Journal of Cosmetics, Dermatological Sciences and Applications
Vol.05 No.02(2015), Article ID:57124,8 pages
10.4236/jcdsa.2015.52013
Treatment of Localized Vitiligo with 1% Pimecrolimus Cream versus 0.05% Clobetasol Propionate Cream―Single, Blinded, Comparative Therapeutic Trial
Khalifa E. Sharquie1*, Hayder R. Al-Hamamy2, Adil A. Noaimi1, Kholod A. Ali3
1Scientific Council of Dermatology and Venereologyl, Iraqi and Arab Board for Medical Specializations, Department of Dermatology, Collage of Medicine, University of Baghdad, Baghdad, Iraq
2Scientific Council of Dermatology and Venereology, Iraqi Board for Medical specializations, Baghdad Teaching Hospital, Medical City, Baghdad, Iraq
3Department of Dermatology & Venereology, Baghdad Teaching Hospital, Baghdad, Iraq
Email: *ksharquie@ymail.com, hayder317@gmail.com, adilnoaimi@yahoo.com, kholod.abbas85@gmail.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 30 March 2015; accepted 8 June 2015; published 12 June 2015
ABSTRACT
Background: Calcineurin inhibitors like tacrolimus and pimecrolimus are new modality of treatment of localized vitiligo. Objective: To compare the effectiveness and side effects of 1% pimecrolimus cream in comparison with 0.05% clobetasol propionate cream as a treatment of localized type of vitiligo. Patients and Methods: Fifty two patients with localized vitiligo were included in this single, blind, comparative therapeutic trial. Full history and examination for each patient was done. Wood’s light was used when needed to confirm the diagnosis. Thirty (57.7%) were females and 22 (42.3%) males, female to male ratio of 1.36:1. Their ages ranged between 3 - 40 (13.15 ± 7.9) years, while disease duration ranged from 6 - 84 (29.62 ± 20.56) months. Total numbers of lesions were 114 lesions with a mean of 2.2 lesions per patient. The surface area of the lesions ranged between 1 - 31 (7.15 ± 6.98) cm². Vitiliginous patches were most commonly located on face 48 (42.1%) lesions, and lower limbs 35 (30.7%) lesions. Patients were divided in to two groups according to the type of therapeutic modality. Group A consisted of 25 patients (52 lesions) receiving 1% pimecrolimus cream and Group B 27 patients (62 lesions) treated with 0.05% clobetasol propionate cream, both used twice daily. The amount of cream per area was applied according to rule of fingertip unit. Measuring the surface area of the lesions and calculating the reduction rate were done every month till the end of the 6th month. Then patients were asked to stop the use of medication to be re-evaluated again after 3 months without any treatment and to record any local or systemic side effects. Results: After 6 months of treatment there was 79.67% reduction in the surface area of lesions in Group A, while in Group B there was 82.59% reduction in the surface area with no statistically significant difference between the two groups. The reduction rate was early as there was statistically significant reduction for both groups after one month of treatment (p value < 0.01). The reduction rates for facial lesions were more than other parts of the body. There was no significant relapse after 3 months of stopping treatment for both groups. Side effects were minimal and tolerable. Conclusions: Topical 1% pimecrolimus is as effective as clobetasol propionate in treatment of localized vitiligo affecting less than 5% of the body surface area but pimecrolimus was more preferred as the side effects of topical steroid could be avoided.
Keywords:
Localized Vitiligo, Pimecrolimius, Clobetasol Propionate

1. Introduction
Vitiligo is an inflammatory skin disorder where many etiological factors are incriminated in its etiopathogenesis commonly the autoimmune reaction. The end result is destruction of melanocytes partially or completely. The histopathology of early lesions often shows inflammatory reaction like T-cell lymphocytic invasion of epidermis even forming Pautrier’s like micro-abscess [1] . The course of the disease is usually unpredictable, but it is often slowly progressive. The activity of the disease is important in relation to the therapy but this activity could be only regional and this does explain why there is very good response in selective area or region but progressive with new lesions elsewhere [2] .
Thus the development of effective treatment modalities depends on studies highlighting the etiology and pathogenesis of vitiligo. The abnormalities in cell-mediated immunity arise as the starting point for the use of immunomodulation agents such as corticosteroids and macrolides [3] .
