic spine, esophageal atresia, diaphragmatic hernia, intestinal atresia, malrotation, bladder duplications, and extensive genital malformations [1-4,40].
U/S, CT, and MRI imaging are very helpful imaging modalities in defining the anatomic borders of a duplication [7]. U/S imaging is the first line imaging choice to confirm the diagnosis of an ADT as the sign of the “double-wall” is highly indicative of enteric duplications [41]. In prenatal diagnosed ATDs, an MRI is more accurate than U/S in detecting the anatomy of a duplication in the fetus [42]. Additional imaging modalities such as barium enema and radionuclide scanning may sometimes prove helpful [5]. In our series, U/S was diagnostic in the majority of cases with CT and MRI being reserved for only two cases (cases 3 and 7, Table 1).
The optimal treatment of ATDs is surgery. The location, size and benign nature of the lesion must be taken into account when a plan of surgery is under consideration. Surgical excision varies between simple removal as in the case of a CIDC [29], or resection of the cystic part along with the adjacent bowel segment with primary anastomosis [43]. In the case of long tubular duplications in which extensive resection of the adjacent bowel may lead to short-bowel syndrome, the Bianchi technique can be used; this involves removing only the duplicated portion, leaving the adjacent bowel intact [44]. Ectopic gastric mucosa should be resected either by stripping or by limited resection [1]. Staged approaches may sometimes be necessary [2]. Although laparotomy and/or thoracotomy are most commonly performed, a large study of French patients has shown that minimally invasive surgery (thoracoscopy or laparoscopy) yields good results with a low rate of adverse effects, and could, therefore, be performed in centers with experience of the procedures [45].
Low mortality rates after surgery of up to 20% have been reported [13], with thoracic duplications being responsible for the greater part of the overall mortality, due to respiratory complications [3] and other coexisting congenital anomalies [2,4,5,13]. In our series, all patients had an uneventful postoperative course.
5. CONCLUSION
Alimentary tract duplications, despite their rarity, are not uncommon in the pediatric population. They most often become symptomatic early in life and require excision to avoid complications such as hemorrhage and cancer. When thoracic masses are involved, there should be a high level of suspicion for duplications. Select cases may represent a surgical challenge. The common blood supply with the native bowel must be taken into consideration during surgery, to avoid the loss of a healthy native bowel. Overall, open surgery is safe: minimally invasive surgery is also feasible in centers with adequate experience.
REFERENCES
- Ildstad, S.T., Tollerud, D.J., Weiss, R.G., et al. (1988) Duplications of the alimentary tract. Clinical characteristics, preferred treatment, and associated malformations. Annals of Surgery, 208, 184-189. http://dx.doi.org/10.1097/00000658-198808000-00009
- Holcomb 3rd, G.W., Gheissari, A., O’Neill Jr, J.A., et al. (1989) Surgical management of alimentary tract duplications. Annals of Surgery, 209,167-174. http://dx.doi.org/10.1097/00000658-198902000-00006
- Stringer, M.D., Spitz, L., Abel, R., et al. (1995) Management of alimentary tract duplication in children. British Journal of Surgery, 82, 74-78. http://dx.doi.org/10.1002/bjs.1800820126
- Iyer, C.P. and Mahour, G.H. (1995) Duplications of the alimentary tract in infants and children. Journal of Pediatric Surgery, 30, 1267-1270. http://dx.doi.org/10.1016/0022-3468(95)90482-4
- Olajide, A.R., Yisau, A.A., Abdulraseed, N.A., et al. (2010) Gastrointestinal duplications: Experience in seven children and a review of the literature. Saudi Journal of Gastroenterology, 16, 105-109. http://dx.doi.org/10.4103/1319-3767.61237
- Anderson, M.C., Silberman, W.W. and Shields T.W. (1962) Duplications of the alimentary tract in the adult. Archives of Surgery, 85, 94-108. http://dx.doi.org/10.1001/archsurg.1962.01310010098014
- MacPherson, R.I. (1993) Gstrointestinal tract duplication: Clinical, pathologic, etiologic, and radiologic considerations. Radiographics, 13, 1063-1080.