Response to therapy depends on the severity, location, and time factors of the disease, but it can be divided into 2 groups: so-called “rapid responders” are patients who respond quickly to drugs that are prescribed for vitiligo, and the so-called “non responders” are patients who do not respond to these drugs [4] .
Unfortunately there is no single therapeutic modality of treatment with high cure rate in vitiligo [4] . Best results are obtained in vitiligo that is recent in onset and when it affects face and trunk. Topical corticosteroids are the first line of therapy for vitiligo. Class 1 (very potent) topical corticosteroids have many adverse effects such as atrophy, telangiectasia, purpura, striae, infection, allergic contact dermatitis, burning, itching, irritation, dryness caused by vehicle, hypopigmentation, hypertrichosis of face [5] .
Topical calcinuein inhibitor: tacrolimus and pimecrolimus are novel topical immunomodulatory drugs that are originally used for treatment of atopic dermatitis [6] .
Pimecrolimus is an ascomycin macrolactam derivative that has been shown in vitro to bind to macrophilin-12 (also referred to as FKBP-12) and inhibits calcineurin. Thus pimecrolimus inhibits T-cell activation by inhibiting the synthesis and release of cytokines from T-cells. Pimecrolimus also prevents the release of inflammatory cytokines and mediators from mast cells [6] .
Pimecrolimus has a similar mode of action to that of tacrolimus but is more selective, with no effect on dendritic Langerhans cells [7] . It has lower permeation through the skin than topical steroids or topical tacrolimus although they have not been compared with each other for their permeation ability through mucosa. In addition, in contrast with topical steroids, pimecrolimus does not produce skin atrophy and telangiectasia especially on the face and intertriginous areas. It has been used in various inflammatory skin diseases like seborrheic dermatitis [8] , cutaneous lupus erythematosus [9] , oral lichen planus [10] , psoriasis [11] vitiligo [12] .
Accordingly, the aim of the present study is to compare the effectiveness and side effects of 1% pimecrolimius cream in comparison with 0.05 clobetasol propionate cream as a treatment of localized type of vitiligo.
2. Patients and Methods
This comparative, single blinded, therapeutic clinical trial was carried out at the Department of Dermatology and Venereology-Baghdad Teaching Hospital, Baghdad, Iraq. For the period extended from March 2013 to March 2014.
Fifty two patients with localized vitiligo were included in the study. Full history was taken from each patient including: age, gender, site of onset, duration of disease, family history of vitiligo and history of other auto immune diseases like diabetes mellitus, thyroid dysfunction, alopecia areata and others. Physical examination was done to evaluate the site and size of the lesion. Wood’s light was used when needed to confirm the diagnosis.
Inclusion criterion was localized vitiligo affecting less than 5% of the body surface area.
2.1. Exclusion Criteria
1) Patients who had new lesions of vitiligo in the last 6 months and patients who had increase in the surface area of their lesions in the last 6 months (active vitiligo).
2) Patients with universal vitiligo.
3) Patients with resistant type of vitiligo, like segmental vitiligo and acrofacial vitiligo.
4) Patients who had taken treatment for their lesions in the last 2 months.
5) Unreliable patients.
Formal consent was taken from each patient before trial after a full explanation about the nature of disease, course and its complications in addition to method of treatment, course, and duration, and duration of follow up, as well as the need of pre and post-treatment photographs.
The ethical approval was obtained from Scientific Council of Dermatology and Venereology―Iraqi Board for Medical Specializations.
The patients were divided into 2 groups of treatment in which each patient had the chance to be in any group.
Group A: Patients were treated with pimecrolimus cream [(Elidel)® Produced by: Novartis Pharma Corporation, GmbH Wehr, Germany; pimecrolimus 10 mg in 1 g, 1% cream, 30 g]. In this group 25 patients were instructed to apply a thin layer of Pimecrolimus cream gently and smoothly twice daily to the vitiliginous patches and to avoid as possible the surrounding normal skin. The amount of cream per area was applied according to rule of fingertip unit (FTU) [13] . FTU is the amount of ointment expressed from a tube with a 5-mm diameter nozzle, applied from the distal skin crease to the tip of the index finger. One FTU weighs approximately 0.5 gm and each 1 gm of cream covers 100 square centimeters of skin. Clinical evaluation was done every 2 weeks till the end of the 6 month and measuring the surface area of the lesions and the calculated reduction rate was done every month. Then patients were asked to stop the use of medication at the end of the 6th month of treatment and to be re-evaluated again after 3 months without any treatment.
Group B: Twenty seven patients were treated with clobetasol propionate cream [(Dermodin)® Produced by: The State Company for Drugs Industry-Medical Appliances SDI Samarra, Iraq; clobetasol propionate, 0.05% cream, 25 g]. Patients in this group were treated, evaluated and followed in the same manner as for patients in Group A.
2.2. Assessment of Response to Treatment
All patients were assessed before the commencement of treatment and subsequently after every 2 weeks and photographs were taken by using Cyber-shot Sony digital Camera (12.1 Mega Pixels) in a good illumination and the same place.
The surface area of each vitiligo patch was calculated using graphic paper. Every month interval, the surface area of every patch was recorded on the same transparent paper. The percentage reduction in the surface area of the patch was recorded. The type of repigmentation was reported in each patch, whether it was perifollicular, marginal, diffuse, or in any combination.
The side effects were recorded in each visit if present, which include: cutaneous atrophy, infection (viral, bacterial, and fungal), acneform eruptions, striae, erythema, telangiectasia, purpura, itching, scaling, burning or stinging sensation.
Statistical Analysis of data was carried out using the statistical package of SPSS-20 (Statistical Packages for Social Sciences―version 20). Data were presented in simple measures of frequency, percentage, mean and standard deviation. Comparison between groups for discrete data was done by using Chi square test. Comparison between visits was done by using paired t-test, and p-value < 0.05 was considered as the level of significance.
3. Results
Fifty two patients with localized vitiligo were included in the study; 22 (42.3%) males and 30 (57.7%) females, female to male ratio of 1.36:1. Their ages ranged between 3 - 40 years with a mean ± SD of 13.15 ± 7.9 years, while the disease duration ranged from 6 - 84 months with a mean ± SD of 29.62 ± 20.56 months. Total numbers of lesions were 114 lesions, with a mean of 2.2 lesions per patient. The surface area of the lesions ranged between 1 - 31 cm2 with a mean ± SD of 7.15 ± 6.98 cm2 (Table 1).
Family history for vitiligo was positive in 11 (21.2%) patients. One (1.9%) patient had hypothyroidism, 2 (3.8%) patients had history of alopecia areata and 2 (3.8%) patients had diabetes mellitus.
Patients Were Divided in to 2 Groups
Group A: 25 patients with 52 lesions; 12 (48%) males, and 13 (52%) females. Their ages ranged between 3 - 40 years with a mean ± SD of 13.6 ± 8.3 years. Duration of disease ranged from 6 - 84 months with a mean ± SD of 32.4 ± 21.4 months. The surface area of the patches ranged between 1 - 27 cm2 with a mean ± SD of 7.02 ± 6.8 cm2.
Group B: 27 patients with 62 lesions, 10 (37%) males, and 17 (63%) females. Their age range between 3 - 32 years with a mean ± SD of 12.74 ± 7.6 years. Duration of disease ranged from 6 - 72 months with a mean ± SD of 27.04 ± 19.8 months .The surface area of the patches ranged between 1 - 35 cm2 with a mean ± SD of 7.3 ± 7.2 cm2 (Table 1).
The location of vitiliginous lesion was as follow: face 48 (42.1%) lesions, lower limbs 35 (30.7%) lesions; trunk 16 (14%) lesions; upper limbs 14 (12.3%) lesions and one (0.9%) lesion on genitalia.
The mean ± SD of surface area of lesions in Group A was decreased from 7.02 ± 6.76 cm2 at baseline visit to 6.11 ± 6.35 cm2 at second visit (after one month) which was statistically significant (p value= 0.01). The mean surface area continued to be reduced till reaching 5.82 ± 6.14 at third visit, 5.20 ± 5.92 at fourth visit, 4.03 ± 5.23 at fifth visit, 2.97 ± 4.65 at sixth visit and 2.12 ± 4.18 at seventh visit. All were statistically significant when compared to baseline visit (Figures 1-3).