- Cavar, S., Bogovic, M., Luetic, T., et al. (2006) Intestinal duplications—Experience in 6 cases. European Surgical Research, 38, 329-332. http://dx.doi.org/10.1159/000094021
- Lawrence, E., Warner, S. and Warner, B. (2000) Gastrointestinal duplications. Seminars in Pediatric Surgery, 9, 135-140. http://dx.doi.org/10.1053/spsu.2000.7565
- Mattioli, G., Buffa, P., Pesce, F., et al. (1999) Pancreatitis caused by duodenal duplication. Journal of Pediatric Surgery, 34, 645-648. http://dx.doi.org/10.1016/S0022-3468(99)90096-9
- Ladd, W.E. (1937) Duplications of the alimentary tract. Southern Medical Journal, 30, 363-371. http://dx.doi.org/10.1097/00007611-193704000-00002
- Kim, E.P. and McClenathan, J.H. (2001) Unusual duplication of appendix and cecum: Extension of the CaveWallbridge classification. Journal of Pediatric Surgery, 36, 18-19. http://dx.doi.org/10.1053/jpsu.2001.26400
- Bower, R.J., Sieber, W.K. and Kiesewetter, W.B. (1978) Alimentary tract duplications in children. Annals of Surgery, 188, 869-874. http://dx.doi.org/10.1097/00000658-197811000-00015
- Hocking, M. and Young, D.G. (1981) Duplications of the alimentary tract. British Journal of Surgery, 68, 92-96. http://dx.doi.org/10.1002/bjs.1800680210
- Li, L., Zhang, J.-Z. and Wang, Y.-X. (1998) Vascular classification for small intestinal duplications: Experience with 80 cases. Journal of Pediatric Surgery, 33, 1243- 1245. http://dx.doi.org/10.1016/S0022-3468(98)90159-2
- Kottra, J.J. and Dodds, W.J. (1971) Duplication of the large bowel. American Journal of Roentgenology, Radium Therapy and Nuclear Medicine, 113, 310-315. http://dx.doi.org/10.2214/ajr.113.2.310
- Bentley, J.F.R. and Smith, J.R. (1960) Developmental posterior enteric remnants and spinal malformations: The split notochord syndrome. Archives of Disease in Childhood, 35, 76-86. http://dx.doi.org/10.1136/adc.35.179.76
- Bremer, J.L. (1952) Diverticula and duplications of the intestinal tract. Archives of Pathology, 38, 132-140.
- Ravitch, M.M. (1953) Hindgut duplication, doubling of colon and genital urinary tracts. Annals of Surgery, 137, 588-601. http://dx.doi.org/10.1097/00000658-195305000-00002
- Mellish, R.P.W. and Koop, C.E. (1961) Clinical manifestations of duplications of the bowel. Pediatrics, 27, 397- 407.
- Fawara, B.E., Franciosi, R.A. and Akers, D.A. (1971) Enteric duplications: thirty seven cases. A vascular theory of pathogenesis. American Journal of Diseases of Children, 122, 501-508.
- Bower, R.J., Sieber, W.K. and Kiesewetter, W.B. (1978) Alimentary tract duplications in children. Annals of Surgery, 188, 669-674. http://dx.doi.org/10.1097/00000658-197811000-00015
- Grosfeld, J.L., O’Neill, J.A. and Clatworthy, H.W. (1970) Enteric duplications in infancy and childhood: An 18- year experience. Annals of Surgery, 172, 83-90.
- Stern, L.E. and Warner, B.W. (2000) Gastrointestinal duplications. Seminars in Pediatric Surgery, 9, 135-140. http://dx.doi.org/10.1053/spsu.2000.7565
- Sherman, N.J., Morrow, D. and Asch, M. (1978) A triple duplication of the alimentary tract. Journal of Pediatric Surgery, 13, 187-188. http://dx.doi.org/10.1016/S0022-3468(78)80019-0
- Pokorny, W.J. and Goldstein, I.R. (1984) Enteric thoracoabdominal duplications in children. Journal of Thoracic and Cardiovascular Surgery, 87,821-824.