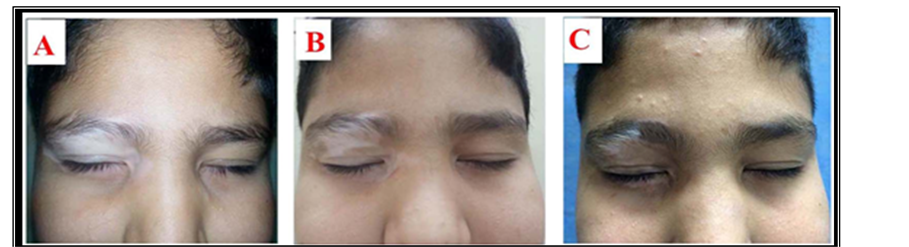
Figure 1. Twelve-year-old male patient with history of localized vitiligo for 4 years treated by Pimecrolimus cream. Lesions on right eyelid at (A) baseline visit; (B) after 3 months and (C) after 6 months.
Table 1. Age, duration of the disease, and surface area of lesions for both groups.
*Independent t-test.
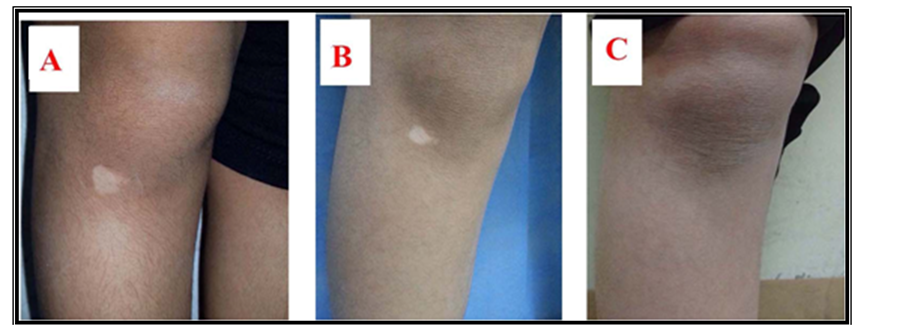
Figure 2. Nine-year-old male patient with history of localized vitiligo for 1 year treated by Pimecrolimus cream. Lesions on right knee at (A) baseline visit; (B) after 3 months and (C) after 6 months.
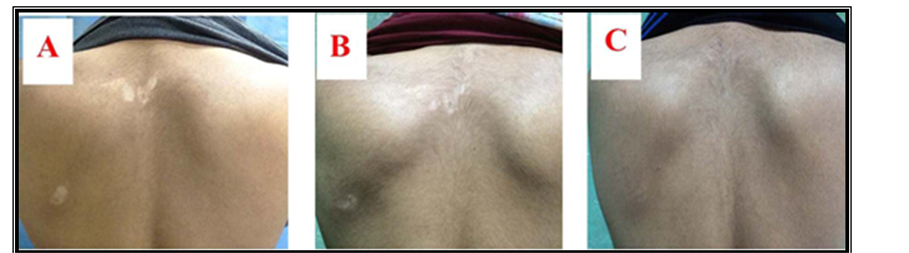
Figure 3. Twenty-year-old male patient with history of localized vitiligo for 2 years treated by Pimecrolimus cream. Lesions on back at (A) baseline visit; (b) after 3 months and (C) after 6 months.
The mean ± SD of surface area of lesions in Group B was decreased from 7.26 ± 7.21 cm2 at baseline visit to 6.32 ± 6.83 cm2 at second visit (after one month) which was statistically significant (p value= 0.01). The mean surface area continued to be reduced till reaching 5.86 ± 6.4 at third visit, 5.31 ± 6.25 at fourth visit, 4.00 ± 5.21 at fifth visit, 2.84 ± 4.21 at sixth visit and 1.8 ± 3.47 at seventh visit. All were statistically significant when compared to baseline visit (Figures 4-6).
After 6 months of treatment there was 79.67% reduction in the surface area of lesions in Group A, while in Group B there was 82.59% reduction in the surface area and there were no statistically significant differences between the two groups (Table 2).
The reduction rates at the end of treatment for facial lesions were 80.51% for Group A and 87.86% for Group B. The reduction in other sites in Group A was as follow: trunk 60.66%, genitalia 40.33%, upper limbs 45.4% and lower limbs 68.5%. While the reductions in other sites in Group B were: trunk 57.27%, upper limbs 70.55% and lower limbs 83.25% (Table 3).
Regarding relapse rate there was no significant relapse after 3 months of stopping treatment as the mean surface area of lesions did not increased significantly in both groups.
In Group A, the pattern of repigmentation was peripheral in 4 (16%) patients, perifollicular in 8 (32%) patients, and mixed in 13 (52%) patients. While in Group B, the pattern of repigmentation was peripheral in 5 (18.52%) patients, perifollicular in 10 (37.04%) and mixed in 12 (44.44%) patient. The pattern of repigmentation between both groups was statistically not significant (p value = 0.862).
The assessment of local side effects for Group A showed burning sensation in 4 (16%) patients. The assessment of local side effects for Group B revealed atrophy in 2 (7.4%) patients, telangectasia in 3 (11.11%) and folliculitis in 1 (3.7%) patient. For both groups, the side effects did not necessitate stopping the treatment.
4. Discussion
Vitiligo is a major health cosmetic problem all over the world that had a bad disfiguring role in a behavior of the
Figure 4. Seven-year-old female with history of localized vitiligo for 2 years treated by Clobetasol propionate cream. Lesions on face at (A) baseline visit; (B) after 3 months and (C) after 6 months.
Figure 5. Seven-year-old female with history of localized vitiligo for 3 years treated by Clobetasol propionate cream. Lesions on lower limb at (A) baseline visit, (B) after 3 months and (C) after 6 months.
Figure 6. Eight-year-old male with history of localized vitiligo for 2 years treated by Clobetasol propionate cream. Lesions on upper back at (A) baseline visit, (B) after 3 months and (C) after 6 months.
Table 2. Percent reduction at each visit for both groups.
*ANOVA test was done between groups. Percent Reduction = (A − B)/A × 100, A is an initial value, B is a final value.
patient. Current treatment modalities of vitiligo are limited as a consequence of conflicting and incomplete responses and high relapse rate [1] . Therapies include topical and oral corticosteroids, psoralen photochemotherapy, narrowband and broadband UVB, khellin, phenylalanine, calcipotriol, tar, vitamins, antioxidants, topical tincture iodine 5%, camouflage cosmetics and surgical methods. Each has its advantages and limitations [1] [14] [15] .
Pimecrolimus cream, an immunosuppressant macrolide which was developed for the treatment of inflamma-
Table 3. Percent reduction according to site for both groups.
*Mean surface area in cm2. **Percent Reduction (PR) = (A − B)/A × 100, A is an initial value, B is a final value.
tory skin diseases, has been approved for atopic dermatitis in adults and children above 2 years of age [16] . The mechanism of action of pimecrolimus is to block T-cell function and to inhibit the synthesis and secretion of inflammatory cytokines. It also targets the secretion of inflammatory cytokines from mast cells and basophils [17] [18] . Also, it has been shown that calcineurin inhibitor like tacrolimus apart from its immunomodulatory action, has an effect on keratinocyte, melanocyte and melanoblasts, thus inducing repigmentation in vitiliginous lesion [19] . In addition to good tolerability, pimecrolimus offers a considerable advantage since it does not cause epidermal atrophy and telangiectasia. In addition, facial and intertriginous application has been found to be safe [20] [21] .
The results of previous studies of pimecrolimus therapy for vitiligo showed different response rates per sites and between studies for the same site. Köse et al. showed 42% mean repigmentation for pimecrolimus in comparison with 65% for mometasone and conclude that pimecrolimus was not effective on the body except for the face in childhood localized vitiligo [22] . Boone et al. found that 72.9% mean repigmentation of vitiliginous lesions in 6 months period [23] . Mayoral et al. found approximately same results as the mean repigmentation was 72.5% [24] . Neslihan et al. showed 65.5% mean repigmentation for face lesions [25] .
The presented work had shown that mean repigmentation after 6 months of treatment with pimecrolimus was 79.67% which was comparable to previous literatures with slightly better results [22] -[26] .
Regarding response of treatment according to sites, in the present work the best reduction rate was on the face (80.51%). Reduction rates in other sites were 68.5% in lower limbs, 60.66% trunk, 45.4% upper limbs and 40.33% in genitalia. These results were similar to Seirafi et al. [27] results in which they found 85.7% repigmentation in trunk, 75% in face and 70% in elbows. While Ho et al. found 58% response in facial lesions and 23% for other sites for those using tacrolimus 01% [28] . Dawid et al. found that facial lesions responded faster than the nonfacial ones [29] .