- Menon, P. and Vaiphei, K. (2004) Isolated enteric duplications. Journal of Pediatric Surgery, 39, e5-e7. http://dx.doi.org/10.1016/j.jpedsurg.2004.04.040
- Sinha, A., Ojha, S. and Serin, Y.K. (2006) Completely isolated, non contiguous duplication cyst. European Journal of Pediatric Surgery, 16, 127-129. http://dx.doi.org/10.1055/s-2006-924004
- Srivastava, P., Gangopadyay, A.N., Kumar, V., et al. (2009) Noncommunocating isolated enteric duplication cyst in childhood. Journal of Pediatric Surgery, 44, E9- E10. http://dx.doi.org/10.1016/j.jpedsurg.2009.03.041
- Steiner, Z. and Mogilner, J. (1999) A rare case of completely isolated duplication cyst of the alimentary tract. Journal of Pediatric Surgery, 34, 1284-1286. http://dx.doi.org/10.1016/S0022-3468(99)90171-9
- Pant, N., Grover, J.K., Madan, N.K., et al. (2012) Completely isolated enteric duplication associated with a classic enterogenous duplication cyst. JIASPS, 72, 68-70.
- Peng, H.L., Chang, C.Y. and Lau, B.H. (2012) Unusual imaging features of completely isolated enteric duplication in a child. Pediatric Radiology, 42, 1142-1144. http://dx.doi.org/10.1007/s00247-012-2380-8
- Nakazawa, N., Okazaki, T. and Miyano, T. (2005) Prenatal detection of isolated gastric duplication cyst. Pediatric Surgery International, 21, 831-834. http://dx.doi.org/10.1007/s00383-005-1517-3
- Oudshoorn, J.H. and Heij, H.A. (1996) Intestinal obstruction caused by duplication of the cecum. European Journal of Pediatrics, 155, 338-340. http://dx.doi.org/10.1007/BF02002724
- Reijnen, J.A.M., Festen, C., Joosten, H.J.M., et al. (1990) Atypical characteristics of a group of children with intussusceptions. Acta Paediatrica Scandinavica, 79, 675- 679. http://dx.doi.org/10.1111/j.1651-2227.1990.tb11534.x
- Rizalar, R., Somoncu, S., Sózübir, S., et al. (1996) Cecal duplication: A rare cause for secondary intussusception. Indian Journal of Pediatrics, 63, 563-565. http://dx.doi.org/10.1007/BF02905736
- Ijaz, L., Husnain, M., Malik, S.I., et al. (2011) Cecal duplication cyst presenting as acute intestinal obstruction in an infant. APSP Journal of Case Reports, 2, 11.
- Shah, A. and Shah, A. (2004) Diagnostic dilemma of cecal duplication. Indian Pediatrics, 41,749-750.
- La Quaglia, M.P., Feins, N., Eraklis, A., et al. (1990) Rectal duplications. Journal of Pediatric Surgery, 25, 980-984. http://dx.doi.org/10.1016/0022-3468(90)90242-2
- Gálvez, Y., Škába, R., Kalousová, J., et al. (2004) Alimentary tract duplications in children? High incidence of associated anomalies. European Journal of Pediatric Surgery, 14, 79-84. http://dx.doi.org/10.1055/s-2004-815852
- Barr, L.L., Hayden Jr, C.K., Stansberry, S.D., et al. (1990) Enteric duplication cysts in children: Are their ultrasonographic wall characteristics diagnostic? Pediatric Radiology, 20, 326-328. http://dx.doi.org/10.1007/BF02013165
- Laje, P., Flake, A.W. and Adzick, N.S. (2010) Prenatal diagnosis and postnatal resection of intraabdominal enteric duplications. Journal of Pediatric Surgery, 45, 1554- 1558. http://dx.doi.org/10.1016/j.jpedsurg.2010.03.017
- Schalomon, J., Schleef, J. and Höllwarth, M.E. (2000) Experience with gastro-intestinal duplications in childhood. Langenbeck’s Archives of Surgery, 385, 402-405. http://dx.doi.org/10.1007/s004230000170
- Bianchi, A. (1980) Intestinal loop lengthening—A technique for increasing small bowel length. Journal of Pediatric Surgery, 15, 145-151. http://dx.doi.org/10.1016/S0022-3468(80)80005-4
- Guérin, F., Podevin, G., Petit, T., et al. (2012) Outcome of alimentary tract duplications operated on by minimally invasive surgery: A retrospective multicenter study by the GECI (Groupe d’Etude en Coeliochirurgie Infantile). Surgical Endoscopy, 26, 2848-2855. http://dx.doi.org/10.1007/s00464-012-2259-7