When compare results of pimecrolimus to previous studies that used tacrolimus in treatment of vitiligo, pimecrolimus showed similar results. Lepe et al., [30] Xu et al., [31] Hartmann et al., [32] and Zeyad et al. [33] found 41.3%, 83.3%, 81% and 70.8% mean repigmentation respectively.
Clobetasol propionate cream was also as effective as pimecrolimus in treatment of vitiligo. The present study showed 82.59% mean repigmentation, while Ho et al. [28] showed 49.3%, Lepe et al. [30] 58% and Giuseppe et al. [34] showed >75% repigmentation. Ho et al. [28] found that facial lesions responded better than other sites which were also proved by this study.
The burning side effects of pimecrolimus was temporary disappeared after few weeks of treatment and was presented in 16% of patients. Köse et al. [22] reported burning sensation in 10% of patients. Köse et al. also reported that 10% of patients treated with clobetasol propionate developed telangiectasia and 10% developed atrophy. While in the present study, telangiectasia was reported in 9% and atrophy in 7.4% [22] .
In the present work, pimecrolimus proved almost as effective as clobetasol propionate to restore skin color in lesions of vitiligo in children. Because it does not produce atrophy, pimecrolimus may be very useful for younger patients and for sensitive areas of the skin such as eyelids, intertriginous areas and genitalia and it should be considered in other skin disorders currently treated with topical steroids for prolonged periods. We recommend further study using combination of therapy like pimecrolimus and clobetasol separately, possibly to have summation response hoping to accelerate the process of repigmentation.
5. Conclusion
In conclusion, topical 1% pimecrolimus is as effective as 0.05% clobetasol propionate propionate in treatment of localized vitiligo. On the anatomical sides that are convenient to prompt development of atrophy such as the face, pimecrolimus may become a useful agent in the treatment of adults and children with vitiligo.
Disclosure
This study is an independent study and not funded by any drug company.
References
- Sharquie, K.E., Mehenna, S.H., Naji, A.A. and Al-Azzawi, H. (2004) Inflammatory Changes in Vitiligo: Stage I and II Depigmentation. The American Journal of Dermatopathology, 26, 108-112. http://dx.doi.org/10.1097/00000372-200404000-00004
- Taieb, A. and Picardo, M. (2009) Clinical Practice. Vitiligo. The New England Journal of Medicine, 260, 160. http://dx.doi.org/10.1056/NEJMcp0804388
- Gawkrodger, D.J., Ormerod, A.D. and Shaw, L. (2008) Guideline for the Diagnosis and Management of Vitiligo. British Journal of Dermatology, 159, 1051-176. http://dx.doi.org/10.1111/j.1365-2133.2008.08881.x
- Sharquie, K.E., Noaimi, A.A. and Al-Mudaris, H.A. (2013) Melanocytes Transplantation in Patients with Vitiligo Using Needling Micrografting Technique. Journal of Drugs in Dermatology, 12, e74-e78.
- Mahmoud, B.H., Hexsel, C.L. and Hamzavi, I.H. (2008) An Update on New and Emerging Options for the Treatment of Vitiligo. Skin Therapy Letter, 13, 1-10.
- Allen, B.R., Lakhanpaul, M., Morris, A., Lateo, S., Davies, T., Scott, G., Cardno, M., Ebelin, M.E., Burtin, P. and Stephenson, T.J. (2003) Systemic Exposure, Tolerability, and Efficacy of Pimecrolimus Cream 1% in Atopic Dermatitis Patients. Archives of Disease in Childhood, 88, 969-973. http://dx.doi.org/10.1136/adc.88.11.969
- Eleston, D.M., James, W.D. and Burger, T.G. (2011) Disturbance of Pigmentation. In: Eleston, D.M., James, W.D. and Burger, T.G., Eds., Andrews Disease of the Skin, Clinical Dermatology, 11th Edition, W.B Saunders Elsevier Company, Philadelphia, 856.
- Firooz, A., Solhpour, A., Gorouhi, F., Daneshpazhooh, M., Balighi, K., Farsinejad, K., Rashighi-Firoozabadi, M. and Dowlati, Y. (2006) Pimecrolimus Cream, 1%, vs. Hydrocortisone Acetate Cream, 1%, in the Treatment of Facial Seborrheic Dermatitis: A Randomized, Investigator-Blind, Clinical Trial. Archives of Dermatology, 142, 1066-1067. http://dx.doi.org/10.1001/archderm.142.8.1066
- Kreuter, A., Gambichler, T., Breuckmann, F., Pawlak, F.M., Stücker, M., Bader, A., Altmeyer, P. and Freitag, M. (2004) Pimecrolimus 1% Cream for Cutaneous Lupus Erythematosus. Journal of the American Academy of Dermatology, 51, 407-410. http://dx.doi.org/10.1016/j.jaad.2004.01.044
- Gorouhi, F., Solhpour, A., Beitollahi, J.M., Afshar, S., Davari, P., Hashemi, P., Nassiri Kashani, M. and Firooz, A. (2007) Randomized Trial of Pimecrolimus Cream versus Triamcinolone Acetonide Paste in the Treatment of Oral Lichen Planus. Journal of the American Academy of Dermatology, 57, 806-813. http://dx.doi.org/10.1016/j.jaad.2007.06.022
- Jacobi, A., Braeutigam, M., Mahler, V., Schultz, E. and Hertl, M. (2008) Pimecrolimus 1% Cream in the Treatment of Facial Psoriasis: A 16-Week Open-Label Study. Dermatology, 216, 133-136. http://dx.doi.org/10.1159/000111510
- Boone, B., Ongenae, K., Van Geel, N., Vernijns, S., De Keyser, S. and Naeyaert, J.M. (2007) Topical Pimecrolimus in the Treatment of Vitiligo. European Journal of Dermatology, 17, 55-61.
- Habif, T.P. (2010) Topical Therapy and Topical Steroids. In: Habif, T.P., Ed., Clinical Dermatology: A Color Guide to Diagnosis and Therapy, 5th Edition, Mosby, Edinburgh, 79.
- Rebat, M.H. and Sumayah, J.T. (2012) Disorders of Melanocytes. In: Wolff, K., Goldsmith, L.A., Katz, S.I., Gilchrest, B.A., Paller, A.S. and Leffell, D.J., Eds., Fitzpatrick’s Dermatology in General Medicine, 8th Edition, McGraw-Hill - Company, New York, 72:616-622.
- Felsten, L.M., Alikhan, A. and Petronic-Rosic, V. (2011) Vitiligo: A Comprehensive Overview. Part II: Treatment Options and Approach to Treatment. Journal of the American Academy of Dermatology, 65, 493-514. http://dx.doi.org/10.1016/j.jaad.2010.10.043
- Esfandiarpour, I. (2009) The Efficacy of Pimecrolimus 1% Cream Plus Narrow-Band Ultraviolet B in the Treatment of Vitiligo: A Double-Blind Placebo-Controlled Clinical Trial. Journal of Dermatological Treatment, 20, 14-18. http://dx.doi.org/10.1080/09546630802155057
- Kovarik, C.L., Spielvogel, R.L. and Kantor, G.R. (2010) Pigmentory Disorder of the Skin. In: Elder, D.E., Johnson, B.L., Elenitsas, R. and Murphy, G.F., Eds., Levers Histopathology of the Skin,10th Edition, Lippincott-Williams & Wilkins, Philadelphia, 1575-1576.
- Coskun, B., Saral, Y. and Turgut, D. (2005) Topical 0.05% Clobetasol Propionate versus 1% Pimecrolimus Ointment in Vitiligo. European Journal of Dermatology, 15, 88-91.
- Lan, C.C., Chen, G.S., Chiou, M.H., Wu, C.S., Chang, C.H. and Yu, H.S. (2005) FK506 Promotes Melanocyte and Melanoblast Growth and Creates a Favourable Milieu for Cell Migration via Keratinocytes: Possible Mechanisms of How Tacrolimus Ointment Induces Repigmentation in Patients with Vitiligo. British Journal of Dermatology, 153, 498-505. http://dx.doi.org/10.1111/j.1365-2133.2005.06739.x
- Paul, C., Graeber, M. and Stuetz, A. (2000) Ascomycins: Promising Agents for the Treatment of Inflammatory Skin Diseases. Expert Opinion on Investigational Drugs, 9, 69-77. http://dx.doi.org/10.1517/13543784.9.1.69
- Shaquie, K.E., Al-Hamamy, H.R., Noaimi, A.A. and Al-Obeidy, M.H. (2014) Treatment of Vitiligo with Topical 5% Tincture Iodine and UVA Light. The American Journal of Dermatology and Venereology, 3, 75-79.
- Köse, O., Arca, E. and Kurumlu, Z. (2010) Mometasone Cream versus Pimecrolimus Cream for the Treatment of Childhood Localized Vitiligo. Journal of Dermatological Treatment, 21, 133-139. http://dx.doi.org/10.3109/09546630903266761
- Boone, B., Ongenae, K., Van Geel, N., Vernijns, S., De Keyser, S. and Naeyaert, J.M. (2007) Topical Pimecrolimus in the Treatment of Vitiligo. European Journal of Dermatology, 17, 55-61.
- Mayoral, F.A., Vega, J.M., Stavisky, H., McCormick, C.L. and Parneix-Spake, A. (2007) Retrospective Analysis of Pimecrolimus Cream 1% for Treatment of Facial Vitiligo. Journal of Drugs in Dermatology, 6, 517-521.
- Şendur, N., Karaman, G., Saniç, N. and Şavk, E. (2006) Topical Pimecrolimus: A New Horizon for Vitiligo Treatment. Journal of Dermatological Treatment, 17, 338-342. http://dx.doi.org/10.1080/09546630601028711
- Eryılmaz, A., Seçkin, D. and Baba, M. (2009) Pimecrolimus: A New Choice in the Treatment of Vitiligo. Journal of the European Academy of Dermatology and Venereology, 23, 1347-1348. http://dx.doi.org/10.1111/j.1468-3083.2009.03228.x
- Seirafi, H., Farnaghi, F., Firooz, A., Vasheghani-Farahani, A., Alirezaie, N.S. and Dowlati, Y. (2007) Pimecrolimus Cream in Repigmentation of Vitiligo. Dermatology, 214, 253-259. http://dx.doi.org/10.1159/000099592
- Ho, N., Pope, E., Weinstein, M., Greenberg, S., Webster, C. and Krafchik, B.R. (2011) A Double-Blind, Randomized, Placebo-Controlled Trial of Topical Tacrolimus 0.1% vs. Clobetasol Propionate 0.05% in Childhood Vitiligo. British Journal of Dermatology, 165, 626-632. http://dx.doi.org/10.1111/j.1365-2133.2011.10351.x
- Dawid, M., Veensalu, M., Grassberger, M. and Wolff, K. (2006) Efficacy and Safety of Pimecrolimus Cream 1% in Adult Patients with Vitiligo: Results of a Randomized, Double-Blind, Vehicle-Controlled Study. Journal der Deutschen Dermatologischen Gesellschaft, 4, 942-946. http://dx.doi.org/10.1111/j.1610-0387.2006.06124.x
- Lepe, V., Moncada, B., Castanedo-Cazares, J.P., Torres-Alvarez, M.B., Ortiz, C.A. and Torres-Rubalcava, A.B. (2003) A Double-Blind Randomized Trial of 0.1% Tacrolimus vs. 0.05% Clobetasol for the Treatment of Childhood Vitiligo. Archives of Dermatology, 139, 581-585. http://dx.doi.org/10.1001/archderm.139.5.581
- Xu, A.E., Zhang, D.M., Wei, X.D., Huang, B. and Lu, L.-J. (2009) Efficacy and Safety of Tacrolimus Cream 0.1% in the Treatment of Vitiligo. International Journal of Dermatology, 48, 86-90. http://dx.doi.org/10.1111/j.1365-4632.2009.03852.x
- Hartmann, A. (2008) Occlusive Treatment Enhances Efficacy of Tacrolimus 0.1% Ointment in Adult Patients with Vitiligo: Results of a Placebo Controlled 12-Month Prospective Study. Acta Dermato Venereologica, 88, 474-479. http://dx.doi.org/10.2340/00015555-0464
- Al-Ani, Z.T. and Kubiasi, T.A. (2012) Treatment of Facial Vitiligo by 0.1% Topical Tacrolimus in the Iraqi Patients. Iraqi Journal of Community Medicine, 265-267.
- Giuseppe, S., Giusto, T., Cinzia, B., Giorgia, G., Sergio De, M., Nicola, M. and Pasquale, P. (2013) Narrow Band-Ul- traviolet B versus Clobetasol Propionate Foam in the Treatment of Vitiligo: A Retrospective Study. Dermatology and Therapy, 3, 95-105. http://dx.doi.org/10.1007/s13555-013-0028-8
NOTES

*Corresponding author.